Emerging Trends in Nickel Sulfide Electrodes for High-Performance Supercapacitors: Synthesis, Mechanisms, and Nanocomposite Innovations
Abstract
Electrochemical supercapacitors (SCs) are increasingly recognized as pivotal in the field of energy storage, distinguished by their substantial specific capacitance, robust cyclic stability, and remarkable power density. These devices are not only environmentally friendly but also cost-effective, which enhances their appeal in sustainable technological applications. One material that has gained significant interest lately is nickel sulfide (NiS). This compound is being explored for its potential in pseudocapacitors due to its unique chemical and physical traits that elevate electrochemical performance. This review focuses on the latest developments in SC electrodes based on NiS. It presents an in-depth analysis of the energy storage mechanisms employed by NiS, along with a comprehensive examination of the different methods used for its synthesis. The versatility of NiS allows for various nanostructural morphologies, which are crucial in optimizing its functionality and efficiency. Additionally, the integration of NiS with other materials is discussed extensively. This includes combinations with carbon, oxides, and other sulfides, forming innovative nanocomposites. These composites are crucial for enhancing the electrochemical properties and performance of SCs. The review also explores how these material integrations influence the overall energy storage capacity and efficiency, presenting a forward-looking perspective on the potential advancements in SC technology.
1. Introduction
In recent years, storage technologies involving batteries and electrochemical supercapacitors (SCs) and full cells have gotten essential interest as potential energy sources for many kinds of applications such as vehicles, electrical equipment, portable electronics, and other electronic devices. Among them, SCs have attracted considerable attention due to good electrochemical performance such as their surface reactions (adsorption/desorption and Faraday reaction), which allow them to produce the required energy faster, and provide high power density with a long life cycle [1–5]. SCs are categorized based on their mechanisms into electric double-layer capacitors (EDLCs), pseudo capacitors, as well as hybrid capacitors if both varieties of capacitors are used. In EDLC, the charge is stored electrostatically while the energy is stored through a pseudo capacitor by Faradic reaction (Figure 1).
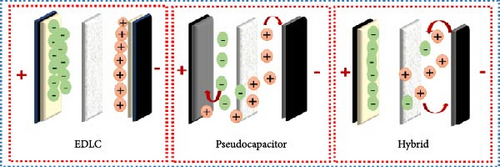
In addition, according to the electrode designs, SCs are classified as symmetric or asymmetric supercapacitor (ASC). The symmetric capacitor has two identical electrodes, but the ASC has two distinct electrodes, one made of pseudocapacitive and the other of capacitive electrode material (Figure 2). Employing diverse electrodes increases the capacitance and energy density of the energy storage system by improving the voltage window [1, 6–10]. One of the most important parts of SCs is the electrode material, and the device’s performance is directly influenced by its characteristics. The considered factors for electrode material that affect their performance are the large surface area to provide great specific capacitance (Cs), strong conductivity which minimizes charge resistance, and good chemical and thermal stability to excellent life cycles [11, 12].
SCs are frequently made of conducting polymers (CPs), transition-metal oxides (TMOs) such as CuO, MnO2, V2O5, Co3O4, and NiO, as well as TMSs such as FeS2, CoS2, MnS, NiS), and CuS. The CPs have a high capacitive performance, even though they have low charge/discharge stability [13–16]. TMOs are utilized as effective materials for SCs. These materials play a role in developing high-energy density devices because they have excellent theoretical specific capacitance and low resistance, in addition to being inexpensive and environmentally friendly [17–21]. However, transition metal oxides have poor cycle stability and low conductivity, resulting in limited mobility of electrons and a low theoretical capacity [22, 23]. On the other hand, TMSs have gotten a significant interest in SC applications [24–28]. Due to its superior characteristics to their oxides, good thermal and chemical stability, a wide range of phases, excellent electrical conductivity, richer redox reactions, lower impact on the environment, and various valence states, it can enable more electrochemical activity and have better capacitance. Furthermore, the efficacy of metal sulfide-based electrode materials can be affected by adjustments to their shape, size, and morphology [29–31]. For example, Shwetha et al. [32] investigated the electrochemical performance of CoS2, revealing high-specific capacitances of 398 F/g at 1 A/g, and it exhibited excellent cycling stability, retaining 94.1% of its capacitance after 10,000 cycles at 5 A/g. Niaz et al. [33] synthesized MoS2/polypyrrole electrodes via a hydrothermal method, reporting a specific capacitance of 654F/g after 5000 cycles with a capacitance retention of 95% at a current density of 3 A/g. Manganese sulfide is also studied by Pujari et al. as a promising material for electrochemical applications. In this study, MnS2/CNT electrodes were fabricated, and the charge storage performance demonstrates superior capacitance in Na2SO4. It achieved a high-specific capacitance of 855 F/g at 1 mA/cm2 and retained 90.1% of its capacitance after 5000 cycles [34]. The CuS/CoS electrode’s electrochemical performance was examined by Raghavendra et al.; the electrode exhibited a specific capacity of 138.75 mAh/g at a current density of 1 A/g. Moreover, it demonstrated excellent cycling stability, maintaining 87.56% of its capacity and achieving 94.28% retention in more than 4000 cycles [35].
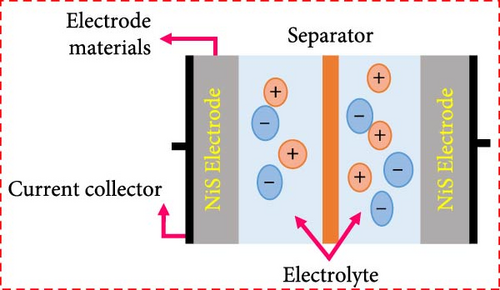
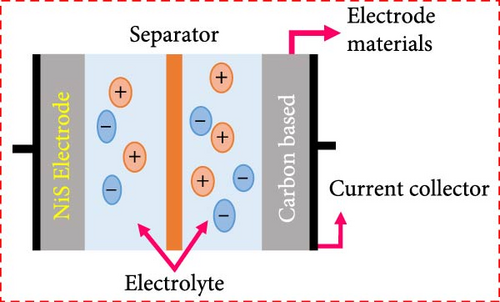
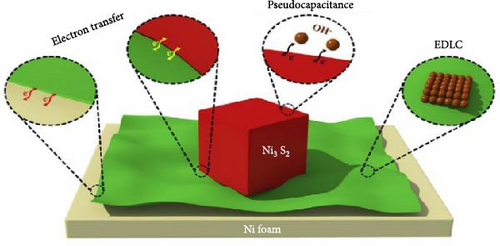
Among metal sulfides, nickel sulfide (NiS) has recently drawn a lot of attention because of its superior physical and chemical features, low cost, less toxicity, and excellent electrochemical performance. Furthermore, NiS appears in a variety of phases, α-NiS, β-NiS, Ni3S4, Ni7S6, NiS2, and Ni9S8 [36–38]. NiS (α-NiS, β-NiS) are the most stable and sulfur rich of these phases. By utilizing the influence of applied temperature, crystal formations can be produced. Where at high temperatures, the α-NiS appears hexagonal. On the other hand, the β-NiS occurs at low temperatures in rhombohedral form [36, 39]. In comparison to other NiSs, the distinct structural properties NiS have a major impact on its charge transfer efficiency. The hexagonal system in NiS’s polycrystalline structure improves its electrochemical characteristics and exhibits excellent electrical conductivity [40, 41]. As a result, NiS employed as electrode material in energy storage systems for its good features, including thermal stability, high electrical conductivity, and lower volumetric expansion during the charge and discharge mechanism (Figure 3) [36, 42]. NiS-based electrode materials are gaining considerable attention in the field of energy storage and conversion due to their unique properties and potential applications. Among the various criteria that determine the efficacy of electrode materials, NiS stands out due to its excellent electrical conductivity, high power density, low resistance, cost-effectiveness, environmental sustainability, robust capability, and impressive chemical and thermal stability. First and foremost, the excellent conductivity of NiS is pivotal for its use in electrode materials. This property ensures that electrons can flow unimpeded through the material, which is crucial for applications such as batteries and capacitors where rapid electron transfer is essential for high performance. This excellent conductivity also contributes to the high-power density of NiS-based electrodes, allowing them to deliver large amounts of power when needed. This characteristic is particularly important in applications requiring quick bursts of energy, such as in electric vehicles or for grid stabilization.
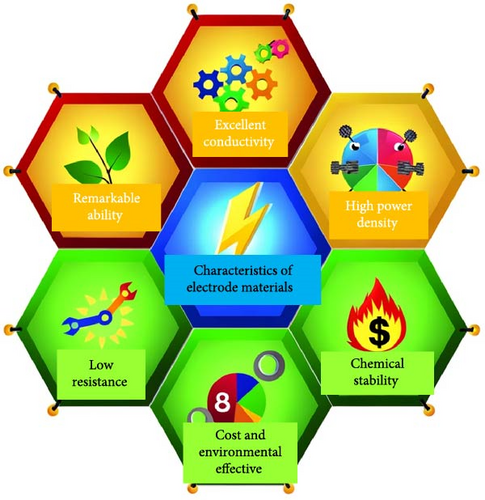
Furthermore, NiS electrodes exhibit low resistance, which minimizes energy losses during operation and improves the overall efficiency of the energy storage system. Low resistance is beneficial because it ensures that the energy being stored can be released with minimal losses, which is crucial for maintaining the efficiency of devices over time. From an economic and environmental perspective, NiS-based electrodes are highly advantageous. These materials are generally less expensive to produce compared to other electrode materials with similar properties. This cost-effectiveness makes NiS an attractive option for widespread commercial applications, thereby facilitating the adoption of green technologies. Additionally, the components used in NiS electrodes are typically more environmentally benign, which aligns with the growing demand for sustainable and eco-friendly technologies in energy storage. The capability of NiS to perform under varying operational conditions also underscores its utility as an electrode material. It can handle different charging and discharging cycles effectively without significant degradation in performance. This robust capability ensures a longer lifespan and consistent performance, which are critical factors for commercial applications and consumer electronics. Chemical and thermal stability are additional important characteristics of NiS electrodes. These properties ensure that electrodes can withstand high temperatures and harsh chemical environments, which are common in many energy storage and conversion applications. Thermal stability is particularly important in preventing overheating and ensuring the safety of the storage devices, while chemical stability protects the electrodes from degrading due to reactions with other substances, thus maintaining their integrity and functionality over time. The NiS-based electrodes are distinguished by their conductivity, high power density, low resistance, cost-effectiveness, environmental sustainability, impressive capability, and remarkable chemical and thermal stability. These properties make NiS an exceptional material for various applications in the field of energy storage and conversion, offering both performance advantages and sustainability benefits. As research and technology continue to advance, the potential applications of NiS in energy systems are expected to expand, further solidifying its role in the future of energy technology. Several studies on NiS with various phases have been synthesized with different methods hydrothermal (solvothermal), chemical precipitation, chemical deposition, sol–gel, and microwave assisted method. NiS, like other sulfides, has poor electrical conductivity and structural instability. As a result, the electron mobility necessary for electrochemical stability in energy storage devices is reduced. As a result, significant efforts have lately been made to research practical strategies for achieving outstanding performance in SCs as electrode materials to overcome these issues [43]. Nanotechnology can increase the electrochemical efficiency of the pseudo-capacitor’s electrode material because of their small size, which results in a lot of active sites and their huge specific surface area. By facilitating electron transport by offering direct pathways for electron migration, increasing electrode/electrolyte contact, promoting adsorption and desorption reactions, and allowing for faster ion diffusion [30, 44]. The most common strategy to develop NiS as an electrode material involves composition with other materials that consist of carbon, metal oxides, or other metal sulfides. High conductive compositions can enhance electrochemical properties by decreasing charge transfer resistance, which results in fast electron transport (Figure 4) [45]. Furthermore, a 3D conductive substrate, such as Ni foam, and porous carbon has been employed as a substrate with pseudocapacitive materials, which optimize the electrochemical characteristics of SCs due to the high surface area and increased active sites, which improve electron transport between electrolyte ions and electrode [36, 46, 47]. Numerous studies have explored the potential of NiSs as pseudocapacitive materials, yielding promising outcomes. For instance, Nayak et al. introduced a straightforward and scalable approach for synthesizing high-surface NiS nanostructures on nickel foam. Electrochemical analysis reveals a high energy density of 27.58 Wh/kg and excellent stability (97% retention after 5000 cycles). The electrodes also enable efficient electron/ion transfer, large surface area, and strong structural integrity. This electrode shows a high specific capacitance of 613 F/g, confirming their potential for advanced SC applications [48]. Thomas et al. report on the microwave-assisted hydrothermal synthesis of 2D NiS nanosheets. The electrode exhibits a high specific capacity of 594.77 C/g in KOH electrolyte and maintains stability over 40,000 cycles. Combined with activated carbon (AC) in an ASC, the device achieves a specific capacitance of 143.58 C/g and an energy density of 29.91 Wh/kg [49]. Chen et al. investigate a novel SC material combining carbon nanotubes (CNTs) and nickel-cobalt sulfide (NiCo2S4) nanoparticles in a three-dimensional structure. This material is synthesized using a simple one-step hydrothermal process. The CNTs/NiCo2S4 electrode exhibits outstanding energy storage performance, achieving a high specific capacitance of 890 C/g at a current density of 1 A/g. It also demonstrates excellent durability, retaining 83.5% of its capacitance after 5000 charge–discharge cycles at 10 A/g. In an ASC setup, the material delivers an energy density of 43.3 Wh/kg and a power density of 800 W/kg, highlighting its potential for high-performance energy storage applications [50]. Moreover, Liu et al. reports the successful synthesis of β-NiS nanoparticles using a simple hydrothermal method. The specific capacity of the synthesized nanoparticles was measured. NiS-15 exhibited a higher specific capacity of 638.34 C/g at a current density of 1 A/g. Additionally, hybrid SCs were assembled using AC as the anode. The NiS-15//AC achieved a superior energy density of 43.57 Wh/kg at 936.92 W/kg. The results highlight the potential of β-NiS nanoparticles as cathode materials [51].
This review demonstrates an overview of the synthesis techniques, charge/discharge process, and strategies for developing the performance of electrode materials based on NiS, involving the various nanomorphology and composite materials for SCs applications.
2. Charge Storage Mechanism in NiS-Based Electrodes
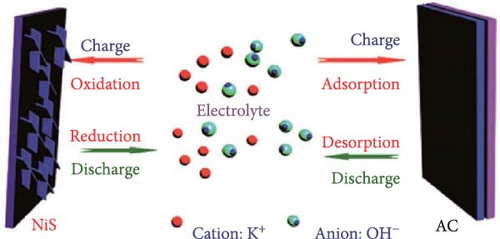
NiS electrode materials are gaining traction in the field of pseudocapacitors due to their efficient energy storage capabilities, which rely on a redox mechanism based primarily on the reversible transfer of electrons. This process plays a critical role during the charge and discharge cycles of the electrodes. During the charging phase, the NiS electrode undergoes an oxidation process. This involves the movement of hydroxide ions (OH−) towards the positively charged electrode, where they interact with NiS to form nickel hydroxide sulfate (Ni─S─OH) (Figure 5). Conversely, during the discharge phase, a reduction reaction occurs where the Ni─S─OH compound reverts to NiS, thus completing a reversible cycle. The chemical reaction can be represented by the following equation [52, 53]:
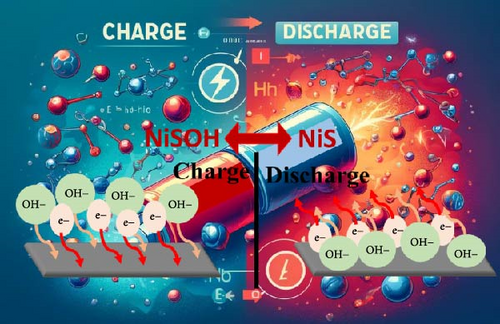
Charge(oxidation) reaction : NiS + OH− → NiSOH + e−
Discharge(reduction) reaction : NiSOH + e− → NiS + OH−
Cs: This is calculated by integrating the discharge current over time and normalizing it by the mass of the active material and the voltage change across the electrodes. It is expressed in farads per gram (F/g) and is given by the Equation (1), where I represents the applied current in milliamperes, dt is the discharge time in seconds, m is the mass of the active material in milligrams, and dV is the change in potential across the electrodes in volts.
Energy Density (E), This quantifies the amount of energy stored per unit mass and is calculated using the specific capacitance and the square of the voltage across the electrodes. It is expressed in watt-hours per kilogram (Wh/kg) and can be determined by the equation (Equation (2)). Here, V denotes the voltage across the electrodes.
Power density (P) measures the rate at which energy can be delivered by the SC and is computed by dividing the energy density by the discharge time (Equation (3)). It is expressed in watts per kilogram (W/kg) and is calculated as follows, where t is the discharge time in seconds.
These equations are essential for understanding and improving the performance characteristics of NiS-based pseudocapacitors. The selection of electrolyte plays a critical role in determining the charge storage mechanism and cycling stability of NiS electrodes. Factors such as pH, ion mobility, and electrochemical window stability significantly impact their performance. Different electrolyte types, including aqueous, organic, and ionic liquids, exhibit distinct properties that influence electrochemical behavior. NiS electrodes exhibit excellent stability in alkaline electrolytes such as KOH, enabling efficient ion intercalation and deintercalation, which significantly improves charge storage capacity [49]. Aqueous electrolytes generally possess a narrow electrochemical window, which may limit the operating voltage of NiS electrodes. However, their ability to maintain structural stability in such environments contributes to an extended cycling lifespan [54, 55]. On the other hand, organic and ionic liquid electrolytes can expand the electrochemical window, allowing for greater energy densities. They might, however, present problems with electrode stability during prolonged cycling [55, 56]. In addition, the electrolyte’s pH plays a crucial role in influencing the redox kinetics and structural stability of NiS electrodes. Alkaline environments with higher pH levels improve hydroxide ion mobility, essential for maintaining charge balance during cycling. Conversely, acidic conditions accelerate electrode material degradation, adversely affecting cycling stability [54, 57, 58].
The degradation of NiS-based electrodes over long-term cycling is a critical issue affecting their electrochemical stability. Several factors contribute to this degradation, including sulfide dissolution, volume expansion, and structural instability. Sulfide dissolution occurs when NiS interacts with the electrolyte, leading to leaching of active material, which reduces the overall capacitance over time. Additionally, the repeated charge/discharge cycles induce volume expansion, causing mechanical stress that leads to structural degradation and loss of active sites. To mitigate these issues, researchers have explored various strategies. Doping with transition metals such as Co or Mo can enhance the structural stability of NiS by reducing lattice strain and improving electron transport. Core–shell structures, such as NiS encapsulated with carbon materials, provide a protective layer that prevents direct contact with the electrolyte, minimizing sulfide dissolution. Moreover, incorporating protective coatings like graphene or metal oxides can further enhance the longevity of NiS-based electrodes by offering additional structural reinforcement and mitigating electrolyte-induced degradation. By optimizing these parameters, researchers and engineers can enhance the efficiency, capacity, and speed of charge–discharge cycles in energy storage devices. The NiS is a promising material for use in pseudocapacitors, with its performance largely hinging on the effective management of redox reactions at the electrodes. Continuous improvements in the synthesis and processing of NiS could lead to even more efficient energy storage solutions, thereby paving the way for advanced applications in both portable electronics and larger-scale energy systems [23, 30, 36, 59].
3. Synthetic Methods
Synthetic techniques play a pivotal role in determining the structural attributes and performance characteristics of materials used in advanced applications, such as SCs. For the synthesis of NiS, methodologies that are both cost-effective and straightforward are particularly favored in research settings [60]. These methods allow for the creation of NiS with diverse morphologies and sizes, typically ranging from a few nanometers up to 100 nanometers. Such variability in size and shape directly influences the electrochemical properties and efficiency of the material when used as SC electrodes. Various tables and summaries in the literature outline the most commonly utilized synthesis methods for NiS (Tables 1 and 2), reflecting a broad spectrum of techniques and their respective advantages. Additionally, the choice of precursors and solvents has been explored extensively, with researchers selecting those that best suit specific desired outcomes in terms of material performance and application feasibility. This selection process is crucial as it impacts the quality and suitability of NiS for energy storage applications, highlighting the intricate balance between synthesis method, material properties, and application requirements. The synthesis conditions of NiS, including temperature, precursor concentration, and pH, play a crucial role in determining its crystallinity, phase purity, and morphology, which ultimately impact its electrochemical performance in SCs. A detailed correlation between these parameters and the resulting structural properties is essential for optimizing NiS-based electrodes. Temperature is a key factor influencing the phase formation, crystallinity, and grain size of NiS. Different temperatures during synthesis lead to the formation of various NiS phases with distinct electrochemical properties.
| Method | Advantages | Disadvantages |
|---|---|---|
| Spray pyrolysis |
|
|
| Sonochemical |
|
|
| Microwave-assisted |
|
–Require reactants with a high absorber of irradiation. |
| Hydrothermal/solvothermal |
|
|
| Chemical bath deposition |
|
|
| Electrode | Methods | precursors | Solvent | Ref. |
|---|---|---|---|---|
| NiS@NF | Hydrothermal | C2H5NS (C)3H7NO2S NiSO4 6H2O | DI water | [36] |
| β-NiS | Hydrothermal | CH4N2S, NH3, (Ni (NO3)2 6H2O) | DI water | [61] |
| H-flower-like NiS | Solvothermal | NiCl2 6H2O (C)H3CSNH2, acetic acid | Ethanol | [30] |
| NiS@Ti substrate | Chemical bath deposition | CH3CSNH2, (Ni (NO3)2 6H2O) | Ethanol | [62] |
| NiS thin film | Chemical bath deposition | Na2S2O3 5H2O, HCl, NiSO4 | DI water | [63] |
| NiS nanoparticles | Sonochemical | Ni (CH3COO)2 4H2O (C)2H5NS | DI water | [64] |
| NiS nanoparticles | Sonochemical | Ni (CH3COO)2 2H2O, TEA (C)2H5NS | DI water | [65] |
| NiS nanoparticles | Microwave-assisted | Ni (CH3COO)2 4H2O (C)S (NH2)2 | Ethylene glycol | [66] |
| NiS films on glass | Spray pyrolysis | Ni (NO3)2 6H2O (C)S (NH2)2, acetic acid | DI water | [67] |
| NiS nanostructures | laser irradiation | Ni (CH3COO)2,CH3CSNH2, (HOCH2CH2)3N | DI water | [68] |
| β-NiS | Solvothermal | NiCl2 6H2O (C)3H6O2S or CS (NH2)2, C2H5NS | Ethylene glycol | [66] |
| NiS nanostructures | Microwave-hydrothermal | Ni (NO3)2 6H2O), NH2CSNH2, sodium dodecyl sulphate (SDS), NaC12H25SO4 | DI water | [22] |
| NiS nanorods | Template-free hydrothermal | NiCl2 6H2O, sodium thiosulphate pentahydrate (Na2S2O3 5H2O | Ethanol | [69] |
| NiS nanoparticles | Hydrothermal | NiCl2 6H2O, thiourea (H2NCSNH2), CTAB | DI water | [70] |
| Spherical-NiS | Solvothermal | NiCl2 6H2O (C)S (NH2)2 | Ethanol+ Ethylene glycol | [52] |
At higher temperatures, the reaction kinetics are accelerated, promoting crystallinity and phase purity. NiS tends to form the hexagonal α-NiS phase, which is more thermodynamically stable. This phase exhibits superior electrochemical performance due to its high electrical conductivity and well-defined grain boundaries, facilitating efficient charge transfer. At lower temperatures, the reaction kinetics slow down, favoring the formation of the rhombohedral β-NiS phase, which has different electrochemical characteristics. While β-NiS can still demonstrate good charge storage properties, it generally has lower conductivity compared to α-NiS. Controlled temperature variation can also influence nanostructure formation. For instance, moderate temperatures during hydrothermal synthesis often yield uniform nanorods or nanosheets, whereas higher temperatures (>200°C) promote the growth of three-dimensional hierarchical structures, which improve ion diffusion and electrochemical stability.
The concentration of precursors, particularly nickel and sulfur sources, significantly affects the particle size, morphology, and aggregation behavior of NiS. Higher precursor concentrations generally lead to faster nucleation and growth, producing larger nanoparticles or densely packed nanostructures. This can enhance electrode conductivity but may also result in agglomeration, reducing the effective surface area for ion interactions. Lower precursor concentrations typically result in the formation of smaller and more dispersed nanoparticles, which can provide a higher electroactive surface area. However, if the concentration is too low, it may limit the connectivity of NiS particles, increasing resistance and reducing electrochemical efficiency. The ratio of sulfur to nickel precursors is also critical. An excess of sulfur sources can lead to sulfur-rich phases (e.g., NiS2 or Ni9S8), which may exhibit different electrochemical behaviors compared to NiS. Carefully tuning the precursor concentration ensures the desired phase composition and nanostructure formation.
The pH of the synthesis solution influences nucleation rate, crystal growth, and phase stability.
In acidic conditions (low pH), the solubility of metal ions is increased, leading to a higher nucleation rate and the formation of small, uniform nanoparticles. However, extreme acidity can hinder the stability of NiS phases, potentially leading to unwanted secondary phases. In alkaline conditions (high pH), metal hydroxide intermediates form, which can slow down nucleation and result in the growth of larger, more complex structures such as nanosheets, nanorods, or flower-like morphologies. These structures can enhance the electrode/electrolyte interface, improving charge storage capacity. Maintaining an optimal pH range ensures a balance between controlled growth, high crystallinity, and stable phase formation, which is crucial for optimizing NiS-based SCs.
3.1. Hydrothermal/Solvothermal
The hydrothermal synthesis technique is widely recognized for its effectiveness in producing metal sulfides, such as NiS, and is highly valued for its ability to generate materials with tailored properties. This method operates under the principle of crystallization of particles within a sealed environment typically a hydrothermal autoclave composed of a Teflon-lined stainless-steel container [71–73]. Here, the reactants are subjected to elevated temperatures and high vapor pressures, which facilitate the formation of materials with distinct geometrical or morphological features such as thin films, single crystals, nanocrystals, and bulk particles. Several factors critically influence the outcomes of the hydrothermal process, including the pH of the solution, the concentrations of reactants, the presence of surfactants, temperature, the nature of the substrate, and the duration of the reaction. These parameters can be meticulously adjusted to achieve desired characteristics in the synthesized product, enabling precise control over the material’s physical and chemical attributes [11, 71, 72, 74]. The solvothermal method, often paired with hydrothermal techniques, shares many of its benefits but uses nonaqueous solvents to offer further versatility. This method is particularly noted for its environmental friendliness, as it typically eschews hazardous catalysts. It also allows for the facile control of particle size and shape while yielding products of high purity. However, it has its drawbacks, including slower reaction rates and challenges associated with in situ monitoring of crystal growth, which can complicate the synthesis process. In the context of SC applications, the hydrothermal method has shown considerable promise in enhancing the capacitance of NiS electrodes [30, 36]. By adjusting process parameters such as reaction temperature, duration, precursor concentration, solvent choice, and elemental composition, researchers can manipulate the size, dimension, and morphology of the resulting NiS crystals. These modifications directly impact the electrochemical performance of the electrodes, optimizing them for specific functions and operational conditions. Extensive research has explored the potential of NiS synthesized via hydrothermal methods for use in energy storage technologies, particularly in SCs. The ability to produce NiS with varied morphologies by tweaking experimental conditions has led to significant advances in this area, demonstrating the method’s adaptability and effectiveness. As such, hydrothermal and solvothermal synthesis remain at the forefront of material science research, driving developments in SC technology through the precise fabrication of innovative electrode materials.
The exploration of NiS for SC applications has been extensively documented in recent research, demonstrating the influence of synthesis techniques and experimental conditions on the material’s electrochemical properties. Al-Abawi et al. utilized a one-step solvothermal process to synthesize sphere-shaped interconnected NiS under varied conditions, including different solvents, sulfur concentrations, and durations of reaction. This meticulous approach resulted in an optimized NiS electrode with an impressive specific capacitance of 694.0 F/g [52]. Similarly, Parveen et al. investigated the impact of varying sulfur amounts on the morphology and electrochemical performance of hierarchical flower-like NiS electrodes. Their findings revealed a notable cyclic stability of 89% after 5000 cycles, with a specific capacitance of 603.9 F/g [30]. Furthermore, Naresh et al. assessed the capacitive performance of NiS under different morphologies created via a hydrothermal method by adjusting the reaction time. Their studies showed that the electrode could achieve an energy density of 23.19 Wh/kg at a power density of 243.9 W/kg [36]. Bhardwaj et al. focused on the synthesis of β-NiS using the hydrothermal method and explored its performance in various electrolytes. They discovered that β-NiS in one-dimensional form exhibited high electrochemical performance in LiOH electrolytes, achieving a specific capacitance of 3.66 F/cm² and maintaining 88% cyclic stability over 1000 cycles [39]. In a similar vein, Manikandan et al. crafted a NiS2@NiV2S4 electrode through the hydrothermal route, employing a KOH electrolyte. This electrode demonstrated a higher capacity of 520 C/g at a current density of 1 A/g, showcasing its potential for high-capacity energy storage [75]. These studies collectively highlight the critical role of synthesis conditions in tailoring the properties of NiS for specific applications in SCs. The ability to manipulate experimental parameters such as solvent type, sulfur content, and reaction duration allows for the fine-tuning of NiS’s electrochemical characteristics, making it a versatile and effective material in energy storage technologies. Each of these research endeavors underscores the potential of NiS as a transformative material for SC electrodes, with advancements in synthesis methods leading to electrodes that not only offer high capacitance but also demonstrate robust cyclic stability under prolonged use. This ongoing research is paving the way for more efficient, durable, and high-performing SCs, which are crucial for meeting the growing demands of energy storage systems.
3.1.1. Microwave Assisted
Microwave-assisted synthesis has emerged as a highly efficient technique for the production of NiS nanoparticles, leveraging the unique properties of microwave radiation to enhance the synthesis process. This method employs microwave irradiation as a direct source of heat, which is distributed almost instantaneously throughout the reaction system. This rapid heating leads to the quick decomposition of starting materials and fast nucleation, resulting in the formation of finely scaled nanoparticles within reduced reaction times [72, 74, 76, 77]. The integration of microwave techniques with other synthesis processes such as hydrothermal, solvothermal, and ultrasonic methods enhances the efficiency of the process, allowing for the production of nanoparticles. One of the critical considerations for the microwave method is that the reactants must efficiently absorb microwave radiation to ensure effective heating and subsequent reactions. The microwave-assisted approach offers several distinct advantages for synthesizing NiS. Firstly, the uniform microwave energy distribution ensures low temperature gradients within the medium, which facilitates the production of nanoparticles that are consistent in size and high in purity. Secondly, the reaction rates are significantly accelerated by microwave irradiation, which not only speeds up nucleation but also reduces overall synthesis time, thus enhancing throughput and energy efficiency.
Additionally, the method is known for its high yield, making it a cost-effective option for large-scale production. The electrochemical properties of NiS electrodes produced through microwave-assisted synthesis are notably superior. These electrodes typically exhibit high specific capacitance, prolonged cycle life, and reduced resistance, which are critical attributes for SCs [78, 79]. For instance, Peng et al. [78] utilized this method to produce α-NiS nanoparticles with a uniform size of 100 nm, demonstrating excellent electrochemical performance characterized by high specific capacitance and extended cyclic stability. Further comparative studies, such as those conducted by Nandhini et al., have explored the impact of different synthesis techniques on the properties of NiS nanostructures. Their research indicated that using a combination of hydrothermal and microwave-assisted methods yielded hexagonal NiS phases, while employing solely microwave synthesis resulted in the formation of Ni9S8. These findings underscore the ability of microwave-assisted synthesis to manipulate the structural phases of NiS, thus influencing its functional properties in SC applications [22]. Overall, microwave-assisted synthesis stands out as a powerful and versatile technique for the production of NiS nanoparticles, offering significant advantages in terms of speed, efficiency, and quality of the final product. Its ability to be integrated with other synthesis methods and its impact on the electrochemical performance of NiS make it a valuable tool in the field of material science and energy storage technologies.
3.2. Chemical Bath Deposition
The chemical bath deposition (CBD) is a highly effective method for the synthesis of metal sulfides, particularly NiS, suitable for SC electrode materials. This technique involves immersing substrates in a precursor solution, where metal ions react to forming a continuous thin film of metal sulfide on the substrate. The deposition occurs in an aqueous bath under controlled conditions of temperature, with the capability to finely tune the composition of the resulting film by adjusting parameters such as pH, concentration of reagents, and the reaction temperature itself [11, 63, 74, 80]. CBD offers significant advantages for the production of NiS films, especially in terms of scalability and cost-effectiveness. The method is notably simple, requiring low operational temperatures, which makes it both energy-efficient and economically viable for large-scale applications. This simplicity does not come at the expense of quality; the films produced are uniform and adhere well to a variety of substrate materials [62, 63]. However, this method results in significant material waste and is unsuitable for producing metallic films [81]. The electrochemical performance of NiS films synthesized through CBD is commendable, characterized by long cycle lives and high specific capacitances. For instance, Patil et al. utilized this method to deposit a thin film of NiS on a stainless-steel substrate using NiSO4, trimethylamine (TEA), and Na2S2O₃ 5H2O as precursors. The resulting electrode, tested in a KOH electrolyte, exhibited an impressive specific capacitance of 750.6 F/g at a scan rate of 5 mV/s, maintaining 92% of its capacitance over 2500 cycles [63]. Such results demonstrate the durability and efficiency of NiS films in SC applications. Similarly, Gaikar et al. employed the CBD technique to fabricate NiS on a titanium (Ti) substrate. The thin film produced showed outstanding electrochemical characteristics, with a specific capacitance of 788 F/g at a current density of 1 mA/cm², along with an energy density of 27.4 Wh/Kg and a power density of 3.05 KW/Kg [62]. These figures highlight the potential of CBD-derived NiS films as high-performance electrode materials in SCs. The success of CBD in these studies underscores its value in the fabrication of advanced materials for energy storage. By enabling the deposition of NiS in a cost-effective and scalable manner, CBD enhances the accessibility of high-quality electrode materials. This contributes significantly to the development of more efficient and durable SCs, crucial to advancing energy storage technology. The consistent results across different research efforts confirm that CBD is a reliable and potent method for producing electrode materials that meet the demanding requirements of modern energy storage solutions.
3.3. Spray Pyrolysis
Spray pyrolysis is a versatile synthesis technique used to produce nanomaterials, such as NiS, in forms including thin and thick films and powders. This method utilizes a relatively simple setup consisting of a precursor solution, a substrate heater, a temperature regulator, and an atomizer. The process begins with the creation of an aerosol from the precursor solution, which could be a metallic salt or colloidal solution [76, 82]. Air, serving as a carrier gas, atomizes the solution into fine droplets that are subsequently transported into a furnace. Here, the aerosol undergoes a series of transformations involving evaporation, precipitation, drying, and pyrolysis to yield the final product [82–84]. One of the primary advantages of spray pyrolysis is its lack of reliance on costly equipment or high-quality substrates, making it an economical choice for material synthesis. Furthermore, the method allows for substantial control over the nanoparticle properties by adjusting various parameters such as pH, flow rate, reactant quantity, and the conditions under which the preparation takes place. These factors can be finely tuned to influence the particle size and shape, directly affecting the material’s physical and chemical properties. Despite its advantages, spray pyrolysis does present some challenges. Controlling the growth temperature can be difficult, and the method may yield relatively lower quantities of the final product compared to other techniques. Additionally, there is a risk of sulfide oxidation when the process is carried out in an air atmosphere, which could potentially alter the material’s desired properties [76, 85, 86]. The application of spray pyrolysis in the synthesis of NiS films has been particularly noted for producing films with large surface areas and excellent substrate adhesion. Altering the concentration of the precursor solution can lead to variations in morphology, which in turn impacts the electrochemical performance of the material [87]. Gahtar et al. conducted a study focusing on the effects of different precursor concentrations on NiS films produced by spray pyrolysis. Their findings indicated that an increase in precursor concentration led to larger crystal sizes and increased roughness on the film surface. These physical changes contributed to enhancing the electrode’s conductivity and specific capacitance, crucial factors for improving the performance of SCs [67]. Overall, spray pyrolysis remains a valuable method for the fabrication of NiS and other nanomaterials, offering a balance between cost-efficiency and the ability to tailor material characteristics. Continued research and optimization in this area are expected to further enhance the capabilities and applications of spray pyrolyzed materials in advanced energy storage systems like SCs.
3.4. Sonochemical
The sonochemical method, utilizing ultrasonic irradiation, is an innovative approach for synthesizing materials with distinctive crystalline structures. This technique capitalizes on the effects of ultrasonic waves on liquids, which lead to the formation of cavitation. These cavities generate localized high temperatures and pressures, alongside rapid cooling rates, creating a unique environment conducive to chemical reactions under extreme conditions [71, 72, 88]. Sonochemistry offers several advantages in the synthesis of nanoparticles, particularly in terms of the rate of production and simplicity of the procedure. It also allows for precise control over the morphology of the nanoparticles by adjusting factors such as temperature and the duration of exposure to ultrasonic waves. However, this method does come with limitations, primarily its unsuitability for heat-sensitive materials and its relatively high energy consumption, which could be a drawback for large-scale applications [71, 89]. In terms of material synthesis, the sonochemical approach has been successfully applied to the production of NiS [64, 65]. The ability to fine-tune the intensity of ultrasonic irradiation enables the manipulation of NiS morphology, thus tailoring its properties for specific applications. For example, Wang et al. [65] utilized a sonochemical method employing nickel acetate (Ni (CH3COO)2) as the nickel source and thioacetamide as the sulfur source, with triethanolamine acting as a complexing agent. The resultant NiS nanoparticles displayed uniform shapes, narrow size distributions, and high purity. Further research by Kristl et al. explored the sonochemical synthesis of NiS using different sulfur precursors alongside nickel acetate tetrahydrate (Ni (CH3COO)2 4H2O). These included thioacetamide, thiourea, and sodium thiosulfate pentahydrate. The study highlighted thioacetamide as the most effective sulfur source, yielding the highest purity and a production efficiency of up to 70%. This indicates the potential of thioacetamide in enhancing the yield and quality of NiS nanoparticles synthesized via sonochemical processes [64]. The exploration of various sulfur sources and adjustments in ultrasonic intensity underscores the versatility and adaptability of the sonochemical method in nanoparticle synthesis. By optimizing these parameters, researchers can achieve desired outcomes in terms of particle morphology, purity, and yield. The ongoing developments in sonochemical techniques continue to push the boundaries of material science, offering promising prospects for the efficient and controlled production of advanced materials like NiS, which are crucial for applications in fields such as energy storage and electronics.
4. Nickel Sulfide Structures
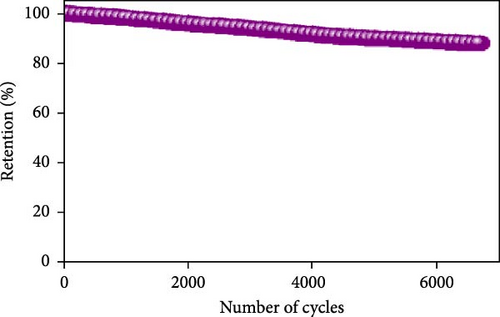
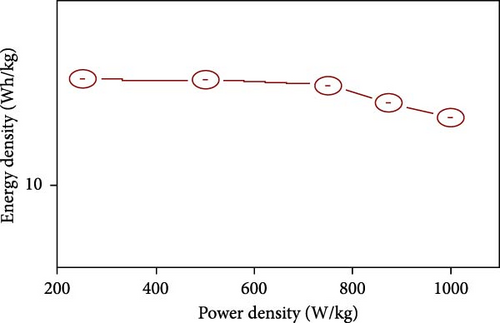
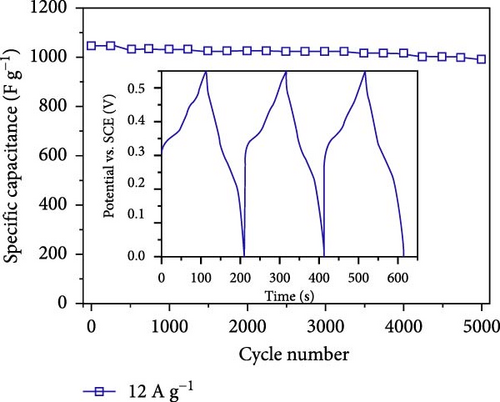
The field of SCs has witnessed significant advancements through the exploration of various NiS nanostructures, each presenting unique electrochemical properties due to their distinct morphologies and phases. These structures range from zero-dimensional spheres to complex three-dimensional forms, each tailored to enhance the performance of SCs in specific ways. One of the most promising nanostructures is the spherical NiS, which provides a large surface area that facilitates more active sites for ion and electrolyte interaction (Table 3) [39]. NiS nanostructures exhibit diverse morphologies, ranging from zero-dimensional nanoparticles to complex three-dimensional architectures, each offering distinct electrochemical advantages and limitations. Zero-dimensional NiS spheres provide a high surface area, promoting increased electroactive sites and efficient ion diffusion, which enhances charge storage capabilities. However, their structural stability can be compromised due to agglomeration during prolonged cycling. One-dimensional structures, such as nanorods and nanowires, facilitate faster electron transport pathways due to their elongated geometry, yet they often suffer from lower surface area compared to 0D structures. Two-dimensional morphologies, including nanosheets and nanoplates, offer a large electrode/electrolyte interface, improving ion accessibility and redox reaction kinetics. Despite these advantages, their tendency to restack can reduce active surface area over time. Three-dimensional architectures, such as hierarchical microflowers and porous frameworks, combine high surface area with structural robustness, ensuring superior electrochemical stability and long-term cycling performance. However, the complexity of fabricating such structures can pose challenges for large-scale production. By systematically analyzing these morphologies, it becomes evident that the choice of NiS structure plays a crucial role in optimizing SC performance, balancing factors such as capacitance, cycling stability, and charge transport efficiency. Al-Abawi et al. synthesized spherical NiS directly on three-dimensional Ni foam using solvothermal methods. The SEM results confirmed the spherical morphology (Figure 6a), which contributed to an enhanced electrochemical capacity. Electrochemical tests on these electrodes in a three-electrode system demonstrated excellent cycle stability, retaining 88% of its capacity after 6700 cycles (Figure 6b) and displaying a power density of 250.93 W/kg and an energy density of 24.9 Wh/kg (Figure 6c) [52].
| Electrode | Morphology | Specific capacitance | Current density | Retention (%) | Number of cycles | Ref. |
|---|---|---|---|---|---|---|
| SS-NiS@3DNF | Spherical shape | 694.0 F/g | 1 A/g | 88 | 6700 | [52] |
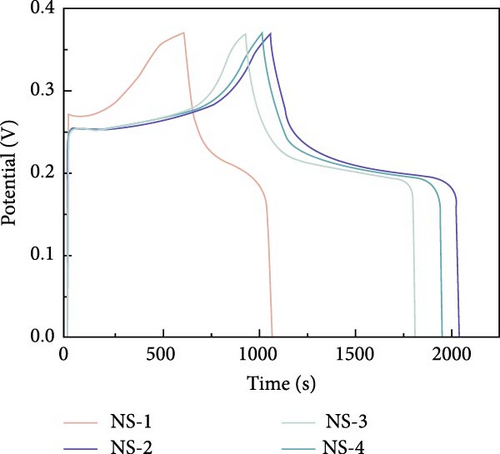
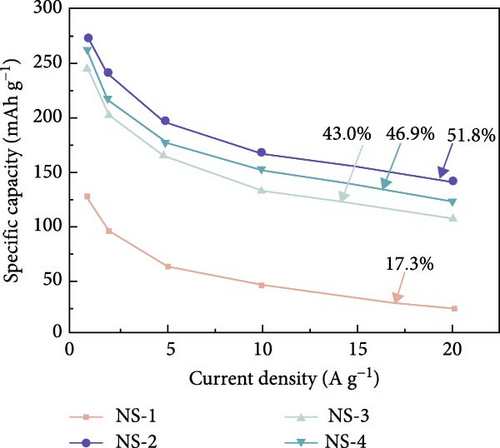
| β-NiS | Microspheres | 273.1 mAh/g | 1 A/g | 51.8 | — | [90] |
| NiS@NF | Hollow spheres | 1553 F/g | 2.35 A/g | 95.7 | 2000 | [91] |
| Ni3S2 | Hollow spheres | 1460 F/g | 4 A/g | 93.6 | 5000 | [92] |
| NiS @Ni foam | Nanosheet | 2587 F/g | 0.2 A/g | 95.8 | 4000 | [93] |
| NiS | Hierarchical-flower-like | 603.97 F/g | 1 A/g | 88.57 | 5000 | [30] |
| NiS/Ni | Nanorods | 1097 F/g | 2 mA /cm2 | 80 | — | [69] |
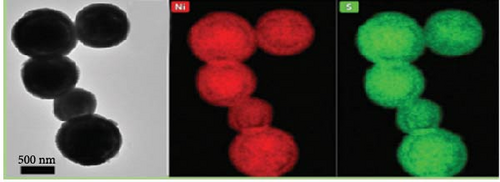
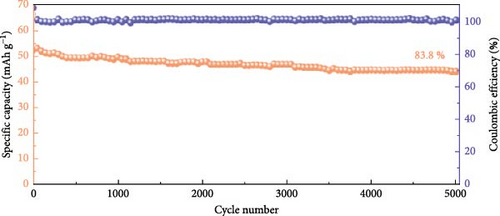
In another innovative approach, Hu et al. developed β-NiS utilizing a ball-in-ball formation process via a vulcanization reaction guided by Ostwald ripening. This unique structure, with sizes ranging from 500 nm to 2 µm (Figure 7a, b), was shown to have a uniform distribution of nickel and sulfur elements across both the core and shell of the microspheres (Figure 7c), as revealed by TEM imaging and elemental mapping. This β-NiS demonstrated outstanding electrochemical properties with a power density of 799.0 W/kg and an energy density of 46.4 Wh/kg, alongside remarkable discharge times and specific capacitance. The cycle stability was also impressive, maintaining 83.8% capacity after 5000 cycles (Figure 7d–f) [90].


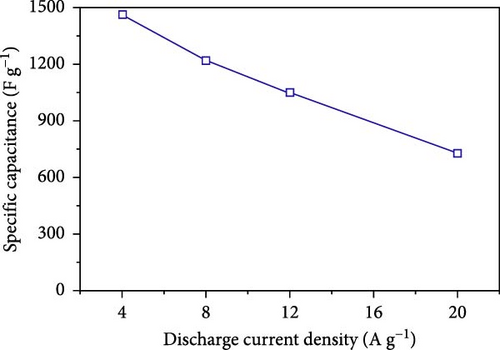
Further exploring the possibilities of nanostructuring, Chen et al. synthesized Ni3S2 hollow nanospheres using a simple template-engaged method (Figure 8a–c). These hollow structures facilitated enhanced ion transport, significantly improving the electrochemical characteristics of the electrodes. The specific capacity achieved was 1460 F/g at 4A/g and 727 F/g at 20A/g (Figure 8d), with a capacity retention of 93.6% over 5000 charge/discharge cycles (Figure 8e) [92]. Tran et al. applied NiS hollow spheres to a nickel foam surface through an electrodeposition procedure, creating SC electrodes with exceptional longevity. These electrodes exhibited a specific capacitance of 1553 F/g at a current density of 2.35 A/g and maintained 95.7% of their initial capacity after 2000 cycles, highlighting the potential of nanostructured NiS in enhancing SC performance [91].
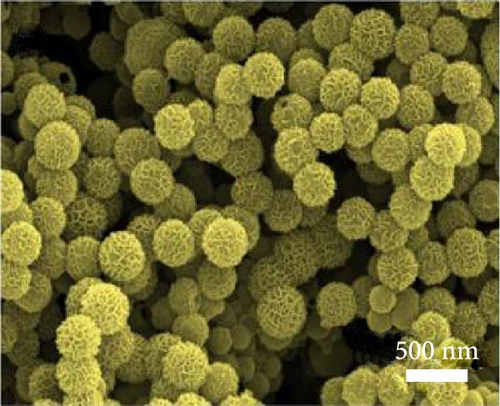
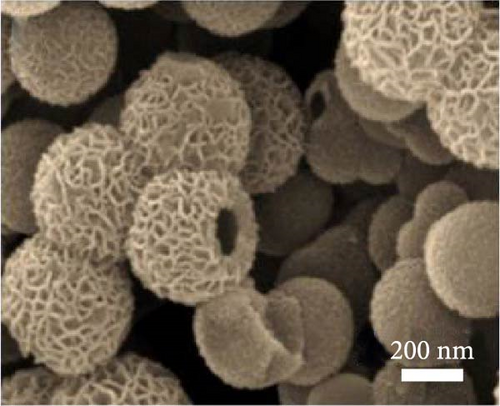

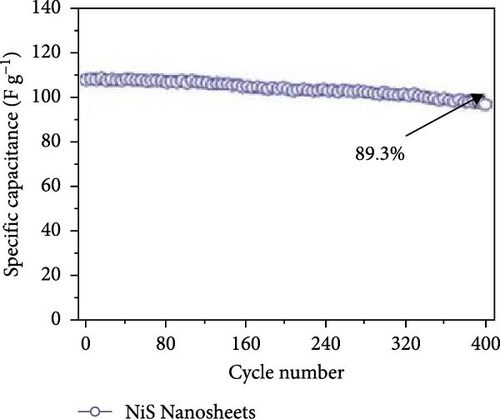
The solvothermal method was also employed by Yan et al. to develop NiS nanosheets on Ni foam. The rough surface of these nanosheets increased the electroactive area, leading to a significant enhancement in capacitance (Figure 9a–d). The electrochemical analysis revealed an outstanding capacity of 2587 F/g at 0.2 A/g (Figure 9e). When used in ASCs paired with active carbon (Figure 9f, g), these nanosheets delivered a capacity of 106 F/g at 2A/g with a cycle stability of 89.3% over 400 cycles (Figure 9h) [93].
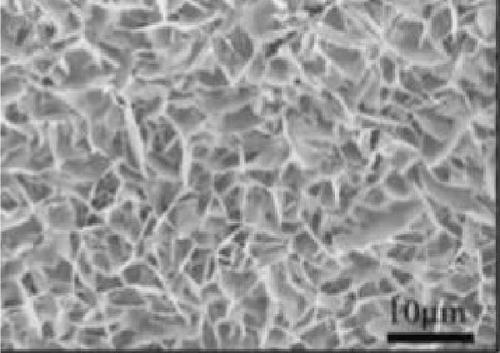
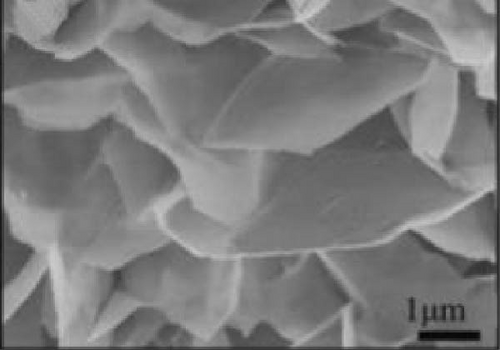
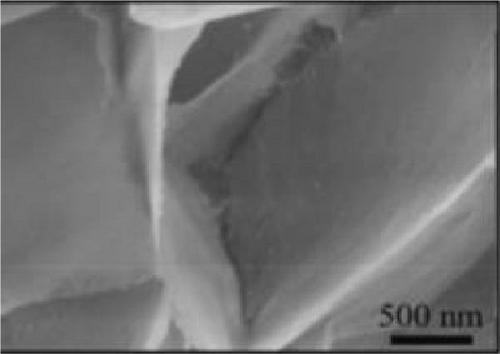
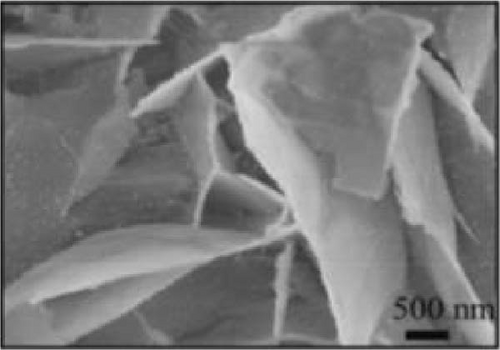
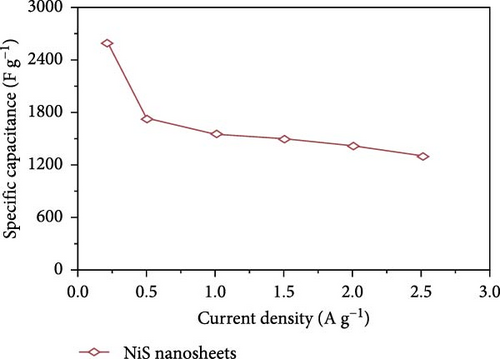
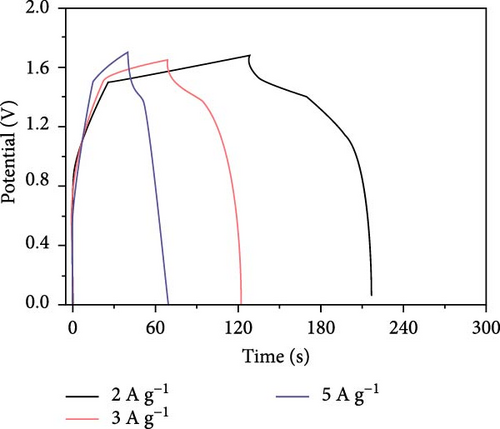
Bhagwan et al. [27] synthesized a three-dimensional micro-flower electrode based on β-NiS with hierarchical geometry, achieving a specific capacitance of 1529 F/g. This structure not only provided high capacitance but also demonstrated excellent cycle stability. Parveen et al. crafted a 3D flower-like morphology of NiS, optimizing experimental conditions to enhance the hierarchical porous structure (Figure 10). This design contributed to a SC device that exhibited a specific capacitance of 11.15 F/g, with energy densities of 0.991 Wh/kg at a power density of 132 W/kg, and 0.329 Wh/kg at 261.11 W/kg, maintaining a cycle life efficiency of 88.57% after 5000 cycles [30].
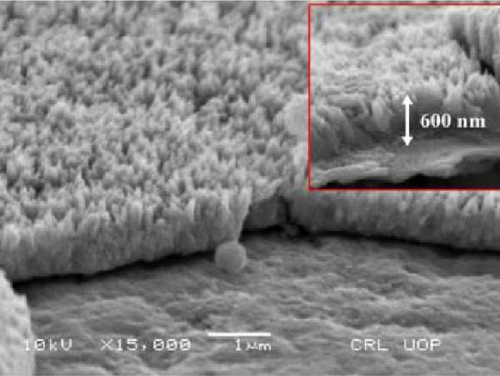
Lastly, Mehmood et al. explored the effects of varying precursor concentrations on the growth of NiS nanorods on Ni foam sheets (Figure 11). These nanorods displayed remarkable charge storage characteristics, with a specific capacitance of 1097 F/g at a current density of 2 mA/cm2, and an energy density of 484 Wh/kg at a power density of 0.79 W/kg [69]. The exploration of these varied nanostructures illustrates the dynamic possibilities within NiS’s application in SCs. Each morphology, from spherical to nanosheet, offers unique benefits in terms of surface area, ion transport, and electrochemical stability, pointing to a rich area of continued research and development in advanced energy storage solutions.
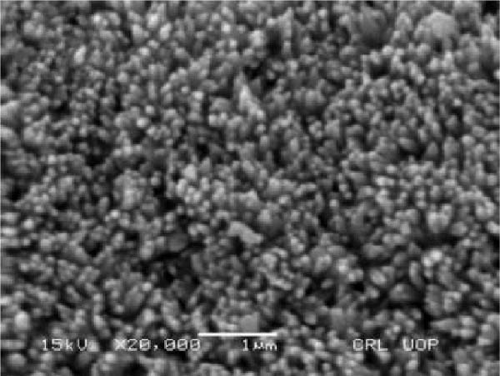
5. NiS Nanocomposites-Based Electrode Materials
NiS nanocomposites have garnered significant attention in the development of advanced electrode materials for SCs due to their enhanced electrochemical characteristics. A variety of composite materials, particularly carbon-based substances such as CNTs, graphene (G), and reduced graphene oxide (rGO), as well as transition metal oxides and sulfides, are frequently combined with NiS to boost its performance. These composites are strategically engineered to leverage the synergistic effects between NiS and the additional components, enhancing properties such as conductivity, electrochemical stability, and specific capacitance. The integration of NiS with these materials not only improves the charge storage capacity but also the overall efficiency and durability of the electrodes [60]. A summarized overview of the electrochemical performance of various NiS-based composite materials can be found in academic resources, providing valuable benchmarks for ongoing research and application in energy storage technologies (Table 4).
| Electrode | Methods | Electrolytes | Specific capacitance | Current density | Retention (%) | No. cycles | Ref. |
|---|---|---|---|---|---|---|---|
| NiS/ACNTs | Hydrothermal-annealing process | KOH (3 M) | 1028 F/g | 10 A/g | 81 | — | [94] |
| NiS/CNTs@NF | Hydrothermal | KOH (3 M) | 732 F/g | 1 A/g | 84.5 | 3000 | [95] |
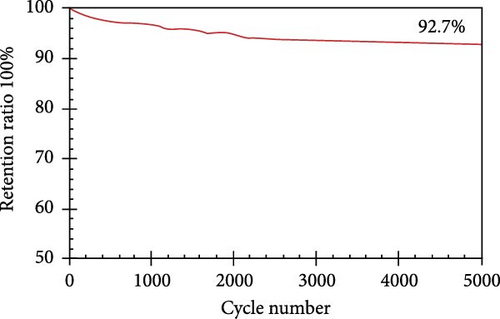
| rGO-Ni3S | Hydrothermal | KOH (6 M) | 616 C/g | 1 A/g | 92.7 | 5000 | [45] |
| Ni9S8/GF | CBD | KOH (1 M) | 2055 F/g | 2 A/g | — | — | [96] |
| a-NiS/Ni3S4 | Hydrothermal | KOH (2 M) | 214.9 mAh/g | 2 A/g | — | — | [38] |
| NiS/Co3S4@Ni | Hydrothermal | LiOH (4 M) | 1893 C/g | 1 A/g | 98.63 | 10,000 | [44] |
| NiS2@MoS2 | Hydrothermal | KOH (6 M) | 848.2 C/g | 1 A/g | 83.1 | 10000 | [97] |
| NiS-PbS | Chemical bath deposition | KOH (3 M) | 125.89 mAh/g | 2 A/g | 88.97 | 3000 | [98] |
| Ni3S2/MnS/rGO | Hydrothermal-anion exchange method | KOH (2 M) | 3374.6 F/g | 1 A/g | 90.7 | 8000 | [61] |
| NiS@CoS | Hydrothermal - Electrodeposition | KOH (2 M) | 1210 F/g | 1 A/g | 80.94 | 2000 | [99] |
| NiS/SnS2@CC | Cation-exchange | KOH (6 M) | 430.38 mAh/g | 1.16 A/g | 82.6 | 1000 | [100] |
| NiO/NiS@CNT | Microwave irradiation | KOH (6 M) | 809.7 F/g | — | 100 | 20,000 | [101] |
| ZnO/NiS/CNT | Solvothermal | KOH (3 M) | 879.0 F/g | 1.5 A/g | 89.7 | 5000 | [102] |
| MnO2@NiS2/Ni (OH)2 | Hydrothermal | — | 785 F/g | 1 A/g | 78 | 3000 | [103] |
5.1. NiS With Carbon Compositions
The integration of NiS with carbon-based compounds has revolutionized the field of SCs by enhancing the electrochemical performance of electrodes through improved electrical conductivity, increased surface area, and enhanced stability [60]. This synergy is well illustrated in various studies, each demonstrating significant advancements in SC technology through the development of NiS/carbon composites (Figure 12). One notable study by Ouyang et al. described the synthesis of NiS/AC nanotube (ACNT) nanocomposites using a combined annealing and hydrothermal approach.
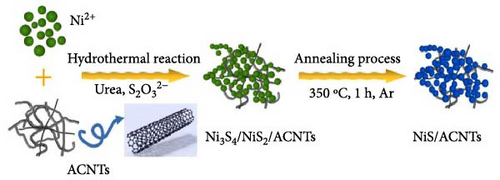
The resulting NiS/ACNT hybrid electrode exhibited a high specific capacitance of 1266 F/g at a current density of 1.0 A/g. Even more impressively, it maintained a capacitance of 1028 F/g at the increased current density of 10 A/g, with 81% of the initial capacitance retention after prolonged cycling. This electrode was also utilized in an ASC setup with active carbon as the anode, where it showed excellent cycling stability, retaining 83% of its capacity after 2000 charge/discharge cycles at a current density of 2.0 A/g. Moreover, this configuration delivered a commendable energy density of 36.0 Wh/kg at a power density of 806 W/kg, showcasing the potential of NiS/ACNTs in high-performance energy storage applications [94]. Similarly, Sabeeh et al. developed electrodes from NiS/CNTs on nickel foam (NiS/CNTs@NF), which capitalized on the enhanced conductive properties and larger surface area provided by the CNTs. This electrode configuration achieved a specific capacitance of 732 F/g, which is a significant improvement over the 405 F/g recorded for NiS@NF alone [95]. The increased surface area and conductivity facilitated by the CNTs evidently play a crucial role in enhancing the electrochemical performance of the electrodes. Further advancing the field, Zhang et al. reported the synthesis of a NiS/rGO nanocomposite using a solid-phase technique. This composite not only exhibited a high specific capacity of 2157.8 F/g at a current density of 2 A/g but also maintained an impressive 92.4% of this capacity after 30,000 cycles. Additionally, the SC device that incorporated NiS/rGO and interconnected hierarchical porous carbon delivered an energy density of 56.1 Wh/kg at a power density of 880 W/kg, highlighting the robustness and efficiency of this material combination [95]. Namdarian et al. took a different approach by synthesizing Ni3S2 nanocubes integrated with rGOs using a straightforward, template-free hydrothermal method. This rGO-Ni3S2 electrode demonstrated a specific capacity of 616 C/g at 1 A/g of applied current density and showcased an exceptional cycling lifetime with a retention of 92.7% after 5000 cycles. The uniform growth of Ni3S2 cubes on rGO sheets not only increased the specific surface area but also enhanced the double-layer capacity and facilitated faradaic redox processes [104, 105], significantly raising the composite’s specific capacity (Figure 13) [45].
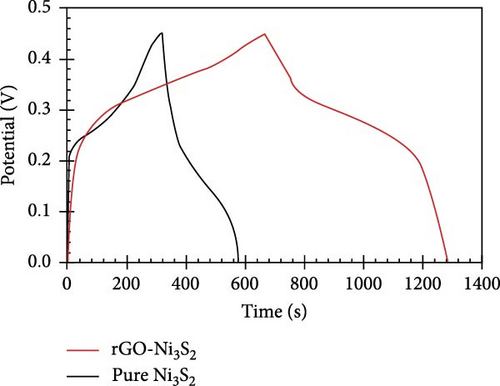
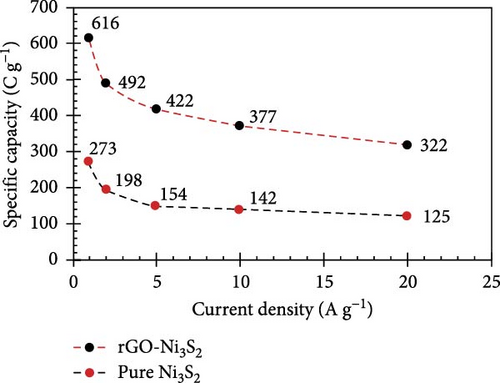
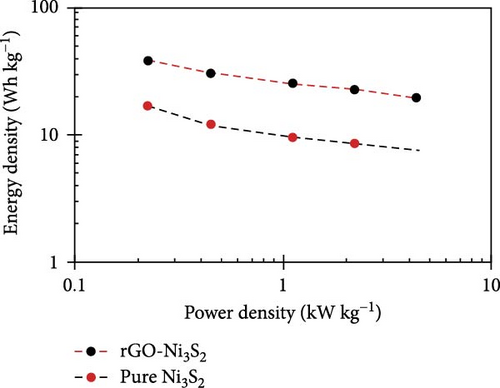
In another innovative approach, Patil et al. utilized a simple CBD technique to develop Ni9S8 on graphene foam (Ni9S8/GF). This material was tested in an ASC device paired with active carbon/nickel foam (AC/NF), achieving a specific capacitance of 143.7 F/g at 3.3 A/g, along with an energy density of 51.11 Wh/kg and a power density of 2.66 kW/kg [96]. The three-dimensional graphene foam not only provided a large surface area but also enhanced the conductivity and facilitated electron transport, significantly improving the supercapacitance of the device. These examples underscore the transformative impact of combining NiSs with various forms of carbon in SC electrodes. The incorporation of carbon materials such as ACNTs, CNTs, rGO, and graphene foam into NiS-based composites has led to remarkable improvements in specific capacitance, energy density, power density, and cycling stability. This synergy not only enhances the functional characteristics of the electrodes but also broadens the potential applications of SCs in energy storage, paving the way for the next generation of high-performance supercapacitive devices.
5.2. NiS With Metal Sulfide
In the evolving landscape of SC technology, the combination of NiS with other transition metal sulfides has demonstrated significant potential in enhancing electrochemical performance. This synergistic approach results in composites that outperform monometallic sulfides in terms of specific capacitance, cyclic life, conductivity, and optical properties due to narrower band gaps and increased faradaic actions [61]. One pioneering study by Hu et al. showcased the development of an α-NiS/Ni3S4 composite electrode, fabricated by finely tuning the molar ratios of thiourea and 2-mercaptopropionic acid, which served as sulfur sources. This methodological precision in synthesis allowed for the formation of a composite that combines the unique properties of both α-NiS and Ni3S4, as evidenced by X-ray diffraction patterns confirming the presence of both phases (Figure 14a). The electrochemical performance of this composite was remarkable, achieving a specific capacity of 214.9 mAh/g at a current density of 2 A/g when the sulfur source ratio was optimized (Figure 14b). Moreover, this composite exhibited robust cyclic performance, maintaining structural integrity and functionality after 5000 cycles (Figure 14c) [38].
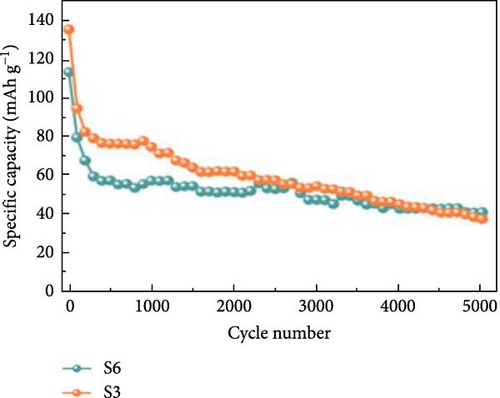
Expanding upon this theme, Zhang et al. developed a Ni3S2/MnS (NMS) composite using a hydrothermal technique followed by an anion exchange process on rGO-coated nickel foam. The NMS/rGO electrode not only provided a high specific capacitance of 3374.6 F/g at a current density of 1 A/g but also demonstrated extraordinary durability with a cycling life of 90.7% capacity retention over 8000 cycles at 10 A/g [61]. This durability underscores the structural and chemical stability imparted by the composite’s design. In another notable investigation, Miao et al. synthesized NiS@CoS by electrodepositing CoS onto a hydrothermally prepared NiS layered sheet on carbon cloth (Figure 15a). This innovative electrode showcased a specific capacitance of 1210 F/g at 1 A/g, significantly higher than that of pristine NiS and CoS, which delivered 1080 F/g and 140 F/g, respectively (Figure 15b). The NiS@CoS electrode maintained 87.2% of its initial capacitance after 2000 cycles at 10 A/g, illustrating excellent cyclic stability. Additionally, when used in an ASC with AC, it exhibited a specific capacitance of 75.9 F/g, alongside an energy density of 24.1 Wh/kg and a power density of 752.15 W/kg, showcasing its effectiveness in a practical device configuration (Figure 15c) [99]. Further illustrating the versatility of NiS composites, Guan et al. employed a facile cation-exchange technique to develop a permeable NiS/SnS2 structure on carbon cloth. This electrode benefited from an increased surface area and enhanced reaction kinetics, which are critical for high-performance energy storage applications. The proximity of the NiS shell to the SnS2 core facilitated beneficial interactions, boosting the redox activity and, consequently, the overall electrochemical performance of the electrode [100].
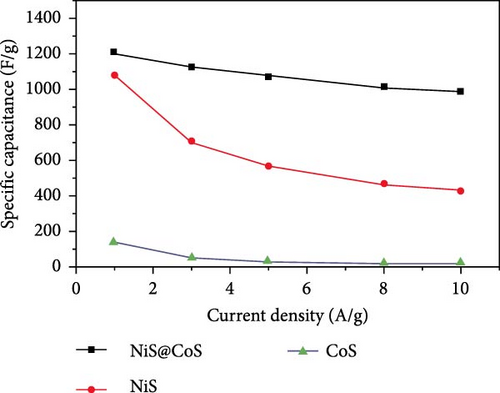
These studies collectively highlight the advantages of integrating NiS with other metal sulfides, which not only enhance the electrochemical properties through synergistic effects but also contribute to the development of SCs with higher energy densities and longer life spans. The innovative combinations of NiS with various sulfides such as Ni3S4, MnS, CoS, and SnS2 demonstrate a promising route towards the next generation of energy storage devices. Each composite brings unique properties to the table, resulting in electrodes that offer high capacitance, exceptional cycle stability, and improved kinetic profiles, thus meeting the increasing demands of advanced SC applications. The performance of NiS-based electrodes in hybrid SC-battery systems, highlighting their potential for energy storage applications. NiS electrodes, when incorporated into asymmetric or hybrid cell configurations, demonstrate a balance between faradaic and non-faradaic charge storage mechanisms. The inclusion of NiS nanosheets on Ni foam in ASCs, coupled with active carbon as the anode, enhances the electrochemical performance by improving the overall specific capacitance and energy density. The study reports a specific capacitance of 2587 F/g at 0.2 A/g, with a stable cycle life of 89.3% retention after 4000 cycles. Further, NiS-based hybrid configurations exhibit enhanced charge transfer kinetics and high-rate capability. The integration of NiS with conductive carbon materials, such as AC nanotubes, has been shown to significantly improve electrochemical properties. In an ASC setup, a NiS/ACNT hybrid electrode paired with active carbon delivered an energy density of 36.0 Wh/kg at a power density of 806 W/kg, maintaining 83% of its capacitance after 2000 cycles. Another approach employs NiS combined with rGO, where the hybrid electrode achieves an ultra-high specific capacitance of 2157.8 F/g at 2 A/g and retains 92.4% capacity after 30,000 cycles. This suggests that optimizing hybrid electrode architectures can significantly improve long-term stability and energy storage performance. A detailed analysis of the charge storage mechanisms within these hybrid configurations, where the faradaic reactions of NiS contribute to high-capacity energy storage, while the non-faradaic contributions from carbon-based materials enhance conductivity and cycling stability. However, further research into optimizing interfacial charge transfer and mitigating volume expansion effects in NiS-based hybrid electrodes is necessary to improve performance in practical applications.
5.3. NiS With Metal Oxides
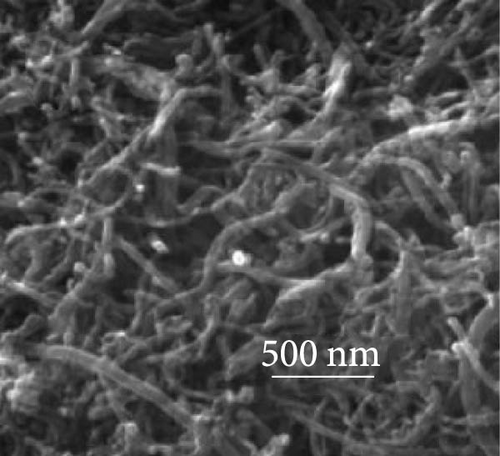
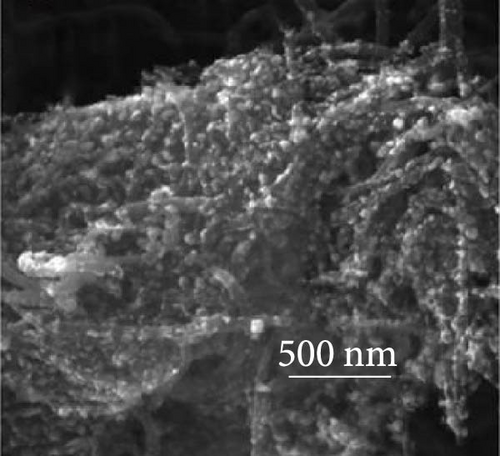
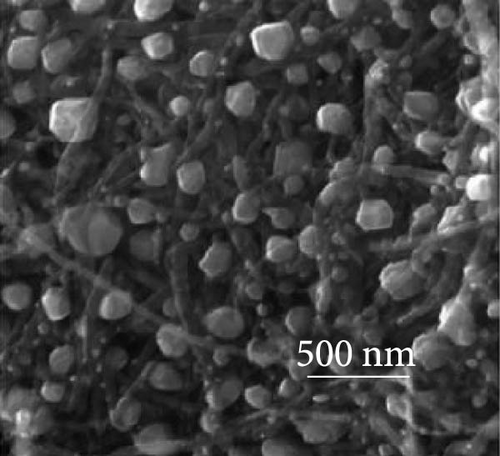
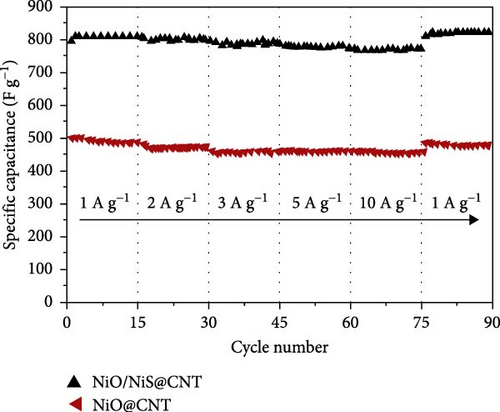
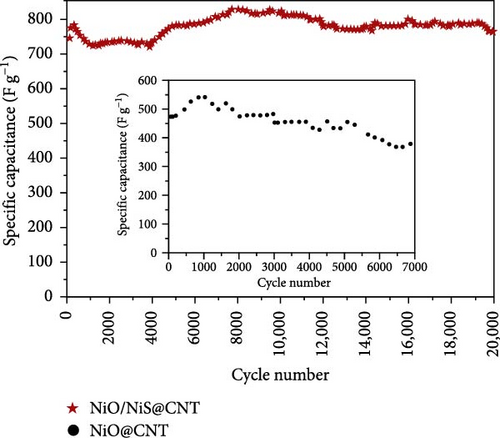
The integration of metal oxides with NiS has emerged as a pivotal strategy in the development of electrodes for SCs, offering enhanced capacitance and superior electrochemical properties [101]. These composite materials combine the unique properties of metal oxides and metal sulfides, resulting in electrodes that exhibit high specific capacitance and excellent cycling stability, essential for high-performance SC applications. A significant contribution to this field was made by Zheng et al., who utilized a one-step rapid microwave synthesis process to develop a NiO/NiS@CNT composite. This method facilitated the uniform growth of multiple NiO nanoparticles on the surface of CNTs, ensuring a symmetrical dispersion. The composite showcased NiS nanoparticles with a relatively larger spherical shape compared to the NiO nanoparticles (Figure 16a–c). The dual presence of NiO and NiS nanoparticles evenly dispersed over the CNT surface significantly enhanced the electrochemical interface. Electron microscopy images provided clear evidence of this well-orchestrated nanostructure. Electrochemically, the NiO/NiS@CNT composite electrodes delivered an impressive specific capacitance of 809.7 F/g at a current density of 1 A/g. Even more notable was the durability of the composite, which maintained considerable cycling stability after 20,000 cycles, underscoring the effectiveness of the microwave synthesis in creating robust electrode materials. In comparison, NiO@CNT composites, while still effective, exhibited a lower specific capacitance of 491.9 F/g and retained 80% of their capacity after 7000 cycles (Figure 16 d, e) [101]. Further expanding the scope of metal oxide integration with NiS, Ji et al. developed a core–shell heterostructure composite consisting of MnO2@NiS2/Ni (OH)2.
This composite acted as a pseudocapacitive material and was characterized by a unique 1D and 2D structural integration where MnO2 served as the substrate. This configuration ensured good cycling efficiency and facilitated electron transport, critical for high-rate performance. The NiS2/Ni (OH)2 layer significantly increased the surface area, which in turn enhanced the charge transfer characteristics. This structural innovation led to a remarkable specific capacitance of 1010 F/g, showcasing the potential of heterostructured composites in advanced electrochemical applications [103]. Adding to these advancements, Srinivasa Rao explored the enhancement of NiS electrochemical performance through a composite with zinc oxide (ZnO) on CNTs. The ZnO/NiS/CNT electrode demonstrated exceptional cycling stability, retaining 89.7% of its capacity after 5000 cycles and achieving a high specific capacitance of 879.0 F/g at a current density of 1.5 A/g [102]. The presence of ZnO not only contributed to the structural integrity of the composite but also improved its electrochemical reactivity, illustrating the benefits of incorporating metal oxides into NiS-based composites. These examples underscore the significant benefits of combining NiS with metal oxides. By leveraging the complementary properties of metal oxides and sulfides within carbon-based frameworks, researchers have successfully developed electrode materials that meet the rigorous demands of SC applications. This strategic material design not only enhances electrochemical performance in terms of capacitance and stability but also paves the way for the next generation of energy storage devices, where efficiency and durability are paramount.
The interfacial charge transfer mechanisms in NiS-based nanocomposites, specifically focusing on NiS-carbon, NiS-metal oxide, and NiS-metal sulfide interfaces. The combination of NiS with these materials significantly enhances conductivity, electrochemical stability, and charge storage efficiency. The integration of NiS with carbon-based materials such as graphene, CNTs, and rGO improves electrical conductivity by facilitating faster electron transport. The large surface area of carbon-based materials provides more active sites for faradaic reactions, enhancing charge storage. Carbon also prevents agglomeration and structural degradation, improving cycling stability. NiS-carbon composites have demonstrated high specific capacitance values, such as a NiS/ACNT hybrid electrode achieving 1266 F/g at 1 A/g with an 81% retention after 2000 cycles. Another study on NiS/rGO composites reported a specific capacitance of 2157.8 F/g at 2 A/g with 92.4% retention after 30,000 cycles, showing exceptional durability and electrochemical performance. The integration of NiS with transition metal oxides such as NiO, MnO2, and ZnO introduces additional redox-active sites, further increasing charge storage capacity. Metal oxides improve structural stability and prevent volume expansion, maintaining electrode integrity over prolonged cycling. The inclusion of NiO in NiS composites enhances redox reactions by introducing multiple oxidation states, while MnO2 provides a wider electrochemical window, leading to improved energy and power densities. A NiO/NiS@CNT composite synthesized using a microwave-assisted method exhibited a specific capacitance of 809.7 F/g at 1 A/g with outstanding cycling stability, retaining its capacitance after 20,000 cycles. Similarly, a ZnO/NiS/CNT hybrid electrode showed a specific capacitance of 879.0 F/g at 1.5 A/g with 89.7% retention after 5000 cycles. The incorporation of metal sulfides, such as CoS, Ni₃S4, and SnS2, into NiS electrodes provides synergistic effects that enhance charge transfer, conductivity, and electrochemical stability. The presence of additional sulfide phases reduces charge transfer resistance and improves overall energy storage efficiency. NiS@CoS composites demonstrated superior electrochemical properties, achieving a specific capacitance of 1210 F/g at 1 A/g with 87.2% capacitance retention after 2000 cycles. Similarly, NiS/Ni₃S4 hybrid structures exhibited a specific capacity of 214.9 mAh/g at 2 A/g, highlighting the effectiveness of metal sulfide-based composites in optimizing charge storage performance. This study now includes an extensive discussion of these interfacial interactions, emphasizing the role of material selection in tuning the electrochemical properties of NiS-based electrodes. By combining NiS with carbon, metal oxides, and metal sulfides, researchers have successfully improved its electrical conductivity, charge storage efficiency, and long-term cycling stability. These findings indicate that NiS-based nanocomposites hold significant potential for high-performance SCs, and further research should focus on optimizing synthesis methods and interface engineering to achieve even greater electrochemical performance [97, 98, 102, 106–111].
6. Conclusion and Future Prospective
The recent advancements in utilizing NiS for SC electrodes represent a significant stride in the evolution of energy storage technologies. NiS, with its versatile morphologies ranging from spheres and nanorods to nanosheets and hierarchical flower-like structures, offers a high surface area critical for enhancing electrochemical performance. These diverse structures facilitate increased active sites for ion interaction and improved charge storage capabilities, positioning NiS as a promising candidate for SC applications. However, despite these advancements, several challenges hinder the full potential of NiS-based electrodes. The primary issues include low energy density, limiting their applicability in high-energy-demand scenarios, and structural degradation over prolonged charge–discharge cycles, which impacts long-term stability and performance. Addressing these challenges requires a multifaceted approach. Integrating NiS with conductive materials such as carbon, metal oxides, and other metal sulfides has proven to enhance electrical conductivity and structural integrity. These composites leverage synergistic effects, reducing charge transfer resistance, enhancing ion diffusion, and improving overall electrochemical behavior. Moreover, refining nanostructures and surface morphologies, along with precise control over size and dimensions, has shown to optimize electrochemical properties, leading to enhanced capacitance and durability.
7. Future Prospective
Looking ahead, the development of NiS-based SCs holds immense promise but demands strategic innovations to overcome existing limitations. Future research should prioritize optimizing fabrication techniques to enhance capacitance, stability, and structural integrity. Advanced synthesis methods capable of controlling morphology and compositional parameters at the nanoscale will be vital in tailoring NiS properties for specific energy storage applications.
Environmental sustainability will also play a pivotal role in the evolution of NiS-based SCs. Greener synthesis methods and the development of recyclable composite materials will not only enhance the environmental appeal of NiS but also align with global sustainability goals. Furthermore, assessing the lifecycle impacts and recyclability of NiS-based devices will be essential to mitigate environmental concerns linked to their widespread commercial adoption.
The integration of computational modeling and machine learning offers another promising avenue for accelerating material discovery and optimization. These tools can predict material behavior under various conditions, reducing the time and cost of experimental trials and guiding the design of more efficient electrode materials. Expanding the applications of NiS-based SCs into emerging fields such as wearable technology, flexible electronics, and large-scale energy storage systems will necessitate further innovations in device architecture and integration strategies. Hybrid systems that combine the high energy density of batteries with the rapid charge–discharge capabilities of SCs could revolutionize energy storage, addressing both power and energy demands. While significant progress has been made in the development of NiS-based electrodes, the future of this field relies on continuous innovation, multidisciplinary collaboration, and sustainable practices. By addressing current challenges and leveraging advanced strategies, NiS holds great potential to contribute to next-generation energy storage solutions. Furthermore, expanding the applications of NiS SCs into new areas such as wearable technology, flexible electronics, and large-scale energy storage systems will necessitate innovations in device architecture and integration strategies. The development of hybrid systems that combine the advantages of batteries and SCs could lead to breakthroughs in both energy density and power delivery. The significant progress has been made in the development of NiS-based electrodes for SCs, the path forward involves a multidisciplinary approach encompassing material science, engineering, and sustainability considerations. Continuous innovation and research will be imperative in overcoming the current limitations and unlocking the full potential of NiS in next-generation energy storage solutions.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Batool Taher Al-Abawi: conceptualization, methodology, writing–original draft. Nazish Parveen: supervision, data curation, methodology, writing–review and editing. Sajid Ali Ansari: investigation, funding acquisition, visualization, writing–review and editing. Haya Abdullah Al-Dosari: writing–review and editing. Haidar Khalid Alshaikh: writing–review and editing. Ahmad Umar: data curation, formal analysis, validation, writing–review and editing. Ahmed A. Ibrahim: formal analysis, validation, writing–review and editing. Sheikh Akbar: formal analysis, validation, writing–review and editing.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. KFU251406].
Acknowledgments
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. KFU251406].
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




