Improved Hydrogen Production Through Electrochemical Dehydrogenation of Tetrahydroquinolines Using TEMPO to Suppress Dimerization
Abstract
As a sustainable and effective strategy for hydrogen production, the electrochemical dehydrogenation of saturated organic heterocycles has recently gained significant attention in energy research. Here, we present an unusual perspective on the electrochemical dehydrogenation of 1,2,3,4-tetrahydroquinoline (THQ) and its derivatives, for example, 2-methyl-1,2,3,4-tetrahydroquinoline (M-THQ), in the presence of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) in acetonitrile. The electrochemical oxidation of THQs led to their dimerization, thereby restricting the efficient electrochemical dehydrogenation of THQs. Detailed electrochemical studies revealed the crucial role of TEMPO in suppressing the side reaction of dimerization, consequently enhancing the electrochemical dehydrogenation of THQs when TEMPO is employed.
1. Introduction
Electrochemical dehydrogenation of saturated organic heterocycles, such as N-heterocyclic compounds, is an effective and environmentally friendly strategy for hydrogen production as well as for organic synthesis. It has thus attracted substantial attention in both fundamental mechanism studies and practical applications [1–3]. This is because the electrochemical dehydrogenation of saturated heterocycles allows the generation of unsaturated compounds with the release of hydrogen under mild conditions (e.g., no toxic and dangerous oxidants, low temperatures, and low pressures) [4]. Pioneering efforts have been made for catalytic electrochemical dehydrogenation of N-heterocycles under mild conditions. For example, the first electrochemical acceoptorless dehydrogenation of six-membered and five-membered N-heterocycles was reported for the synthesis of various N-heteroarenes under oxidant-free conditions with high synthetic values [1, 5]. The pioneering work reports the use of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) in the electrochemical dehydrogenation as a redox mediator for catalytic indirect electrolysis of the N-heterocycles [1, 5]. The TEMPO-electrocatalyzed dehydrogenation was also investigated using 1,2,3,4-tetrahydroquinoline (THQ) as a model compound of N-heterocycles by in situ extractive electrospray ionization mass spectrometry (MS) [6]. While the pioneering efforts have shown significant advancements, it is crucial to attain a more comprehensive understanding of the electrocatalytic pathways. This is essential due to the diverse nature of the reaction pathways involved in the anodic oxidation of N-heterocycles [7, 8]. Specifically, in TEMPO-electrocatalyzed dehydrogenation, TEMPO is commonly perceived as a redox mediator for indirect oxidative electrolysis of N-heterocycles. This indirect process necessitates that the oxidation potential of the mediator must be lower than that of the N-heterocycles [1]. However, the common assumption is not always true because some of the N-heterocycles, such as THQs derivatives, used in TEMPO-mediated electrochemical dehydrogenation have a lower oxidation potential than that of TEMPO [6].
In this regard, the present study aimed to understand the enhanced electrochemical dehydrogenation of THQ and its derivatives in the presence of TEMPO for efficient hydrogen production. While many previous studies have highlighted TEMPO’s role as a redox mediator in the process of electrochemical dehydrogenation, this study investigated its additional role of suppressing dimerization side reactions. Specifically, the electrochemical dehydrogenation of THQs exhibited side reactions involving dimerization products through C─C coupling at the 6-ring positions. The presence of TEMPO suppressed the dimerization side reactions, which were verified with detailed electrochemical experiments. The TEMPO’s role in suppressing dimerization led to the enhanced yield of quinolines with the release of hydrogen in controlled-current electrolysis in the presence of TEMPO via the electrochemical dehydrogenation of THQs.
2. Experimental
2.1. Chemicals and Materials
1,2,3,4-Tetrahydroquinaldine was received from Tokyo Chemical Industry (Japan). Pyridine, imidazole, tripropylamine, triethanolamine, ethylenediamine, pyrrolidine, anhydrous acetonitrile (MeCN), tetrabutylammonium hexafluorophosphate (TBAPF6), sodium perchlorate (NaClO4), lithium perchlorate (LiClO4), TEMPO, ferrocene (Fc), and acetonitrile-d3 were obtained from Sigma–Aldrich, Inc. (USA). Hydrogen peroxide and sulfuric acid were received from DAEJUNG Chemical & Metals Co., Ltd. (Korea). Nafion 212 membrane (NR212) and 1,4-BTMSB-d4 were obtained from NARA cell-tech (Korea) and FUJIFILM Wako Chemicals (USA), respectively. All of the chemicals were commercially available and used without further purification.
2.2. Electrochemical Experiments
All electrochemical experiments were carried out with a standard three-electrode configuration using a WaveDriver 200 bipotentiostat (Pine Instrument Co., USA). Cyclic voltammetry was performed in a single-compartment electrochemical cell using a standard three-electrode configuration with a glassy carbon disk working electrode (dia. 3 mm), a Pt wire counter electrode, and a nonaqueous Ag/Ag+ (0.01 M AgNO3) reference electrode. Note that we used Fc as an internal standard for the electrochemical experiments, and thus referenced reported electrode potentials against the Fc couple at 0 V vs. Fc/Fc+ [9]. Similarly, hydrodynamic voltammetry was performed with a rotating ring-disk electrode (RRDE glassy carbon disk and Au ring, Model #: AFE7R9GCAU, Pine Instrument Co., USA) as a working electrode. The working electrodes were polished successively with 1.0 and 0.3 μm alumina powders on polishing clothes and rinsed with deionized water, followed by ultrasonication in water for the removal of any residual alumina particles. The polished electrodes were rinsed again with deionized water and dried under flowing N2 gas. Bulk electrolysis was conducted in an H-type electrochemical cell with a Nafion 212 membrane between two compartments at a constant current of 20 mA for 4 h under vigorous stirring at room temperature. The electrolysis used a reticulated vitreous carbon electrode (RVC, Model # MF-2077, Bioanalytical Systems Inc., USA) and a Pt mesh electrode as a working and a counter electrode, respectively. After the bulk electrolysis finished, electrolyzed mixtures were analyzed using both high-performance liquid chromatography (HPLC) (KNAUER, Germany) with a LUNA 5u C18 column (Phenomenex, USA) and NMR (Bruker Avance 400, Germany) with 1,4-BTMSB-d4 as a standard for quantification of electrolysis. In addition, the products of the electrolysis were identified using HPLC/MS (Agilent 1100 Series with a Zorbax RR Eclipse XDB-C18 column (3.0 × 100 mm, 3.5 μm), Agilent 6130 Series Single Quadrupole). As-received Nafion membranes were pretreated as follows: Nafion membranes were slightly boiled in 5% H2O2 (aq) at 70°C for 3 h and washed with 70°C deionized water. Successively, the membranes were boiled in 0.5 M H2SO4 (aq) for 2 h and in deionized water for 1 h, respectively, at 70°C. Then, they were washed with deionized water and dried at 40°C.
3. Results and Discussion
We started to investigate the electrochemical oxidative behavior of THQ in acetonitrile (MeCN) containing 0.1 M TBAPF6. As shown in Figure 1A(A1), the cyclic voltammogram (CV) of THQ shows an irreversible anodic peak (aox) in the first positive scan at a potential of 0.37 V (vs. Fc/Fc+) with no reversal cathodic peak in the subsequent negative scan. Note that subscript “ox” and “red” denote oxidation and reduction reactions. The single irreversible anodic peak, which became broader and anodically shifted as the concentration of THQ increased (Figure S1), suggests that the electrochemical oxidation product, that is, THQ•+, is unstable and thus subject to subsequent side reactions. Additional new peaks (box1/bred1 and box2/bred2) were observed in the negative and second positive scans (Figure 1A(A1), which indicates two redox processes of the side reaction product of THQ•+. This product can be attributed to the dimerization product of THQ•+ via ring–ring coupling, similar to what was reported in early studies on the electrochemical oxidation and subsequent C─C coupling of N-heterocycles and aromatic amines (Figure S2) [7, 10–12]. The peak potential separations, ΔEp, for box1/bred1 and box2/bred2 were both ca. 59 mV, indicating a reversible single-electron transfer. In addition, we investigated the electrochemical oxidative behavior of several THQ derivatives in MeCN. Because 2-methyl-1,2,3,4-tetrahydroquinoline (M-THQ) was reported as the most effective dehydrogenation substrate with complete selectivity among methyl-substituted THQs [13], M-THQ was investigated in some detail (Figure 1A(A2)). Nevertheless, we found M-THQ to behave in a similar manner to THQ. Figure 1A(A2) shows an irreversible anodic peak of M-THQ and subsequent two redox processes of a dimerization product of the M-THQ•+ radical cation, suggesting that M-THQ followed the same reaction pathway. Indeed, the dimerization of M-THQ during its electrochemical oxidation was confirmed by HPLC-MS (Figure S3), which is consistent with the previous mass spectrometric studies on the electrochemical dehydrogenation of THQ and aromatic amines [6, 14]. However, it is worth noting that no indication of further coupling of the dimerization product to form polymers was observed under our experimental conditions. A general ECE (heterogeneous electron transfer, homogeneous chemical reaction, and heterogeneous electron transfer, in sequence) reaction pathway is thus proposed in Figure 1B. While there is some uncertainty regarding the way of the coupling reactions of the M-THQ•+ radical cations (e.g., C─C, C─N, and N─N couplings), it can be ruled out that N─N and C─N coupling reactions occur under our experimental conditions, which involve neutral MeCN solutions, low concentrations of THQ, and short electrolysis time scales [7, 15]. To verify the proposed dimerization of M-THQ via C─C coupling at the 6-ring positions experimentally, we carried out critical control experiments with 6-M-THQ and 1,2-dimethyl-1,2,3,4-tetrahydroquionoline (1,2-diM-THQ) under the same conditions. Figure 1A(A3) shows no redox peaks of the dimerization product formed by following the electrochemical oxidation process of 6-M-THQ. In contrast, we observed subsequent redox peaks of the dimerization product formed from the electrochemical oxidation of 1,2-diM-THQ despite the fact that 1,2-diM-THQ is N-methylated (Figure S4). These results indicate that the dimerization reaction of THQs proceed predominantly via C─C coupling at the 6-ring positions under our experimental conditions, which confirms the mechanistic assumption proposed in Figure 1B.
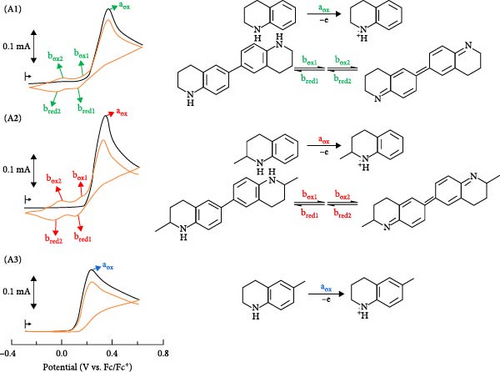
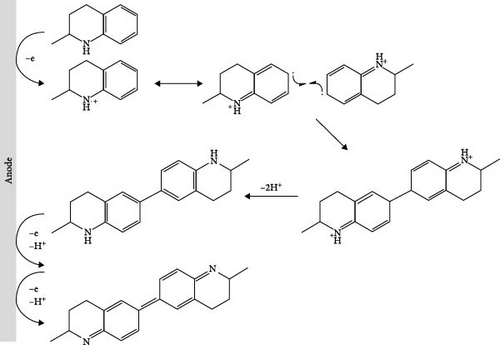
Furthermore, we investigated the electrochemical dehydrogenation of M-THQ in the presence of TEMPO because the use of TEMPO has been proposed for efficient electrochemical dehydrogenation of N-heterocycles [16]. Interestingly, Figure 2A shows the disappearance of the redox peaks of the dimerization product of the M-THQ•+ radical cation in the presence of TEMPO despite the appearance of the irreversible anodic peak of M-THQ, suggesting the suppression of the dimerization reaction of M-THQ. Although the majority of previous works on the electrochemical dehydrogenation of N-heterocycles with TEMPO focused on the role of TEMPO as a redox mediator for catalytic indirect electrolysis [1, 17, 18], TEMPO might not play a significant role in indirectly catalyzing the oxidation of M-THQ via redox mediation in our present study. This is because the oxidation potential of M-THQ is as low as that of TEMPO (Figure 1A(A2) and inset in Figure 2A) [6], and thus, we cannot rule out direct electrochemical oxidation of M-THQ to generate M-THQ•+ even in the presence of TEMPO. Nevertheless, we observed the disappearance of the reversible cathodic peak of TEMPO during the electrochemical dehydrogenation of M-THQ in the presence of TEMPO (Figure 2A). We also observed that THQ behaves similarly in the presence of TEMPO (Figure S5). Instead of catalytic indirect electrolysis of M-THQ in the presence of TEMPO, we hypothesized that TEMPO suppresses the formation of the dimerization product of M-THQ•+ via proton-coupled oxidation of the M-THQ•+ radical cation, accompanied by hydrogenation of TEMPO to generate TEMPOH and eventually TEMPOH2+ during the dehydrogenation of M-THQ•+. Figure 2B shows the proposed mechanism for electrochemical dehydrogenation of M-THQ in the presence of TEMPO. The proposed mechanism is highly plausible because hydrogenation of TEMPO to TEMPOH was reported in a recent MS study of electrochemical dehydrogenation of THQ in the presence of TEMPO [6]. In addition, we observed that the deteriorated proton-coupled oxidation of M-THQ•+ in acidic acetonitrile containing sufficient L-(+)-tartaric acid leads to less suppression of the formation of the dimerization product of M-THQ•+ even in the presence of TEMPO (Figure S6). This experimentally verifies the proposed mechanism involving proton-coupled oxidation of the M-THQ•+ radical cation.

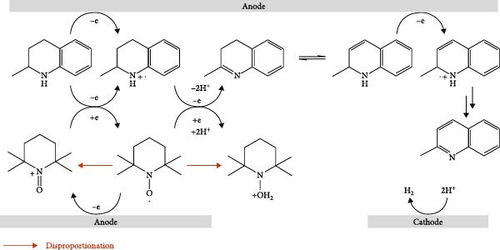
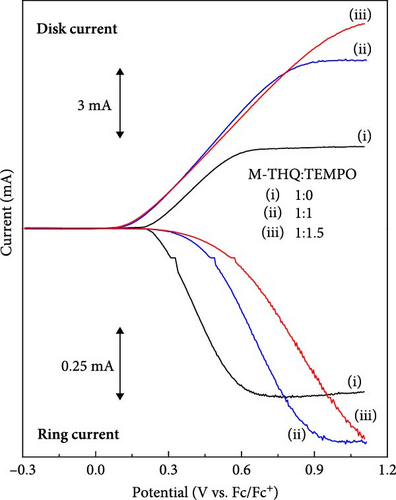
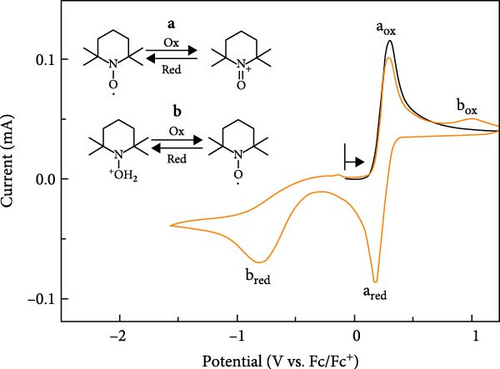
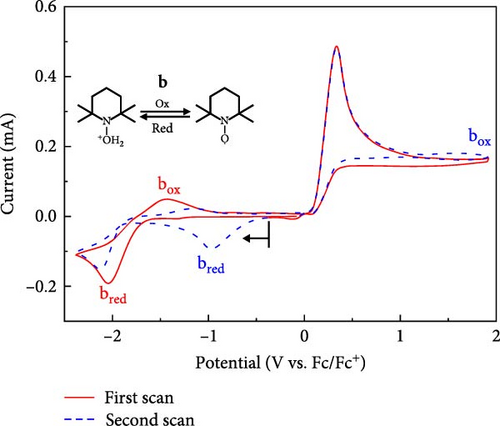
To further validate the mechanistic hypothesis, we conducted RRDE experiments. Figure 3 shows linear sweep voltammograms for electrochemical oxidation of M-THQ in the absence and presence of TEMPO on RRDE. The ring potential was fixed at −0.06 V, sufficient to induce the electrochemical reduction of the dimerization product while sweeping the disk potential in an anodic direction with a rotation speed of 1800 rpm at a sweep rate of 100 mV/s. In the absence of TEMPO, the oxidation onset of M-THQ at the disk electrode occurred at ca. 0.19 V, and the reduction onset on the ring electrode also occurred at ca. 0.21 V due to the reduction of the dimerization product (Figure 3(i)). On the contrary, the ring electrode exhibited a negligible reduction current in the presence of TEMPO near the potential of 0.21 V, even with the same ring potential of −0.06 V, until the disk potential was swept toward a more positive region (Figure 3(ii) and (iii)). This can be attributed to the suppressed formation of the dimerization product in the presence of TEMPO via the proton-coupled oxidation of M-THQ•+ (Figure 2B), which is consistent with the cyclic voltammetry results of M-THQ described above (Figure 2A). Indeed, as the concentration of TEMPO increased, the reduction onset on the ring electrode displayed the anodic shift, which also verified the dimerization suppression. We also obtained RRDE voltammograms of only TEMPO as a control experiment (Figure S7). It is worth noting that the reduction process on the ring electrode at −0.06 V is not only the reduction of the dimerization product but also the reduction of TEMPO+ excessively formed in the presence of TEMPO (Figure S8). This is the reason that we observed increasing diffusion-limited current of the ring electrode as the concentration of TEMPO increased (Figure 3(ii) and (iii)). Additionally, we conducted detailed cyclic voltammetric studies of TEMPO and M-THQ to verify the proposed mechanism further for the electrochemical dehydrogenation of M-THQ in the presence of TEMPO. As shown in Figure 4, the CV of TEMPO in acidic acetonitrile containing sufficient L-(+)-tartaric acid displays not only the reversible electrochemical redox peaks of TEMPO (aox and ared) but also the proton-coupled reduction peak of TEMPO to TEMPOH2+ (bred). Notably, the anodic peak of TEMPOH2+ (box) on the second positive scan appears at a potential higher than that observed for the electrochemical oxidation of TEMPO under the acidic condition, as reported previously [19]. The small anodic peak current of TEMPOH2+ also indicated its slow electrochemical oxidation in the acidic aprotic solution. We observed that the electrochemical oxidation of TEMPOH2+ (or TEMPOH) to TEMPO displays a significant pH-dependency while the redox of TEMPO/TEMPO+ is insensitive to the pH (Figure S9). Accordingly, we expected that the electrochemical dehydrogenation of M-THQ in the presence of TEMPO would result in the oxidation of TEMPOH2+ at potentials higher than that for the oxidation of TEMPO. Figure 5 shows a CV obtained during the electrochemical dehydrogenation of M-THQ with TEMPO. The electrochemical dehydrogenation of M-THQ caused the reduction peak potential of TEMPO (bred) to shift to a more positive position on the second negative scan than that observed on the first scan. We also observed negligible oxidation of TEMPOH2+ on the second positive scan up to 1.59 V (vs. Fc/Fc+) while it was observed at −1.43 V (vs. Fc/Fc+) on the first scan, indicating slow comproportionation of TEMPOH2+ and TEMPO+ during the dehydrogenation of M-THQ with TEMPO [19]. Considering acid-promoted disproportionation of TEMPO in acidic aprotic solutions [20], the results also indicate insignificant recycling of the catalytic process by TEMPO because of the gradual consumption of TEMPO during the electrochemical dehydrogenation of M-THQ.
Based on the above scenario, we conducted controlled-current electrolysis of M-THQ in the presence of TEMPO in a two-compartment cell for 4 h under stirring at room temperature. The electrochemically dehydrogenated quinoline product was identified using NMR and HPLC-MS analyses and quantified by HPLC analysis. Without TEMPO, the yield of quinoline product was 9.2 (±0.8)%. In contrast, the yield almost tripled to 28.5 (±1.2)% in the presence of TEMPO (1 mol equiv) (Figure S10). In addition, we observed that the presence of TEMPO decreased the amounts of dimeric side products compared to the amount of the dimers generated in the absence of TEMPO during the electrolytic dehydrogenation of M-THQ (Figure S11).
4. Conclusion
In summary, the electrochemical dehydrogenation of THQ and its derivatives was investigated for efficient hydrogen production through enhanced electrochemical dehydrogenation, utilizing TEMPO for dimerization suppression. The dehydrogenation process involved dimerization side reactions via C─C coupling at the 6-ring positions of the electrochemical oxidation products of THQs. The electrochemical dehydrogenation was enhanced in the presence of TEMPO, taking advantage of the unique role of TEMPO suppressing the dimerization side reactions. The formation of dimerization side products derived from the electrochemical oxidation of THQs was suppressed via proton-coupled oxidation of the electrochemical oxidation products of THQs in the presence of TEMPO. The TEMPO’s role in suppressing the dimerization side reactions was experimentally verified. Furthermore, the unique role of TEMPO was utilized for the improved electrochemical dehydrogenation of THQs in the presence of TEMPO. These findings highlighted the unique role of TEMPO, that is, not only as a generally known redox mediator but also as a dimerization suppressor, which offers a new insight into the TEMPO-based electrochemical dehydrogenation of saturated N-heterocycles.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Aeri Choo and Jaehwan Cheong contributed equally to this work.
Funding
This work was financially supported by the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (RS-2023-00251597 and RS-2024-00343620) and the KIST Institutional Program (2E33282-24-048).
Acknowledgments
This work was financially supported by the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (RS-2023-00251597 and RS-2024-00343620) and the KIST Institutional Program (2E33282-24-048).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The original contributions presented in this study are included in the article/supporting information. Further inquiries can be directed to the corresponding author.




