Green Energy: Power Generation Improvement in Microbial Fuel Cells Using Bio-Synthesized Polyaniline-Coated Co3O4 Nanocomposite
Abstract
The sharp increase in global energy consumption in recent years has intensified the focus on replacing traditional nonrenewable energy sources with sustainable and clean energy. Microbial fuel cells (MFCs) are emerging as a viable solution, generating clean energy through the biological breakdown of organic materials. The efficacy of MFCs is highly reliant on the design of their anode electrodes. In this study, a pencil graphite electrode (PGE) modified with the polyaniline-cobalt oxide (PANI-Co3O4) nanocomposites was made up by the in situ polymerization method, used as the anode for the single-chamber MFC (SCMFC), and a mixed culture inoculum was used as the biocatalyst. In addition to PANI-Co3O4 nanocomposites, Co3O4 NPs and PANI were synthesized by combustion and chemical oxidative polymerization methods, respectively, to investigate the performance of MFCs. The desired materials were characterized via UV–Vis, X-ray diffraction (XRD), FT-IR, and SEM. MFCs’ performance tests were performed via open-circuit voltage (OCV) and closed-circuit voltage (CCV) measurements. OCV measurement of bare PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE electrodes produces maximum results of 238, 394, 565, and 638 mV, respectively. PANI-Co3O4/PGE produces a maximum OCV, which was 2.68 times higher than bare PGE. Furthermore, a CCV measurement was performed at external resistance in the range of 100 Ω –100 kΩ. The continuous test on bare PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE was evaluated in glucose-fed mixed culture-based SCMFC. The results indicate that PANI-Co3O4/PGE can generate maximum power densities up to 413.07 mW/m2, which is equivalent to a current density of 2429.56 mA/m2. Applying PANI-Co3O4 nanocomposites to PGEs significantly enhanced the power and current density by 6.78 and 3.92 times, respectively. This study highlights the effectiveness of modified PGE anodes in generating electricity with MFCs. The results demonstrate a potential for using artificial wastewater across various regions to produce green energy from waste.
1. Introduction
In recent years, there has been a notable upsurge in energy consumption globally. Conventional nonrenewable energy reservoirs, including fossil fuels and nuclear power, have experienced extensive utilization on a global scale [1]. Traditional energy reservoir depletion is progressing at a significant rate, while renewable energy reservoirs remain underutilized. The combustion of nonrenewable energy sources results in the release of a multitude of greenhouse gases, particularly carbon dioxide, which contribute to profound environmental repercussions [2]. Furthermore, nuclear fuel power production is radioactive and harmful to the environment [3]. To overcome this entry problem, the search for alternative sources of energy generation that are inexpensive and eco-friendly has become a prime necessity [4]. Microbial fuel cells (MFCs), emerging as novel devices, exhibit substantial promise in addressing these challenges by effectively generating sustainable clean energy through the biological degradation of organic matter by microorganisms [5].
However, the main impediment to the practical implementation of this technology continues to be the relatively low power density obtained from MFCs [6]. Over the past decade, substantial advancements have been made in enhancing MFC performance by adjusting parameters, such as concentration, pH, electrode materials, electrolyte types, and reactor configurations [7]. However, the pivotal focus remains on enhancing power generation capabilities and refining cost-effective electrode materials within real-time MFC technology. Despite notable progress, the quest for an optimal electrode architecture and its modifications remains unresolved [8]. Recent strides in nanofabrication present a promising avenue for engineering efficient electrode materials using the exceptional structural, electrical, and chemical properties offered by nanomaterials (NMs). Overall, for anode modification using NMs, various properties of these compounds, including redox properties, conductivity, surface area, biocompatibility, and cost, should be considered. Considering this property, carbon-based electrodes, conductive polymer NM, and inexpensive transition metal oxide (TMO) NMs could be regarded as excellent choices [9]. Additionally, it should be noted that the effect of NMs could differ for each species of exoelectrogen, as shown by Chen et al. [10], and should be taken into consideration during experimental design.
In recent years, anode electrodes have typically been made of carbon-based materials due to their excellent electron transfer kinetics, high conductivity, large surface area, low porosity, good biocompatibility, high chemical and thermal stability, and ease of availability [11]. Pencil graphite electrodes (PGEs) are among the best anode materials for MFCs because they meet all of the requirements listed above, making them an excellent choice for enhancing MFC performance. PGE and other traditional carbon electrodes still have low conductivity, few surface reaction sites, and inadequate biocompatibility, such as graphite felt, graphene foil of carbon fiber, and Toray carbon paper [12]. These factors impede bacterial biofilm production, reduce electron transfer rates (ETRs), and hinder the electrokinetic mechanisms essential for effective electrochemical processes during MFC operation.
In response to the challenges mentioned above, the issue of low conductivity in PGE anodes can be mitigated by surface modification using TMO NMs such as cobalt oxide (Co3O4) [13], and conducting polymers, such as polyaniline (PANI), followed by a green synthesis approach in the form of NCs [14]. Therefore, Co3O4−based composites, that is PANI-cobalt oxide (PANI-Co3O4), are expected to improve the power generation in MFC due to the bacterial adhesion ability, and these criteria be able to increase ETR toward anode surface. Co3O4 has received increasing amounts of attention from researchers because of their relatively low cost and excellent catalytic properties, which have led to their preference over other catalysts for enhancing the performance of MFCs [14, 15]. However, TMOs are chemically and mechanically unstable. In order to solve this problem, Co3O4 can be improved by enhancing its mechanical strength with PANI, forming PANI-Co3O4 NCs [16, 17]. Co3O4 enhances the stability of PANI electrodes, which typically have low cycle stability. The Co3O4 and PANI composite structure synergistically improves the in-situ PANI-Co3O4 electrode’s performance. Co3O4 boosts PANI’s active sites, facilitating lower energy electron transitions by reducing the band-gap in the PANI structure. Strong interactions between Co3O4 particles and PANI’s quinoidal sites lead to improved electron exchange, thus increasing the electrocatalytic activity of the PANI-Co3O4 nanocomposite. This might significantly improve the nanocomposite’s electrochemical performance [18]. Overall, the integration of PANI with Co3O4 in MFCs contributes to enhanced performance and efficiency in converting organic substrates into electrical energy [14, 19]. Recently published works provide testimony to the enhanced power generation achieved through the combination of PANI with α-MnO2 [16], MoO2 [20], Co3O4 [21], and carbon felt [6].
Designing protocols for reactors and the types of microorganisms used in MFCs has a significant effect on power generation. MFCs are typically configured as either single-chamber or double-chamber systems [20]. Double-chamber MFCs (DCMFCs) are more efficient in terms of power generation; however, they have a complex design, require oxygen purging or catholyte, and higher costs. In contrast, single-chamber MFCs (SCMFCs) offer several advantages, including simpler and more compact setups that facilitate portability and straightforward monitoring, lower costs, and less space requirements, making them economically advantageous. The closer microbial-anode interactions in SCMFCs enhance electron transfer, making them a more attractive option compared to DCMFCs. These benefits position SCMFCs as a practical choice for specific research applications and energy generation needs [17, 22]. Furthermore, the use of mixed bacterial cultures creates a synergistic environment in specialized reactors, enhancing the performance of SCMFCs by improving electron transfer, boosting metabolic activity, optimizing substrate utilization, and promoting biofilm formation on the electrodes. In the current study, the use of a combined electrode PANI-Co3O4/PGE; the system results in improved conductivity, higher catalytic activity, and enhanced biocompatibility, leading to increased power output and efficiency. These advancements make SCMFCs more viable for sustainable energy generation and wastewater treatment applications [6]. A comparison of power densities achieved using different inoculum sources in SCMFCs revealed that systems utilizing soil exhibited the lowest power density of 70 mW/m2 [21]. This low performance is likely due to reduced biofilm formation on the electrode surface, a limitation supported by previous research. For example, Mishra and Jain [23] reported a power density of 1125.4 mW/m2 using a mixed culture, while Zhang et al. [24] achieved an even higher output of 2065 mW/m2. These results show that mixed bacterial culture is efficient for MFC power generation.
Preparations of efficient TMOs nanocatalysts with chemical wet methods strongly influence environmental safety because of the corrosive nature of chemicals. To solve this problem, researchers have used a green method because they are cost-effective and environmentally friendly, and could enable the reduction of expensive and toxic reducing chemical constituents. Aloe barbadensis Miller (Aloe vera) is a tropical and subtropical succulent cactus-type plant that used to belong to the Liliaceae family. It grows in warm and dry climates. The leaves of A. barbadensis Miller have a dense epidermis enveloped by cuticles, encasing the mesophyll [25]. This botanical marvel comprises a rich array of more than 75 different compounds, including essential vitamins (A, C, E, and B12), enzymes (such as amylase, catalase, and peroxidase), essential minerals (zinc, copper, selenium, and calcium), sugars (comprising monosaccharides like mannose-6-phosphate and polysaccharides like glucomannans), anthraquinones (like aloin and emodin), fatty acids (like lupeol and campesterol), plant hormones (like auxins and gibberellins), and an assortment of other substances (including salicylic acid, lignin, and saponins) [26].
Due to the presence of natural phytochemicals in the A. barbadensis Miller leaf extract, which serve as inherent reducing and capping agents, this extract represents the optimal choice for the synthesis of Co3O4 nanoparticles. The extract comprises components that deter nanoparticle aggregation by inducing steric repulsion among individual particles. Since A. barbadensis Miller has many chemical constituents, its use as a surface-active agent reduces the overall surface energy result, thereby preventing nuclei aggregation [27]. According to recent studies, the synthesis of ZnO nanoparticles using Miller leaf extract from A. barbadensis yielded 90% of the nanoparticles, while the use of NaOH only produced 50%. Even the reaction time of the ZnO synthesized nanoparticles was quicker than that of NaOH when Miller leaf extract from A. barbadensis was used [28]. Therefore, the main objective of this research is to identify a sustainable approach to green energy production that is highly efficient, user-friendly, affordable, and environmentally benign.
This study achieved a power density of 413.07 mW/m2 using PANI-Co3O4 nanocomposites, outperforming many conventional synthesis methods, such as chemical methods, which often involve toxic reagents [15], and hydrothermal synthesis, which requires high energy consumption. A. vera-assisted synthesis offers a green and cost-effective alternative [29, 30]. While ion deposition provides precise control, it involves complex procedures. These results are comparable to biosynthesized Fe3O4-PANI-modified electrodes (424.51 ± 6.86 mW/m2) [31], but lower than PANI nanofiber-modified electrodes (1091 ± 5% mW/m2) [32]. Overall, A. vera-based synthesis provides an eco-friendly route with promising MFC performance, balancing efficiency and sustainability [33]. So, in this study, we prepared the first biocompatible hybrid nanocomposite material from A. barbadensis Miller (A. vera) assisted Co3O4 NPs and PANI matrix by chemical polymerization method to increase the PGE anode catalytic activity by proposing SCMFC bioreactor system. The test was done with mixed bacteria as a microbe and glucose as a carbon source for wastewater and bioelectricity generation applications. This study addresses critical knowledge and technology gaps in MFC research, particularly in the optimization of anode electrode design, which has been a significant barrier to the widespread adoption of MFC technology. While MFCs offer immense potential for sustainable energy generation, their development has been hindered by limitations in electrode efficiency, scalability, and cost-effectiveness [33]. Traditional high-performance anode materials often rely on expensive fabrication methods or rare materials, making them impractical for large-scale applications. Furthermore, many existing studies fail to explore affordable and readily available alternatives that maintain competitive performance metrics.
To bridge existing gaps in MFC technology, this study introduces a novel approach utilizing PGEs modified with PANI-Co3O4 nanocomposites. PGEs are inexpensive, widely accessible, and straightforward to modify, making them a practical choice for cost-effective electrode development [16]. Testimony, the incorporation of PANI-Co3O4 NCs significantly enhances the electrochemical properties of the anodes, resulting in a power density increase of 6.78 fold increases and a current density increase of 3.92 fold increases compared to unmodified PGEs, which has an impactful meaning on overcoming both efficiency and economic barriers in MFC technology. This improvement can be attributed to the unique synergistic effects of PANI and Co3O4 [18]. PANI contributes high conductivity and mechanical stability, while Co3O4 nanoparticles provide superior redox activity. Furthermore, the straightforward synthesis process and the use of low-cost materials underscore the scalability of this approach for practical applications, particularly in decentralized energy production from artificial wastewater systems. The combination of cost-effectiveness, enhanced performance, and scalability positions PANI-Co3O4-modified PGEs as a promising solution to overcoming efficiency and economic barriers in MFC technology, thereby paving the way for sustainable energy generation.
2. Materials and Methods
2.1. Chemicals and Reagents
The following materials were used exactly as supplied: cobalt nitrate hexahydrate (Co [NO3]2·6H2O; purity 99.9%, Sigma–Aldrich), aniline (C6H5NH2; purity 99.5%, Merck), ammonium persulfate (APS) ([NH4]2S2O8; purity 98%, Sigma–Aldrich), ethanol (C2H5OH; purity 98%, Merck), hydrochloric acid (HCl; purity 37%, Merck) and sewage chemicals. All necessary solutions were prepared using distilled water, and the chemicals, nutrients, and substrates suitable for MFCs are of analytical grade.
2.2. Sample Collection Site
Fresh leaves of A. barbadensis Miller were collected from the Kembata Zone, Regional state of Central Ethiopia Regional state, Ethiopia (latitude: 7°14′60.00′ North and longitude 37°49′ 59.99′ East). Effluent wastewater samples were obtained from the wastewater treatment plant at the main campus of Hawassa University in Hawassa, Ethiopia. Samples were collected using glass bottles wrapped with light steel rods for secure fastening.
2.3. Extraction of A. barbadensis Miller Leaf Gel
The extraction of A. barbadensis Miller leaf gel was carried out following the procedures outlined in the guidelines referred to [29], though with little modification. Initially, freshly harvested A. barbadensis Miller leaf gel was washed, followed by carefully removing the edges of the leaf using a knife. Subsequently, the leaves were longitudinally sliced to expose the gel inside, which was then delicately scooped out with a spoon and transferred to a sterile Petri dish. Subsequently, approximately 50 g of Miller leaf gel of A. barbadensis was finely chopped into small fragments after a thorough rinse with distilled water. The chopped gel was boiled in 50 ml of distilled water for 20 min and subsequently allowed to cool. The resulting gel-like liquid was then filtered and stored at 4°C in a refrigerator for further analysis.
2.4. Biosynthesis of Co3O4 NPs
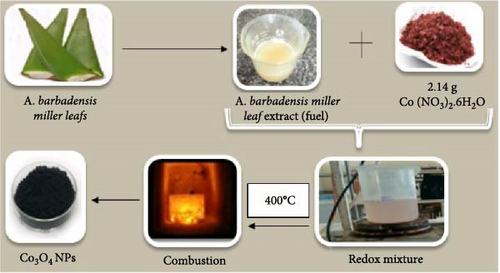
Co3O4 nanoparticles were synthesized using A. barbadensis Miller extract, ensuring an environmentally friendly approach. Cobalt nitrate (Co [NO3]2·6H2O) was used as a precursor, while Miller gel from A. barbadensis Miller gel was used as a fuel source in a low-temperature self-propagating combustion technique to synthesize cobalt oxide nanoparticles. This method, with minor adjustments, was derived from the reference literature [34]. Initially, 2.14 g of cobalt nitrate hexahydrate was placed in a 100 ml beaker, followed by the addition of 10 ml of Miller gel of A. barbadensis. The mixture was then stirred on a magnetic stirrer for 10 min to ensure homogeneity. Subsequently, the gel fuel–oxidizer mixture was transferred to a ceramic crucible and placed in a preheated muffle furnace set at 400°C. Upon boiling and the formation of the mixture froth, the Co3O4 nanoparticles were ultimately reduced to a black powder, as shown in Figure 1.
2.5. Synthesis of PANI
The following procedures were followed in the preparation of PANI using the chemical polymerization by oxidation method: taken from [16, 35], the literature. In summary, 1 M (100 ml) HCl aqueous solution was mixed with 0.5 ml of aniline monomer in a beaker and stirred for 30 min. Next, 50 ml of distilled water was used to dissolve 5.71 g of APS. For 1 h, both solutions were stored at room temperature. The two solutions were kept apart at room temperature for 1 h. The two solutions were then combined and agitated in a beaker using a magnetic stirring stick until a color shift from colorless to dark green was observed, signifying the formation of PANI. The mixture was then left to polymerize for 4 h. After filtration, the resulting PANI precipitate was collected and then cleaned with 0.2 M HCl until the filtrate became clear. The mixture was then repeatedly washed with distilled water to eliminate the acid and with ethanol to remove the monomer and oligomer. The PANI powder was dried for the last time for 1 h at 60°C in an oven (Figure S1).
2.6. Biosynthesis of PANI-Co3O4 Nanocomposites
PANI was polymerized via chemical oxidative polymerization, followed by in situ incorporation of Co3O4 to form the final PANI-Co3O4 nanocomposite [34], with little modifications. First, to create aniline hydrochloride, 0.5 ml of aniline monomer (0.1 M) was dissolved in 100 ml of 1 M HCl aqueous solution in a beaker and stirred for 30 min. Secondly, to maintain Co3O4 uniformly suspended in the solution, 0.5 g (0.5 wt%) of green synthesized Co3O4 NP was dispersed by ultrasonication for 5 min in 25 ml of distilled water. To complete the polymerization process, homogeneously suspended Co3O4 nanoparticles were slowly added dropwise to a doped aniline hydrochloride solution containing 2.86 g of APS (codopant) at ambient temperature. The mixture was constantly agitated for a period of 4 h. Following the transformation of the reaction mixture into a dark green precipitate, polymerization was initiated at room temperature. On the second day, the obtained materials were collected by filtering and repeatedly washed with a 0.2 M HCl solution until the filtrate became clear. Additionally, ethanol was used several times to remove oligomers and monomers, and distilled water was used multiple times to remove acid. Finally, the product was dried for 24 h at 60°C in the oven to produce PANI-Co3O4 nanocomposites (Figure S2).
2.7. Anode Modifications
A Topsky HB-228 PGE was used as the base anode. The PGE surface was modified with PANI-Co3O4 nanocomposites via drop-casting to enhance microbial adhesion and electron transfer efficiency. The modified anodes were dried at 60°C to ensure stability. Before modifications, four identical HB PGE were created by hand using a pencil lead that measured 0.2 cm in diameter and 1 cm in length. When the fuel cell was assembled, the lead was immersed in distilled water for a full day and then washed with 0.2 M phosphate buffer solution (PBS). Then, using the drop-cast coating method, biosynthesized Co3O4, NP, and PANI-Co3O4 nanocomposites were created, along with a synthesized PANI powder catalyst and polyvinyl alcohol (PVOH) polymer binder solution. The job of the binder is to strengthen the bond between the bare PGE and the prepared catalyst. After adding the necessary amount of NP to the beaker, distilled water was added, and the mixture was left in an ultrasonic water bath for 15 min at room temperature to create a uniform sol after the binder step. After adding PVOH in a 1:5 ratio (PVOH–NPs wt/wt%) and dissolving it in 10 ml of distilled water, the mixture was mixed for an hour at 70°C with magnetic stirring. To achieve a uniform distribution of the nanocatalyst within the solution, the mixture was sonicated before being coated onto the PGE surface. Subsequently, a microsyringe was used to facilitate the drop-cast method, allowing the application of 100 μl of a homogeneously dispersed nanocatalyst-based PVOH solution onto the surface of PGE. Lastly, it was oven dried for 12 h at 60°C to strengthen the bonding forces and the stability of the modified layer interface [16].
2.8. MFC Setup and Operation
SCMFCs were fabricated using modified PGEs as anodes. The experimental setup included a working volume of 500 ml, which was achieved by using a cubic chamber measuring 3 cm in length, 3 cm in width, and 3 cm in height. PGE, measuring 1 cm in length and 0.2 cm in diameter, were used in each MFC as anode and cathode electrodes. Four different types of anode electrodes with pencil graphite cathode electrodes were tested: unmodified PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE electrodes. For every MFC, artificial wastewater containing 150 mgL−1 of glucose substrate, 3000 mgL−1 of NaCl, 140 mgL−1 of NH4Cl, 2000 mgL−1 of K2HPO4·12H2O, 1000 mgL−1 of NaH2PO4·2H2O, 100 mgL−1 of FeCl3, 1 mgL−1 of MnSO4·H2O, 20 mgL−1 of Co (NO3)2·6H2O, and 100 mgL−1 of sweet potato to fulfill the micronutrient deficiency. The anodes were inoculated with anaerobic activated sludge (biogas plant, Hawassa, Ethiopia) to enrich the biofilm at the anode surface for each MFC. The microbial growth medium could be the anolyte with a pH of 7.40. There was some air exposure on the cathodic side. The cathode PGEs were exposed to air. There were 1000 mL in the entire volume. Lastly, for 3 days, the MFC operation was run at room temperature (~18–24°C).
2.9. Electrochemical Calculations
Electrochemical performance was assessed by open circuit voltage (OCV), closed circuit voltage (CCV), polarization curves, and power density curves. OCV was measured every 20 min after a 20-min stabilization period using a DT9205M multimeter from CE China over a 4-h daily duration. Polarization curves were generated by adjusting the resistances from 100 kΩ to 100 Ω using an ANSHUMAN DRB-7T decade resistance box at 30-min intervals, measuring the CCV against the decrease in external resistors. The calculation of current (I) across each resistor utilized Ohm’s law (I = V/R), where V represents the cell voltage, and R is the external resistance (Rext). Current (I) and power densities (PD) were determined by applying the anode’s surface area (0.6914 cm2) as the ratio of current and power. The calculation of the area of the platinum graphite electrode (PGE) was based on the right circular cylinder formula (AT = 2πr2 + 2πrh) [36].
3. Results and Discussion
3.1. UV–Vis Analysis
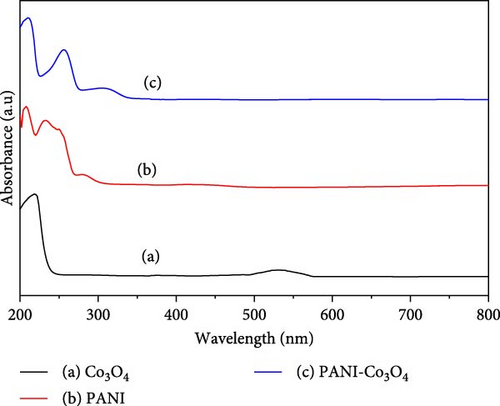
The successful synthesis of nanoscale Co3O4 NPs, PANI powder, and PANI-Co3O4 nanocomposites using a green approach was confirmed by UV–Vis absorption spectra analysis in the surface plasmon resonance (SPR) region, as depicted in Figure 2a–c, respectively. The formation of biosynthesized Co3O4 NP was identified by distinct absorbance peaks at 218 and 532 nm. These peaks correspond to the O2−–Co2+ and O2−–Co3+ charge transfer processes, indicating the dual oxidation states of cobalt elements, as reported in previous studies [37–39]. In the UV–vis absorption spectrum of PANI, a characteristic peak at around 208 nm signifies the absorption behavior of PANI in high oxidation states [40]. Additionally, the absorption band at 232 nm corresponds to the transition from π to π ∗ of C═C to C in the benzenoid rings [16], while the band in the 410–420 nm range indicates the polaron to π ∗ transitions [14]. Recent research [36] suggests that a blue shift observed in the UV region may result from a strong interaction between the orbitals of the metal oxide d and π orbitals within the polymeric material. The UV–Vis absorption spectrum of the PANI-Co3O4 nanocomposites (Figure 2c) showed a blue shift in the UV region at 306 nm, showing an intense and broad peak compared to Co3O4 and PANI individually. This change indicates a clear combination of Co3O4 within the PANI conducting matrix, highlighting the formation of the PANI-Co3O4 nanocomposite with enhanced optical properties and structural characteristics.
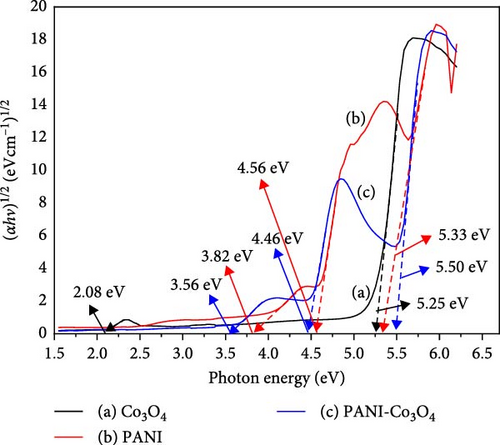
| Samples | Eg1 (eV) | Eg2 (eV) | Eg3 (eV) |
|---|---|---|---|
| Co3O4 | 2.08 | 5.25 | — |
| PANI | 3.82 | 4.56 | 5.33 |
| PANI-Co3O4 | 3.50 | 4.46 | 5.50 |
3.2. X-ray Diffraction (XRD) Analysis
XRD was used to analyze the crystal structures of Co3O4 nanoparticles, PANI powder and PANI-Co3O4 nanocomposites. The XRD patterns for each material are shown in Figure 4a–c. The Co3O4 NP XRD pattern revealed prominent peaks at 2θ values of 18.9726°, 31.2093°, 36.7721°, 38.5349°, 44.6992°, 55.5059°, 59.1996°, 65.0552°, and 77.1793°, corresponding to the 111, 220, 311, 222, 400, 422, 511, 440, and 533 lattice planes of Co3O4 (JCPDS No. 00-042-1467) [43]. These findings are consistent with the XRD patterns expected for synthesized Co3O4 NPs.
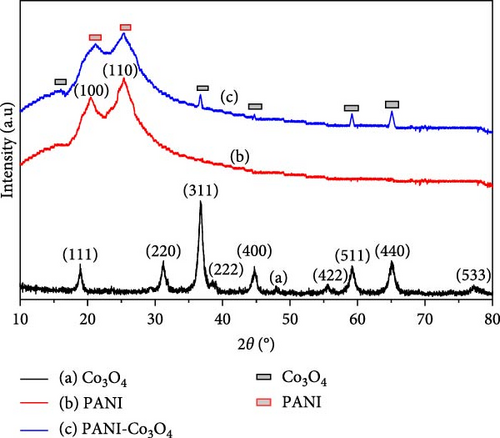
The XRD pattern of PANI exhibited broad peaks indicative of a semicrystalline structure [44]. Specifically, diffraction peaks were observed at 20.48° and 25.38° (Figure 4b), corresponding to the (100) plane of quinoid units with π–π stacking-induced partial periodicity arrangement and the (110) plane of benzenoid units. These results are consistent with data from the existing literature on PANI [45].
The XRD pattern of the PANI-Co3O4 nanocomposite (Figure 4c) exhibits overlapping peaks from both Co3O4 NCs and PANI, indicating the formation of a polymer-Co3O4 complex on the surface of the nanoparticles. This suggests that the presence of PANI does not significantly alter the crystal structure of cobalt oxide. The XRD analysis of composite material reveals subtle shifts in the characteristic diffraction peak positions of both Co3O4 and PANI, with broader and shorter intensities upon coupling with PANI. These shifts indicate changes in lattice parameters and potential interactions between PANI and the cobalt oxide surface. The alteration in peak symmetry compared to PANI alone suggests a modification in the crystal structure of Co3O4 during the polymerization reaction [16], indicating a transformation from crystalline Co3O4 to the amorphous phase of PANI. These observations confirm the successful incorporation of Co3O4 into the PANI matrix. These findings provide insights into the structural modifications induced by PANI coupling on Co3O4 NPs, offering valuable information for optimizing the properties of the composite for enhanced electrocatalytic activity.
The average crystal sizes (D) determined (Table S1) were found to be 12.29, 2.07, and 2.68 nm for Co3O4 NPs, PANI powder, and PANI-Co3O4 NCs, respectively. These distinct crystal sizes among Co3O4 NPs, PANI powder, and PANI-Co3O4 NCs signify differing structural features at the nanoscale, highlighting the beneficial impact of the PANI compound on the surface of Co3O4 NPs to enhance their unique properties. The larger crystal size observed for Co3O4 NPs compared to PANI powder and PANI-Co3O4 NCs suggests the presence of larger crystalline domains, potentially influencing properties such as stability and catalytic activity. On the contrary, the smaller crystal sizes of PANI powder and PANI-Co3O4 NCs indicate the potential for enhanced surface areas and reactivity, which could be advantageous in applications such as sensing or energy storage.
3.3. FTIR Analysis
The FT-IR spectra of biosynthesized Co3O4 nanoparticles and PANI-Co3O4 nanocomposites, as well as the synthesized PANI powder, were obtained in the spectral range of 4000–400 cm−1. In Figure 5a, depicted by black lines, the prominent bands observed at approximately 3409 and 1634 cm−1 are indicative of the O─H stretching and OH bending vibrations, respectively. These vibrations are attributed to the presence of surface adsorbed water molecules in Co3O4 nanoparticles [47]. Additionally, the band at 1380 cm−1 results from residual nitrogen groups that were created during the combustion process [48]. The prominent absorption bands at 560 and 654 cm−1, attributed to the stretching vibrations of the metal–oxygen bond corresponding to ν (CoIII–O) and ν (CoII–O) vibrations, respectively. These observations provide strong evidence for the successful preparation of Co3O4 NP [49–51]. Furthermore, a recent study [52] noted the presence of two distinct bands indicative of the vibrations of Co3+ and Co2+ ions occupying octahedral and tetrahedral sites, respectively, further confirming the structural composition of the synthesized materials.
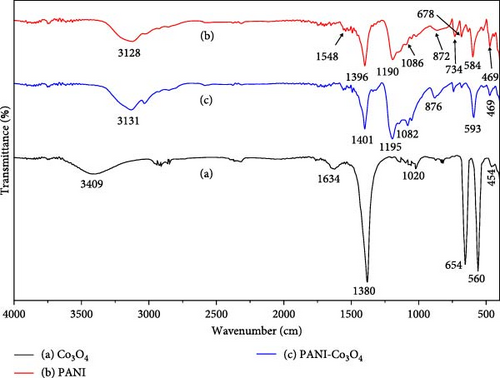
In Figure 5b, the red line represents the typical FT-IR spectra of pure PANI, exhibiting absorption bands at 3128 cm−1 corresponding to the N─H stretching vibrations of secondary amine [35]. Furthermore, characteristic peaks are observed at 1548 cm−1 (C═C stretching of quinoid ring [N = Q = N]), 1396 cm−1 (C═C stretching vibration of the benzenoid ring [N–B–N]), 1190 cm−1 (C─N stretching of secondary aromatic ring), 1086 cm−1 (aromatic C─H in-plane bending vibrations), and 678, 734, and 872 cm−1 attributed to aromatic C─H out-of-plane bending vibrations of C─H out of the plane. These spectral features are also evident in the PANI-Co3O4 nanocomposites shown in Figure 5c as the blue line, with peak positions that closely match those reported in the literature [53, 54]. The infrared spectra of the PANI-Co3O4 nanocomposites show minor band shifts toward the blue side compared to both cobalt oxide and PANI, suggesting changes in chemical environment and bonding interactions, but absorption spectra closely resemble that of pure PANI. These shifts indicate stronger or altered chemical bonds within cobalt oxide due to PANI coating. These findings suggest that PANI and Co3O4 nanoparticles can interact in certain ways [15].
3.4. SEM Analysis
The morphology of Co3O4 nanoparticles, PANI powder, and PANI-Co3O4 nanocomposites was examined using SEM at a magnification scale of 5 µm, as depicted in Figure 6a–c. In Figure 6a, the SEM image shows low-aggregated Co3O4 NP with a flower-like nanostructure, which was synthesized using A. barbadensis Miller leaf extract. The morphology of pure PANI, shown in Figure 6b, revealed agglomerated, nonuniform, rough and densely packed microstructures. According to Dessie et al. [16], aniline monomer concentrations and oxidant type have a significant impact on the form and type of PANI SEM micrograph. Thus, a globular (granular) PANI morphology is produced when strong oxidants and high aniline concentrations are used during the highly acidic PANI synthesis process. This study suggests that the interweaving of PANI in Co3O4 improved surface morphology, as shown in Figure 6c.
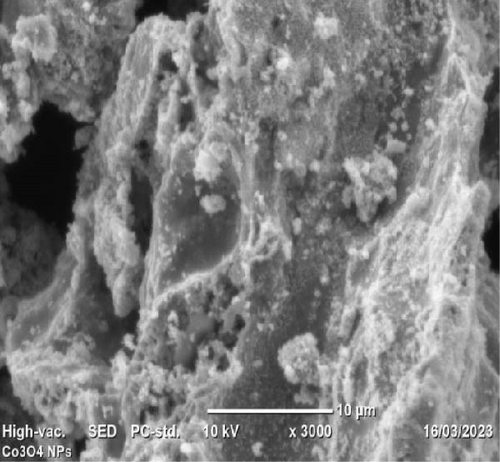


SEM images clearly demonstrate distinct differences in morphology between Co3O4 NP, pure PANI powder, and PANI-Co3O4 NCs. The intertwining of PANI in Co3O4 significantly influences the morphology of PANI-Co3O4 NCs. SEM analysis reveals that PANI-Co3O4 NCs exhibit higher porosity and superior particle dispersion compared to Co3O4 nanoparticles and PANI powder. The enhanced porosity and particle dispersion are promising properties that can potentially enhance the catalytic performance of the synthesized nanoparticles. The porous structure of PANI-Co3O4 NCs provides a large surface area, facilitating bacterial growth and the formation of active biofilm on the anode surface.
3.5. MFC Performance Test
This section evaluates three key parameters: OCV, polarization curves, and power density curves for each MFC. Table 2 presents data from four MFCs used to quantify the generated “power” output.
| MFC’s compartment | MFC 1 | MFC 2 | MFC 3 | MFC 4 |
|---|---|---|---|---|
| Substrate | Wastewater | Wastewater | Wastewater | Wastewater |
| Anode | PGE | Co3O4/PGE | PANI/PGE | PANI-Co3O4/PGE |
| Cathode | PGE | PGE | PGE | PGE |
3.5.1. OCV of SCMFC With Different Anode Catalyst Types
To evaluate the catalytic activity of the prepared materials and improve the MFC performance, all MFCs were initially run in an open circuit, which means that no resistive load was connected between the anode and cathode. Every 20 min, the OCV was recorded and the data was averaged every 240 min. OCV and average OCV responses, respectively, obtained by operating PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE for 3 days, are shown in Figure 7a, b. A continuous 4-h measurement was carried out every day with a 20-min stabilization interval.
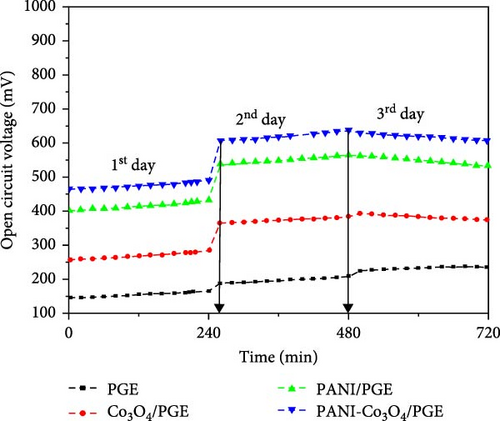
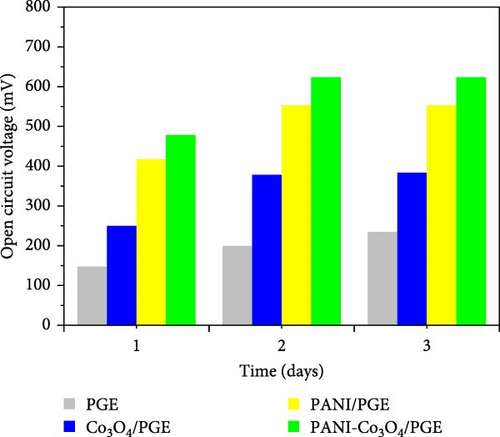
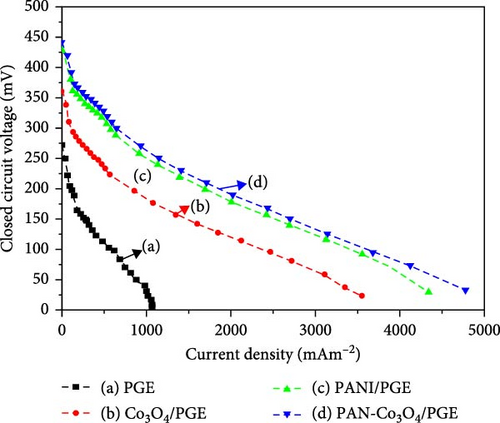
The changes in OCV over time, with the exception of bare PGE, as seen in Figure 7a, revealed three distinct phases. The growth after the first day of operation (240 min) denotes the development of a microbial community (biofilm) on the surface of the anode electrode [55]. The first day of operation (240 min) saw a fairly steady phase after the system’s microbial growth saturates the anode, and the three anode catalysts reach their maximum OCVs. For the anode with a bare PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE, the maximum OCVs were 238 (day 3), 394 (day 3), 565 (day 2), and 638 (day 2) mV across the anode and cathode, respectively. Based on the research findings, the enhanced bacterial adhesion facilitated by the modified anode, resulting in the formation of a conductive biofilm, was identified as the primary factor leading to the higher OCV observed with the PANI/PGE and PANI-Co3O4/PGE anode catalysts on the second day of operation. According to Figure 7b, PANI-Co3O4/PGE improves MFC performance over the course of the operation days by increasing active surface area formation and speed of electron transfer, showing a maximum of 638 mV for PANI-Co3O4/PGE, significantly higher than bare PGE (238 mV) [53]. A similar work supporting our findings was reported by Dessie et al. [16], which indicated that α-MnO2/PANI/PGE improves MFC performance over the course of 3 days compared to the remaining options. Additionally, recently published work by Tekalign et al. [31] highlighted that the Fe3O4/PANI-coated PGE significantly increased the OCVs of the modified electrodes compared to those of the bare and PANI/PGE electrodes throughout all operational days. According to this report, the highest OCV of 645 ± 24.5 mV was achieved with the Fe3O4/PANI/PGE electrode, slightly surpassing our finding of 638 mV for PANI-Co3O4/PGE on day two. This difference may be attributed to the superparamagnetic properties of Fe3O4 nanoparticles compared to Co3O4 nanoparticles [31]. As shown in Figure 7a, the slight decline in MFC performance observed on the third day indicates a progressive reduction in the available food sources for microorganisms, commonly referred to as chemical oxygen demand (COD) [56]. The results indicate good stability of the nanocomposites, with minimal fluctuations in OCV over the testing period. This demonstrates the robustness and durability of the modified NCs for potential long-term applications.
3.5.2. Polarization and Power Density Curve
The polarization and Power density curves serve as a direct indicator of the performance of the MFC. Polarization curve depicts the relationship between current density and voltage, where the voltage exhibits a higher value at lower current densities [13], illustrating an inverse relationship between these two parameters. The electrocatalytic effectiveness of Co3O4 nanoparticles, PANI powder, and PANI-Co3O4 nanocomposite modified on PGE to improve MFC performance is visually represented in Figure 8a,b, respectively. In Figure 8a, four polarization curves exhibit distinct regions. Each plot presents a linear section (region 2) characterized by a consistent voltage drop. The recorded voltage values of 272, 361, 433, and 441 mV correspond to the peak voltage or OCV for the bare PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE configurations, respectively. These polarization curves for PANI-Co3O4/PGE configurations is closely align with the reported works by Rani and Kumar for MoO2/PANI/CC anode [20], Dessie et al. [33] for α-MnO2/NiO/PANI anode, and Tekalign et al. [31] for PANI-Fe3O4/PGE anode. Importantly, the MFCs with PANI-Co3O4/PGE experienced a significant voltage drop through the plateau slope curve, with current densities ranging from approximately 500 to 4752 mA/cm2 before the final potential decreased than remaining anodes. In comparison, our findings demonstrate good performance with voltage drops due to the excellent catalytic properties of Co3O4 NPs over MoO2 NPs, α-MnO2/NiO NCs, and Fe3O4 NPs, which might result in a lower internal resistance, leading to improved MFC performance compared to the reported works for PGE anodes modified in the literature [20, 31, 33]. As highlighted by Balkç and Zgün, these voltage values of MFC are observed in four regions as resistance approaches infinity [57]. In the first region (i), the voltage drops significantly from the OCV at zero current due to overpotential (activation polarization), which requires a higher electric potential to overcome the activation energy for electrochemical reactions at the catalytic interface. This loss primarily occurs at lower current densities, as shown in Figure 8a. In the second region (ii), voltage decreases due to charge transport (ohmic polarization) and becomes nearly linear with current as internal resistance from the electrode material and wiring causes further drops. In the third region (iii), a rapid voltage decrease occurs at higher currents due to concentration polarization, stemming from inadequate mass transfer of reactants to the anode and the accumulation of H+ ions that reduce pH in the anodic chamber, as supported by references [4, 58].
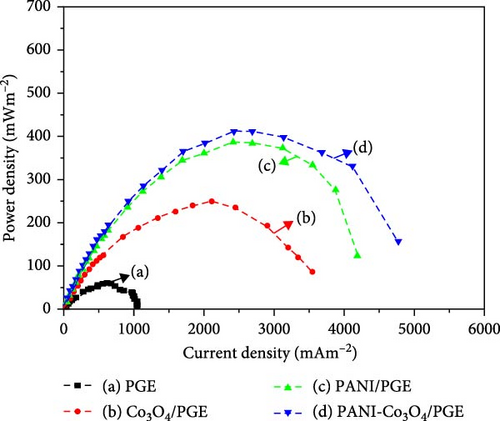
The power density curves shown in Figure 8b can be used to calculate the highest power density that can be produced by an MFC. It is calculated from polarization data Figure 8a and plotted against current density as a function of power density. The surface power density, Pₛ, in mW/m2 can be calculated using the equation Pₛ = V2/(R × A), as expressed in the literature [58], where V is the measured fuel cell voltage in mV, R is the external resistance in Ω, and A (m2) is the projected surface area of the anode. 1000 are needed to maintain the units. The SCMFC power density curve for the bare PGE, Co3O4/PGE, PANI/PGE, and PANI-Co3O4/PGE anodes is shown in Figure 8b.
It illustrates the comparison of power densities achieved by different electrode configurations in an SCMFC. The results indicated that the SCMFC utilizing a bare platinum graphite electrode (PGE) exhibited the lowest power density of 60.94 mW/m2. This low performance can be attributed to the inefficient electrical conductivity of PGE, a limitation that has been corroborated by several researchers. For instance, Dessie et al. [16, 33] reported similar power density values of 60.94 mW/m2 (2021a), 65.74 ± 0.96 mW/m2 (2021b), and additionally, Tesfaye et al. [31] reported 64.85 ± 0.86 mW/m2. To overcome the limitations associated with the PGE anode in MFCs, researchers have focused on modifying the anode using nanoparticles and nanocomposites to leverage their remarkable optical and catalytic properties for enhanced performance. The incorporation of nanoparticles has led to reports of power densities exceeding three times that of bare PGE when modified with various nanoparticles. Furthermore, modifications using binary, ternary, and quaternary nanocomposites have resulted in power densities more than six times greater than those achieved with bare PGE [9, 11, 12, 17, 22, 31, 33, 59–62]. This highlights the significant potential of NMs like Co3O4 NPs in enhancing improved power and current densities in the performance of MFC due to its ability to facilitate rapid electron transfer between bacterial colonies and the electrode surface [13]. Similarly, the PANI/PGE setup showed enhanced power and current densities, due to PANI outperforms Co3O4 in MFCs by its superior electrical conductivity, redox-active sites, and increased surface area, which enhance electron transfer and microbial interaction. PANI’s stability further contributes to consistent energy conversion performance. Electroactive bacteria are more abundant in PANI due to its superior conductivity, porous structure, and redox-active sites, which enhance electron transfer and microbial adhesion. These properties create a more favorable environment for bacterial growth compared to Co3O4 [10, 16].
In particular, the SCMFC equipped with a PANI-Co3O4/PGE anode achieved the highest power density of 413.07 mW/m2 and a corresponding current density of 2429.56 mA/m2. This is due to, PANI-Co3O4 composite modified PGE exhibits superior activity compared to bare PGE, Co3O4-PGE, and PANI-PGE due to its synergistic properties. This composite combines the high electrical conductivity of PANI with the catalytic capabilities of Co3O4, significantly enhancing electron transfer [16]. The presence of redox-active sites in both materials facilitates efficient electron exchange, boosting overall electrochemical activity [10]. Additionally, the composite’s increased surface area provides more sites for microbial attachment and enhances electrochemical reactions, fostering a robust environment for electroactive bacteria. Furthermore, the PANI-Co3O4 composite demonstrates improved chemical and electrochemical stability, ensuring consistent performance over time. These unique features collectively create an optimal environment for energy conversion in MFCs, highlighting the effectiveness of PANI-Co3O4 composites as anode materials in enhancing power generation [10].
Increasing the anode materials’ conductivity may be able to reduce contact resistance, ohmic losses, and slow ETRs [16]. Furthermore, the interaction between PANI and Co3O4 resulted in significant changes in electrode morphology, including increased defect sites, high porosity, and a rough surface, ultimately enhancing electrode surface area [21]. When the microorganisms are immobilized, the porous surface facilitates direct electron transfer in an effective manner. Hollowness reduces internal resistance and ohmic loss. Increases in porosity promote the growth of bacteria and biofilm by indirectly increasing the surface area of the anode, this increase overall performance of MFC [11]. All these modifications have a positive result in MFC power production. Because PANI involvement in Co3O4 has high electron mobility, stability, biocompatibility, anticorrosion properties, and excellent electrokinetics, it improves anode performance through enhanced microbial adhesion. General observations from the results clearly indicate that coating PGE with PANI integrated Co3O4 resulted in anode surface area improvement, allowing for more biofilm growth and bacteria adhesion than the unmodified bare anode electrode [58]. As a result of this architecture, the PANI-Co3O4/PGE anode surface has a large surface area for enhanced electron collection from fast glucose decomposition during mixed bacteria respiration, making it the best anode among the three electrodes.
This study presents a balanced perspective on the performance metrics of PGEs modified with PANI-Co3O4 nanocomposites in MFCs, comparing them with state-of-the-art materials. Advanced anode materials, such as rGO/MnO2/CF, often achieve higher power density 2065 mW/m2, which exceeds our findings by a significant margin; however, it comes with notable drawbacks, including high costs, limited availability, and complex fabrication requirements [23]. In contrast, the PANI-Co3O4-modified PGEs demonstrated a power density of 413.07 mW/m2 and a current density of 2429.56 mA/m2, representing 6.78 and 3.92 times improvements over unmodified PGEs, respectively. While these metrics are modest compared to cutting-edge materials, the key advantage lies in the practicality and scalability of this approach. PGEs are inexpensive, readily available, and easy to process, and the incorporation of PANI-Co3O4 nanocomposites significantly enhances their electrochemical properties without the need for costly manufacturing methods. This balance between performance and cost-effectiveness makes PANI-Co3O4-modified PGEs a practical alternative for large-scale applications, particularly in decentralized energy systems using wastewater. By addressing both efficiency and economic viability, this study highlights the potential of these modified electrodes to bridge the gap between high-performance laboratory results and real-world implementation in sustainable energy generation.
Recent advancements in MFC technology emphasize the significance of electrode modification with NMs to enhance power generation. Our 2025 study, published in Scientific Reports, investigated biosynthesized PANI-coated Fe3O4 on PGEs, achieving a power density of 424.51 ± 6.86 mW/m2, marking a substantial improvement over unmodified electrodes [31]. This power density is greater by 9 mW/m2 compared to the current study, which may be attributed to the superparamagnetic properties of Fe3O4 nanoparticles over Co3O4 nanoparticles. In contrast, a study conducted by Dhillon and his team explored PANI-Co3O4 nanocomposites on graphite felt anodes for soil-based MFCs. Their research reported a peak power density of 70 mW/m2, which is three times higher than that of unmodified graphite felt. This enhancement was facilitated by improved microbial attachment and stability [21]. However, their reported power density is significantly lower than our findings (413.07 mW/m2), indicating a reduced availability of microbial in soil compared to wastewater for electron transfer.
Earlier studies, such as the research conducted by Lai et al. [59], highlighted the enhancement of carbon cloth anodes with HSO4⁻-doped PANI, achieving a remarkable power density of 5160 mW/m2. This improvement was attributed to enhanced biofilm formation and increased electrochemical activity [59]. In a subsequent study in 2013, Wang et al. [60] investigated the use of PANI/mesoporous tungsten trioxide (PANI/m-WO3) composites, reporting a power density of 980 mW/m2. They attributed this improvement to the synergy between the biocompatibility of WO3 and the electrical conductivity of PANI [60].
More recently, a 2022 study by Lascu et al. [61] focused on nitrogen-containing carbon nanostructures derived from PANI, demonstrating a 2.15-fold increase in power density (40.4 mW/m2) compared to unmodified carbon cloth anodes. This enhancement was attributed to the improved surface area and conductivity of the nanostructures [61]. In a 2019 study, Geetanjali et al. [20] utilized PANI-coated molybdenum oxide nanocomposite anodes, achieving a power density of 1101 mW/m2 under sewage treatment plant, which was attributed to the high specific surface area and uniform nanopore distribution of the material. Further studies corroborate the findings reported in a 2021 investigation by team Dessie et al. [16], which demonstrated that α-MnO2-based PANI binary nanocomposite anodes in MFCs improved power density to 426.26 ± 38.89 mW/m2 under E. coli medium, facilitated by enhanced electron transfer and microbial adhesion. Additionally, this team in 2021 study on α-MnO2/NiO-based PANI ternary composite-modified PGE achieved a power density of 506.96 mW/m2, under E. coli medium, indicating promising results for practical MFC applications [33]. Extended work by Dessie et al. [14] encapsulated MnO2 composite-based anodes under E. coli MFCs, yielding enhanced electron transfer capabilities and higher power densities compared to unmodified PGEs. Another study reported by Kumar et al. [63–65] rGO/PPy, PNIPAM-GO-CNT, and rGO/PANI/Pt modified CC anode under E. coli medium produce maximum power density of 1068, 434, and 2059 mW/m2 respectively. Extended work by Kirubaharan et al. [66] Au@PANI composite-based anodes under E. coli medium MFCs, yielding power density of 804 ± 73 mW/m2, enhanced electron transfer capabilities, and higher power densities compared to unmodified CC. Moreover, a 2014 study involving α-MnO2/GO modified CC cathode under anaerobic sludge medium reported a peak power density of 3359 mW/m2, which is 7.8 fold higher than that of the unmodified electrode [67]. Lastly, a study on PANI/carbon felt modified electrodes achieved a peak power density of 216 mW/m2 [6]. These collective findings underscore the significant advancements in electrode modifications using various nanocomposites, highlighting their potential to substantially enhance the performance of MFCs.
This study, which uses 0.5 wt% of Co3O4 in a PANI matrix nanocomposite on PGE, achieved a maximum power density of 413.07 mW/m2 and an OCV of 638 mV. This is comparable to the 424.51 ± 6.86 mW/m2 achieved by PANI-Fe3O4 modified PGE and the 426.26 ± 38.89 mW/m2 from α-MnO2-based PANI PGE. However, it is lower than the values of 804 ± 73, 1068, 1101, 2059, 2065, and 5160 mW/m2 achieved with the Au@PANI/CC, rGO/PPy/CC, MoO2/PANI/CC, rGO/PANI/Pt/CC, rGO/MnO2/CF, and HSO4⁻/PANI/CC anodes, respectively, as summarized in Table 3. This reported finding, even if it exceeds the current work by a significant margin, comes with notable drawbacks associated with cost-effectiveness, limited availability, and complex fabrication requirements. Nevertheless, our work shows a significant improvement with 0.5 wt% of Co3O4 in a PANI matrix nanocomposite over unmodified PGE, achieving a 6.78-fold increase in power density and providing a cost-effective solution for MFCs. The combination of PANI and Co3O4 in this study improves conductivity, biocompatibility, microbial adhesion, and electron transfer, making this research highly relevant for scalable MFC applications.
| Anode catalyst | Cathode material | Pmax (mW/m2) | Imax (mA/m2) | OCV (mV) | Source of inoculums | References |
|---|---|---|---|---|---|---|
| Co3O4/CC | CC | — | 6.4 | 750 | Dairy wastewater | [13] |
| CC | α-MnO2/GO/CC | 3359 | — | — | Anaerobic sludge | [67] |
| rGO/PPy | CC | 1068 | — | 400 | Escherichia coli | [63] |
| PNIPAM–GO–CNT | CC | 434 | 3606 | — | E. coli | [64] |
| Au@PANI/CC | CC | 804 ± 73 | — | — | E. coli | [66] |
| rGO/PANI/Pt/CC | CC | 2059 | 9559 | — | E. coli | [65] |
| α-MnO2/PANI/PGE | PGE | 426.26 ± 38.89 | 2485.51 ± 397.31 | 650.61 ± 10.11 | E. coli | [16] |
| PANI/CF | CF | 216 | 3300 | — | Mixed culture pretreated with Chaetoceros | [6] |
| MoO2/PANI/CC | CC | 1101 | — | — | Sewage treatment plant | [20] |
| PANI-Co3O4-GF | GF | 70 | 143 | — | Soil | [21] |
| MWCNT-MnO2/PPy | CNT | 1125.4 | 3350 | — | Mixed sewage culture | [23] |
| rGO/MnO2/CF | CF | 2065 | — | — | Mixed bacterial culture | [22] |
| PGE | PGE | 64.85 ± 0.86 | 431.96 ± 0.84 | 220.36 ± 8.16 | HU student’s cafeteria sludge | [31] |
| Fe3O4/PGE | PGE | 301.6 ± 86 | 1558 ± 0.00 | 340.58 ± 42.04 | HU student’s cafeteria sludge | [31] |
| PANI/PGE | PGE | 422.79 ± 21.77 | 2475.36 ± 0.00 | 469.17 ± 89.82 | HU student’s cafeteria sludge | [31] |
| PANI-Fe3O4/PGE | PGE | 424.51 ± 6.86 | 2476.52 ± 0.00 | 645 ± 24.5 | HU student’s cafeteria sludge | [31] |
| PGE | PGE | 60.94 | 620.500 | 238 | Anaerobic sludge | This work |
| Co3O4/PGE | PGE | 248.41 | 2114.34 | 394 | Anaerobic sludge | This work |
| PANI/PGE | PGE | 386.27 | 2418.06 | 565 | Anaerobic sludge | This work |
| PANI-Co3O4/PGE | PGE | 413.07 | 2429.56 | 638 | Anaerobic sludge | This work |
3.5.3. Mechanism of Power Generation
Figure 9 illustrates the power generation mechanism in an MFC using a PANI-Co3O4 nanocomposite-coated PGE. The mechanism of power generation in an MFC using a PANI-Co3O4 nanocomposite-coated PGE involves several key processes [2, 13, 16, 62].
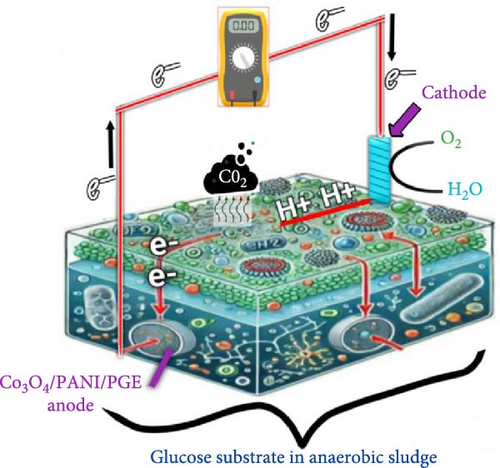
3.5.3.1. Anode Reaction (Oxidation)
3.5.3.2. Cathode Reaction (Reduction)
Oxygen (O2) acts as the final electron acceptor, facilitating the reduction reaction at the cathode. As a result, water (H2O) is formed as the final product, completing the electron transfer process in the MFC.
3.5.3.3. Overall Cell Reaction
This reaction demonstrates how chemical energy from organic matter is converted into electrical energy, making MFCs a promising technology for sustainable bioelectricity generation.
4. Conclusions
This study successfully demonstrates the synthesis of a PANI-Co3O4 nanocomposite through in situ polymerization, aimed at enhancing the performance of anodes in MFCs. The integration of Co3O4 with PANI significantly improves the conductivity of PGE electrodes, leveraging the excellent catalytic properties of Co3O4. Characterization techniques, including UV–Vis, XRD, FTIR, and SEM, confirmed the successful formation of the nanocomposite, with XRD analysis revealing average particle sizes between 2.07 and 12.29 nm. Morphological investigations showed that the Co3O4 nanoparticles exhibited a flower-like structure, while PANI presented a closely packed granular structure. The PANI-Co3O4 composites displayed a porous and irregular morphology, beneficial for enhancing electrode performance. Band-gap energy analysis indicated that the presence of Co3O4 influences the electronic properties of PANI, with PANI-Co3O4 composites showing band gaps of 5.50 and 4.46 eV, alongside an additional band at 3.50 eV. The MFC tests revealed a remarkable increase in power density, with the PANI-Co3O4 coated electrode achieving a maximum of 413.07 mW/m2, an enhancement of 6.78 times compared to the unmodified graphite electrode. This substantial improvement underscores the effectiveness of the biomediated PANI-Co3O4 nanocomposite as an anode catalyst for power generation. Future recommendations include exploring alternative MFC configurations, such as double-chamber and up-flow designs, to optimize performance. Investigating ternary nanocomposites could further enhance catalytic activity, while long-term stability studies will assess viability for commercial use. Additionally, scalable production methods for PANI-Co3O4 electrodes and incorporating biomass-derived materials could improve biocompatibility and microbial efficiency, contributing to the advancement of sustainable MFC technologies.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tesfahun Eyoel: conceptualization, writing – original draft, conceptualized and designed experiments, collect data, validation, investigation, formal analysis. Yohannes Shuka: conception and design of the experiments, performed the experiments, analyzed and interpreted the data, contributed reagents and materials, analysis tools and software, validation, writing – review and editing. Sisay Tadesse: conceptualization, conceived, writing – review and editing, analyzed and interpreted the data, supervised overall work. Mesesle Mengesha: validation, investigation, analyzed and interpreted the data, formal analysis. Tekalign Tesfaye: analysis tools or data, validation, writing – review and editing.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors thank Hawassa University, Department of Chemistry, for providing laboratory facilities, such as instruments and chemicals support during laboratory analysis.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data and material used to support the findings of this study are available from the corresponding author upon request.




