Surface Modification of Graphene Oxide With HDTMA: Advancing Energy-Efficient Technologies for Sustainable Nitrate Removal in Water Treatment
Abstract
The adsorption of hexadecyltrimethylammonium (HDTMA) on graphene oxide (GO) was investigated to probe the molecular interaction of HDTMA adsorbed GO (GO-HDTMA) with nitrates. Physicochemical techniques including scanning electron microscope (SEM), transmission electron microscope (TEM), fourier transform infrared spectrometer (FTIR), Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), and Raman spectroscopy were used to characterize GO-HDTMA, and the effect of GO functionalization on nitrates adsorption was examined. Unmodified physical GO exhibited the weakest adsorption capability (~1.0 mmol g−1). However, nitrate adsorption was markedly enhanced by chemical GO modified with 10 mmol HDTMA (GO-HDTMA-10 mM). Which can be attributed to the various functional groups on GO and increased active sites inducing HDTMA longer chain and higher carbon content. The nitrates adsorption process attain equilibrium in 3 h with maximum adsorption density of 16 mM g−1. HDTMA adsorption was enhanced by pH changes, with pH 6 exhibiting the highest adsorption. It was found that the negative charges on GO results in the retention of HDTMA, while the hydrophobic phase created by the alkyl chain in HDTMA enables the adsorption of nitrates. The X-ray photoelectron spectrometer (XPS) analysis revealed a chemical shift caused by the adsorption of HDTMA and nitrates on the surface of GO. The reusability of the adsorbent was evaluated over four consecutive cycles. GO-HDTMA showed good removal efficiency for up to third regeneration cycles. Results reveal that the nitrates can be adsorbed more efficiently by modifying the HDTMA’s surface coverage on GO.
1. Introduction
Achieving net-zero CO2, the major greenhouse gases (GHGs), by 2050 to mitigate climate change is the significant challenge of this century [1]. Rapid deforestation due to overpopulation, industrialization, and transportation is having a devastating effect on ecosystems and water supplies [2]. Nitrate concentrations in groundwater sources have been on the rise globally mainly due to agricultural intensification in the past few years [3]. High levels of nitrates (>10 mg L−1) are associated with various human health disorders, including thyroid dysfunction, colorectal cancer, and bladder cancer [4, 5].
Ion exchange, reverse osmosis, electrodialysis, electrochemical treatment, membrane filtration, and adsorption are the commonly used physical treatment techniques for nitrate removal from wastewater [6]. Although ion exchange have several advantages, such as operational simplicity, high selectivity, and minimal solid waste generation, however, instability of organic resins under high radiation, disposal issues (landfill and incineration) related with discarded resin leading to environmental pollution and economic issues [7]. Reverse osmosis is another pressure driven simple, effective, and proven technology, but it generates secondary high concentration wastewater and scales. Further, membrane fouling due to membranes surface clogging by accumulation of several contaminants in wastewater increases technology costs [8]. Electrodialysis involve the separation of various ions in water passing through selective permeable anionic and cationic membranes under an applied direct current (DC) electric field. Electrodialysis technology has economic and automated performance, but this method does not reduce nitrates to nitrogen; instead, it separates and purifies high concentration nitrate wastewater, resulting in secondary brine, requiring additional costs for posttreatment [9]. The effectiveness and simplicity of the process make adsorption the most popular method among aforementioned options. Activated carbons are commonly used at commercial adsorbent due to high porosity and surface area (500–2000 m2 g−1) [10]. However, high production cost and regeneration over high pressure stream further increase the operation cost for treatment systems [11, 12]. The high prices of current adsorbent materials have prompted research into more economical and effective alternatives for the removal of pollutants from water.
Graphene and its derivatives, particularly graphene oxide (GO), have received attention for their prospective applications in environmental remediation and energy systems due to their high surface area, chemical stability, and functional diversity [13, 14]. In water purification, GO’s abundant surface functional groups, such as hydroxyl and carboxyl, facilitate the efficient adsorption of pollutants like heavy metals, radionuclides, and organic contaminants. However, magnetic materials, such as magnetic graphene oxide (MGO) and gadolinium-doped graphene oxide (GGO) alleviated the recovering dispersed GO from aqueous solutions. These composites effectively combine efficient pollutant removal with magnetic separation, as demonstrated by GGO’s successful extraction of Pb (II) and Cr (III) from aqueous solutions, in accordance with US EPA standards, while also exhibiting reusability over multiple cycles [15, 16].
GO surface characteristics can be tuned by various functionalization approaches, such as introducing functional groups, aggregation, and doping with heteroatoms [17, 18]. These modifications optimize the interaction between graphene and catalytic species, improving energy conversion processes. In energy applications, metal-doped graphene, including nitrogen-doped variants incorporating Ni, Pd, and Pt, exhibits improved catalytic performance in oxygen reduction reactions (ORRs), essential for fuel cell efficiency. Additionally, surfactants like hexadecyltrimethylammonium (HDTMA) are employed to modify graphene surfaces, further enhancing their functionality for diverse applications [19]. HDTMA-modified materials are highly effective in adsorbing nitrates from water due to their positively charged surface, which selectively attracts and retains negatively charged nitrate ions while minimizing interference from other ions [20, 21]. This selectivity makes them ideal for water treatment, as they ensure efficient nitrate removal without affecting other ions [22]. Additionally, these materials can be regenerated through rinsing and reactivation, enabling multiple usage cycles [23]. Compared to chemical treatments, HDTMA-based adsorption technologies are more environmentally friendly, as they avoid the use of toxic chemicals and the generation of harmful by-products, supporting sustainable water treatment practices [23]. Adsorption with HDTMA-modified materials can be a more cost-effective option compared to treatments like ion exchange or reverse osmosis, especially for large-scale operations [24]. HDTMA-modified materials can be easily tailored for multiple water conditions and applications. This adaptability allows for the design of adsorbents with enhanced nitrate selectivity or adsorption capacity.
This study reports the functionalization of GO with HDTMA to achieve significantly enhanced nitrate removal efficiency, marking a departure from conventional approaches. The influence of various factors, such as HDTMA loading, pH, and initial nitrate concentration on the adsorption performance of the modified GO was examined. The synergistic mechanism combining chemical interactions and electrostatic forces, supported by comprehensive structural analyses (X-ray photoelectron spectrometer [XPS], Raman, and X-ray diffraction (XRD), provides deeper insights into the material’s adsorption behavior. Moreover, the reusability of the adsorbent over multiple cycles demonstrates its practical applicability, which is rarely explored in comparable studies. The findings demonstrate that the surface modification with HDTMA significantly improves the nitrate adsorption capacity of GO, offering insights into optimizing adsorption processes for effective water treatment.
2. Experimental Materials and Methods
2.1. Materials
HDTMA, sod. nitrate (NaNO3 99.9%), sod. chloride (NaCl 99%), and methanesulfonic acid (99%), are purchased from Sigma–Aldrich. GO powder was purchased from Ios Technology Co., Ltd, Taiwan. While hydrochloric acid (HCl 36.5%−38.0%), sod. hydroxide (NaOH 98.7%), and sod. sulfate anhydrous (Na2SO4, 99%) were purchased from J.T. Baker and Macron Fine, respectively. Sod. sulfate hydrated (Na2SO4. 10 H2O) and sod. phosphate hydrated (Na2SO4. 12 H2O) were purchased from Thermo Scientific. All the chemicals are analytical research grade and used without any further purification.
2.2. Equipment
Ion chromatography (IC) system with Dionex IonPacAS20 anion-exchange column from Thermo Fisher Technology Co., Ltd, Taiwan was used to analyze the cations released into solution during the nitrate reduction rection (NRR).
An XRD analysis was performed to determine the changes in the crystalline structure of GO after modification with HDTMA and subsequent nitrate adsorption. XRD experiments was conducted using a German made Bruker D2 Phaser X-ray diffractometer with Cu-Kα (λ = 1.5418 Å, 30 KV) as the X-ray radiation emission source. D2 Phaser uses a sealed tube X-ray lamp, providing efficient, low-maintenance operation with a detection limit typically around 1%–2% for phase composition analysis. The scanning 2θ range from 10°–50°.
Raman spectroscopy is a nondestructive, highly sensitive, and powerful technique to identify the microscopic structure of nanoscale, carbon-based materials including graphite, graphene, nanotubes, etc. Raman spectra of the samples were recorded using a RL633 (RM System 1000 Mk1, Renishaw, UK). A 633 nm HeNe laser was used as the excitation source for the nondestructive analysis of samples to elucidate the molecular and material properties of the GO before and after the HDTMA and nitrate adsorption. Extended scans were used to show peaks where the Raman shift was between 1000 cm−1 and 2000 cm−1. The system measures the inelastic scattering of light to provide detailed information about molecular vibrations, offering chemical fingerprinting capabilities.
A HITACHI S-3400N (Japan) scanning electron microscope (SEM) equipped with a Schottky field emitter electron gun operating at 10 KV was used to collect SEM images. SEM was used to analyze the surface morphology of unmodified (pristine) GO and HDTMA modified GO before and after the nitrate adsorption. A PerkinElmer Spectrum ONE (USA) fourier transform infrared spectrometer (FTIR) used to detect the nature of various functional groups attached with the GO before and after HDTMA modification and nitrate adsorption. A specific surface area and porosimetry analyzer; model: Micromeritics Instrument Corporation/ASAP 2020 was employed to measure the specific surface area (Brunauer–Emmett–Teller [BET] m2 g−1) and pore size distribution of GO and modified GO. A JEOL JEM-2100 LaB6 transmission electron microscope (TEM) from Japan was used at various magnifications to study the internal structure and in depth morphology of the GO. XPS, PH1 5000 VersaProbe lll with variable Al Kα X-rays adjustable was used to analyze the material surface chemistry and variations in oxidation states of the unmodified, modified and nitrate adsorbed GO. Before XPS measurements, copper glue was used to fix the GO powder, facilitating subsequent instrument analysis.
3. Methodology
A schematic representation of the GO modification with HDTMA and nitrate removal process is shown in Figure 1. To modify GO, three different concentrations of HDTMA solution were prepared: 1 × 10−3 M, 5 × 10−3 M, and 10 × 10−3 M. A 50 mL volume of each HDTMA solution was transferred into separate 50 mL centrifuge tubes. Subsequently, 20 mg of GO powder was added to each tube. The samples were placed on a horizontal shaker set at 200 rpm and allowed to equilibrate for 8 h. Modified GO achieve equilibrium in approximately 3 h. The modified GO was finally separated from the solution using vacuum filtration and underwent oven drying for further physicochemical characterization and nitrate reduction experiments.
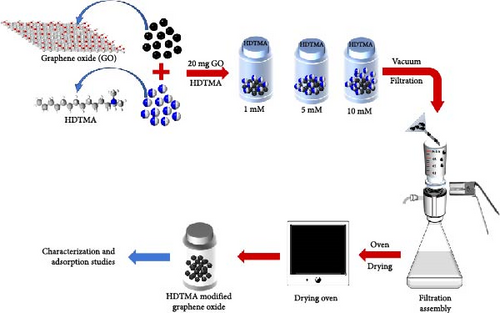
4. Results and Discussion
4.1. XRD
XRD analysis was performed to characterize the crystalline nature and phase purity of the GO and as-synthesized GO-HDTMA and GO-HDTMA-NO3− adsorbents. All the XRD patterns shown in Figure 2 exhibit a diffraction peak within the range of 2θ = 13°–15° accredited to (001), corresponds to the interplanar distance between GO sheets [25]. All the Peaks at 2θ = 16°–18° corresponds to (002) ∗ plane of GO [26, 27]. A small peak at 2θ region from 25° to 26.4° accredited to (002) in all samples corresponds to graphitic carbon in GO [28, 29]. Another weak peak at 2θ of approximately 43° can also be identified, which corresponds to the reflection from GO (1 0 0). After the adsorption of HDTMA and nitrates on GO, a slight peak shift from 2θ ~13.97° to 2θ ~14.16° is observed for GO-HDTMA-10 mM and GO-HDTMA-NO3− respectively which confirms the adsorption of nitrate on GO-HDTMA surface. Furthermore, GO-HDTMA-10 mM exhibits peaks at lower angles (2θ ≈13.97°), which implies an increase in interlayer spacing resulting from the intercalation of HDTMA. In contrast, GO-HDTMA-NO3− displays a peak shift at position (2θ ≈ 14.16°) and an increase in peak intensity, which can be attributed to the structural changes resulting from the adsorption of nitrate ions onto GO.
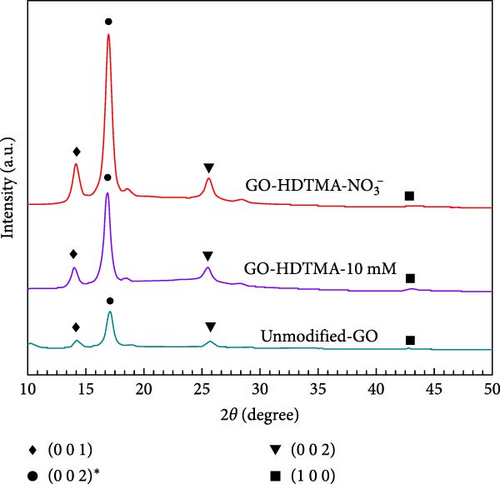
The functionalization of GO with HDTMA and subsequent nitrate adsorption did not demonstrate a significant impact on the X-ray diffraction patterns of GO. Furthermore, neither the duration of the reaction nor the concentration of HDTMA significantly altered the crystallite structure of GO. This suggests that HDTMA reactions took place solely at the surface of GO [29]. The introduction of HDTMA enhances surface functionalization, whereas the presence of nitrate ions leads to a marginal decrease in d-spacing relative to GO-HDTMA-10 mM, indicating a competitive interaction between nitrate and HDTMA. Therefore, it can be concluded that the adsorption of HDTMA onto GO plays a key role in modifying the material’s surface properties, which could have a significant impact on the efficiency of NRR processes.
4.2. SEM
Figure 3a–c illustrates the SEM analyses of unmodified graphene oxide (unmodified GO), 1 mM HDTMA-modified graphene, and 10 mM HDTMA-modified graphene, respectively. The SEM images of GO depicted in Figure 3a,b represents a loosely packed structure of GO compared to the single-layer structure observed in modified GO-HDTMA-10 mM.
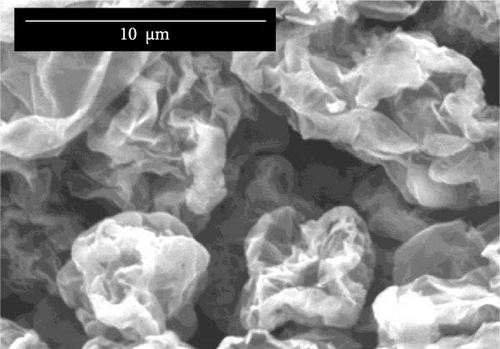

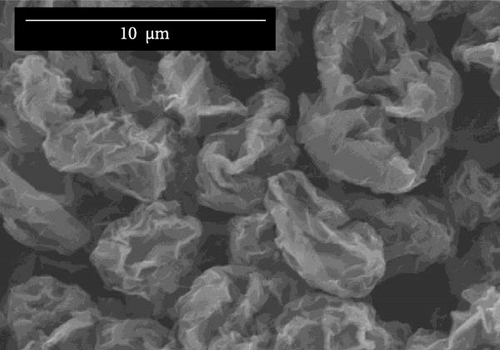
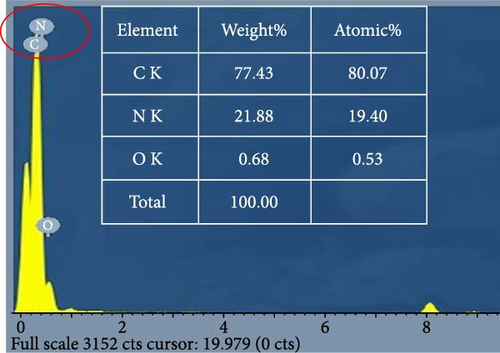
Figure 3a,b represents a similar structure for both unmodified GO and GO-HDTMA-1 mM modified GO. Both shows sheets of GO exhibiting a smooth surface with folds and creases, irregular edges, and rough surfaces. The particle sizes are relatively uneven, and mostly, the sheets appear in a flake-like form. Figure 3c shows GO-HDTMA-10 mM after modification. SEM images of modified GO-HDTMA-10 mM reveal two-dimensional flake sizes of approximately 5 μm. The cationic surfactant enters the graphite layer, filling the micropores, and the particles tend to aggregate, resulting in a spherical shape for GO [30]. Some wrinkles can be observed in these sheets, possibly due to the repulsive negative charge of deprotonated carboxylic acid groups in GO [31]. Additionally, it is observed from energy dispersive X-ray spectroscopy (EDS) that the introduction of the surfactant increases the nitrogen content in GO, Figure 3d.
4.3. BET
Figure 4a,b shows the absorption–desorption isotherms and pore size distribution of unmodified GO and GO-HDTMA-10 mM. It can be seen that the modification of GO with the surfactant does not have a substantial effect on the pore size distribution, as seen in Figure 4a. Both unmodified GO and HDTMA-modified GO exhibit a mixed mesoporous structure as shown by a type IV isotherm [32]. Typically, this region should essentially capture the interactions between the adsorbent and the adsorbate, especially at lower partial pressures.
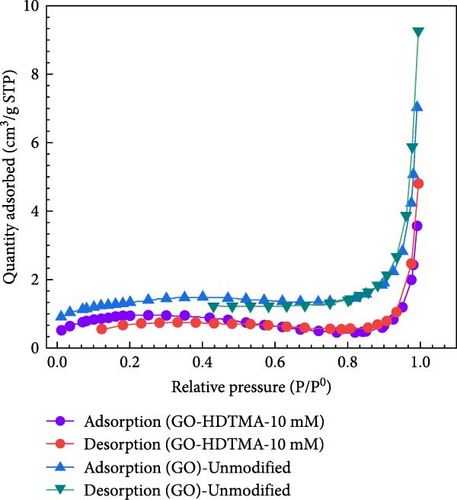
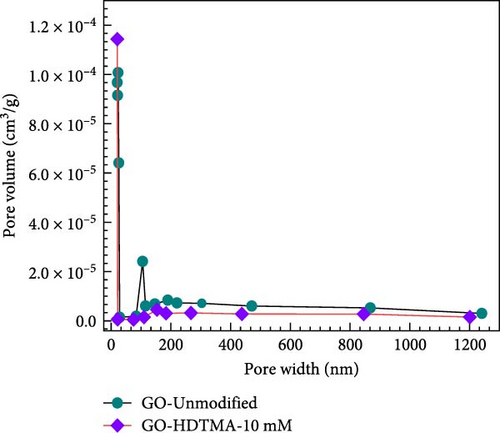
The N2 adsorption–desorption isotherms indicate that the presence of mesoporous structures leads to pore filling at lower pressures. The relationship between pore size and pore volume on GO-unmodified and GO-HDTMA-10 mM is shown in Figure 4b.The pore size of unmodified GO is 11.88 m2 g−1 while after modification with HDTMA, it reduced to 8.35 nm. BET specific surface area of GO could be as low as 8 m2 g−1, possibly due to the stacking of GO layers and agglomeration of GO [33, 34] which is in close agreement with surface area determined for GO in this study (11.88 m2 g−1). Further reduction in pore size along with surface area can be attributed to the covering of GO pores with HDTMA. Consequently, the specific surface area of modified GO (3.55 m2 g−1) is lower than that of unmodified GO (4.82 m2 g−1). Results are in close agreement for GO with the research reported by Chen et al. [35].
Consistent with the SEM results, the images shows that unmodified GO exhibit relatively uneven particle sizes, appearing in a flake-like form. Subsequent to HDTMA modification, the surfactant diffuses into the GO layers, promoting the clogging of micropores and causing the GO to adopt a spherical structure [30].
4.4. XPS
The study investigates the changes in unmodified GO and the variations induced by HDTMA and nitrates adsorption. Figure 5a–c shows the C 1s XPS spectra for unmodified, 10 mM HDTMA modified GO, and GO-HDTMA with nitrate adsorption. It can be clearly seen from Figure 5a that the C 1s spectrum of unmodified GO contains three peaks at 282.54, 284.60, and 286.44 eV corresponding to the sp2 carbon, the epoxide, and the carboxyl functional groups, respectively. As shown in Figure 5a–c, C─C/C═C, C─O, and C═O signals were detected in unmodified GO, GO-HDTMA. and GO-HDTMA-NO3−. All the signals were obviously enhanced after addition of 10 mM-HDTMA to GO because HDTMA contains a large number of C─C bonds. There is a positive peak shift for all the samples. However, after functionalization with HDTMA, peak shifts of 0.1, 1.24, and 0.14 eV was calculated for peak 1, peak 2, and peak 3 respectively for C 1s XPS spectra of GO-HDTMA-10 mM. It is further evidenced by a high intensity for these peaks which revealed high concentration of C─C bonds due to addition of HDTMA having extensive C─C (Sp2) bonding. Furthermore, a reduction in oxygen content was measured after loading HDTMA on GO surface because HDTMA does not contain any oxygen. It also confirmed the adsorption of HDTMA on GO subsequently used for nitrate reduction.
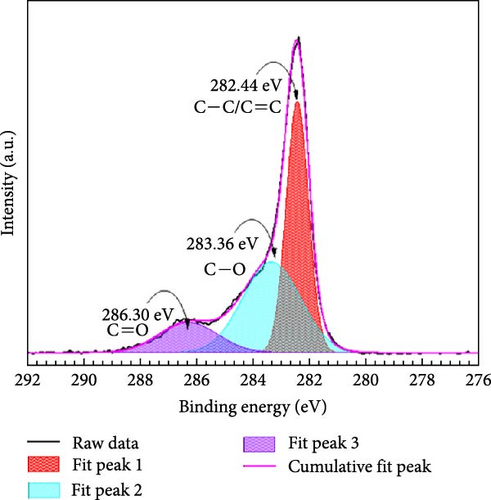
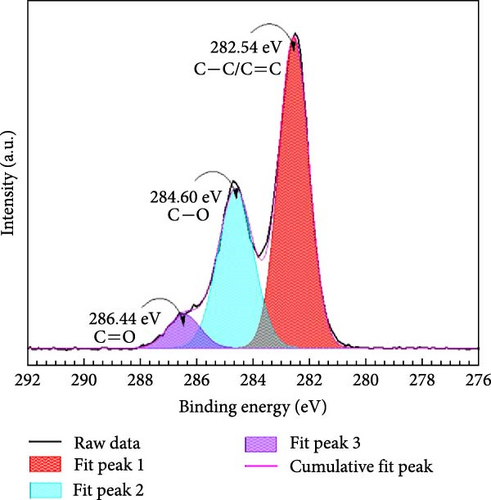
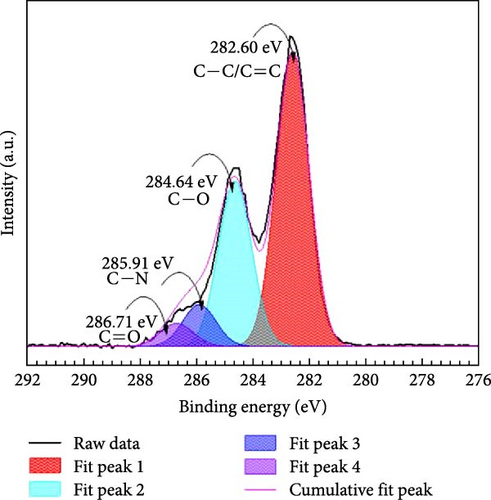
After addition of 30 mM nitrate solution to HDTMA functionalized GO, a slight positive peak shift was calculated for all the peaks which confirmed the adsorption of nitrates on the surface of GO-HDTMA-10 mM. However, there is no prominent change observed in the intensity of the peaks. A similar trend have been reported by Kuang et al., [36] for HDTMA loaded GO sponge for nitrate reduction. However, a new peak appeared at 285.91 eV for GO-HDTMA-NO3− which can be assigned to C─N (quaternary ammonium groups) [37].
Before modification with HDTMA, GO contains elements, such as C and O. After modification, GO shows an increase in N content, consistent with the results of XPS elemental analysis shown in Table 1. However, the nitrogen content in unmodified GO is determined to be <0.1%, indicating the absence of nitrogen in GO. After modification with HDTMA, the N content in GO-HDTMA increases to 1.4%, indicating that HDTMA can effectively load onto GO.
| Adsorbent | C% | O% | N% |
|---|---|---|---|
| GO-unmodified | 78.1 | 20.5 | <0.1 |
| GO-HDTMA-10 mM | 73.4 | 25.2 | 1.4 |
4.5. TEM
The TEM analysis in Figure 6a,b provides structural insights into unmodified GO and 10 mM-HDTMA modified GO. As shown in Figure 6a unmodified GO exhibits a characteristic two-dimensional layered structure with many wrinkles representing the typical morphology of GO as reported in many preliminary works [38, 39]. After the addition of the surfactant, the GO layers looks dense, which make the layered structure less distinct. HDTMA’s positive charge can interact with the negative functional groups on GO’s surface, altering the charge distribution. This interaction can impact GO’s conductivity by modifying the charge transfer properties of GO [40]. Adsorption of HDTMA molecules between the layers of GO can increase the interlayer spacing. This expansion of the interlayer distance can reduce the π-π stacking interactions between adjacent GO sheets, potentially affecting the conductivity.

4.6. FTIR
Cationic surfactants were used to modify the surface of GO with HDTMA (1–10 mM). A comparative FTIR analysis of unmodified GO, GO-HDTMA, and GO-HDTMA-NO3− was conducted to further understand the functional groups attached at the sample.
Figure 7 shows the combined FTIR spectra in the wavenumber range of 600–4000 cm−1. A broad peak at 3435 cm−1 in high frequency area is attributed to O─H stretching, which reveals the presence of hydroxyl groups on GO [41]. A peak around 2915 cm−1 is attributed to C─H stretching vibration [42]. The peak near 2850 cm−1 is attributed to the symmetric stretching mode of CH2. The absorption bands from 1633–1664 cm⁻1 is due to C═C vibrations. The peaks around 1400 cm−1, 1204 cm−1 (C─O─C), and 1049 cm−1 (C─O) are associated with C─O─C and C─O stretching respectively [43, 44], suggesting the presence of alkenes in the GO. The peak near 872 cm−1 corresponds to the CH2 bond, and the peak around 598 cm−1 is attributed to the COO─bond.
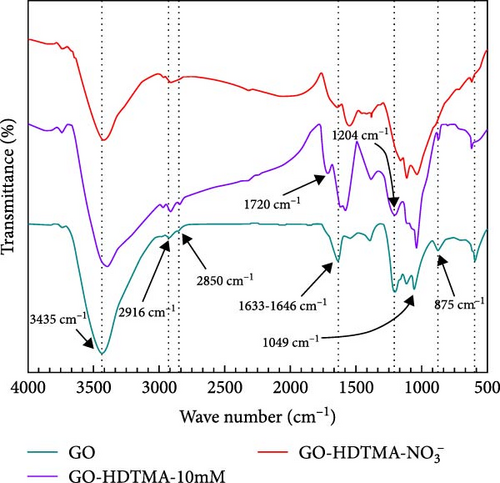
After modifying GO with 10 mM HDTMA, changes in the spectra before and after modification are observed. An enhanced absorption peak is noticed around 1580 cm−1, possibly due to the introduction of HDTMA, enhancing the carboxylate anion on the modified GO. The band observed at 1720 cm−1 was assigned to the carboxyl group [45]. However, there are no significant changes in other regions, indicating that there is not a substantial difference between modified and unmodified GO.
Subsequently, the GO modified with HDTMA is used to adsorb nitrate ions in water. The adsorption of nitrate ions leads to changes in the chemical properties of the GO surface. Specifically, the symmetric stretching of COO─ around 1580 cm−1, the C─O─C bond near 1204 cm−1, and the C─O─H bending vibration around 900 cm−1 show significant changes. The peak of C─O─C at 1204 cm−1 decreases slightly after nitrate adsorption, and a new COO─ peak appears at 1540 cm−1, suggesting an increase in oxygen functional groups in GO [30]. Furthermore, peak around 900 cm−1 related to the C─O─H bond disappear in both HDTMA modified and unmodified GO after nitrate adsorption, indicating a possible consumption of oxygen functional groups due to the interaction with nitrate ions [30].
4.7. Raman Analysis
Raman spectroscopy is extensively employed to investigate the ordered and disordered crystalline structures of carbonaceous materials. Figure 8 shows Raman spectra for GO, GO-HDTMA, and GO-HDTMA-NO3−. Peaks represent the standard vibrational modes for GO structure, with characteristic D and G bands. The typical Raman spectrum of GO can be observed in Figure 8, comprising two main bands at 1329 cm−1 and 1588 cm−1 known as the D- and G-bands, respectively [46]. The G-band at 1329 cm−1 is a characteristic of all sp2-hybridized carbon networks, originates from the first-order scattering from the doubly degenerate E2g phonon modes of graphite, while the prominent D peak comes from the structural imperfections created by the attachment of oxygenated groups on the carbon basal plane [47]. The D-band at approximately 1329 cm−1 corresponds to the breathing mode of k-point phonons of A1g symmetry. While G-band approximately 1588 cm−1 corresponds to the E2g phonon of sp2 carbon atoms. It represents the in-plane stretching of the carbon–carbon bonds. In GO Raman spectra, the D-band intensity shows oxidation on the GO, while the G-band represents the graphitic structure. The tiny dislocation of G and D band of GO-HDTMA and GO-HDTMA-NO3− samples suggested more defects and surfactant adsorption on GO surface [46]. After the adsorption of HDTMA on the GO, changes in the Raman spectrum can be observed. The G-band slightly shift and increase in intensity due to charge transfer between HDTMA-Br and GO. The adsorption of HDTMA can interact with the sp2 domains in GO, influencing the vibrational modes. Similarly, D-band might show increased intensity, indicating an increase in disorder due to the HDTMA adsorption which is consistent with literature citing the disruption in the structure caused by the surfactant molecules [28].
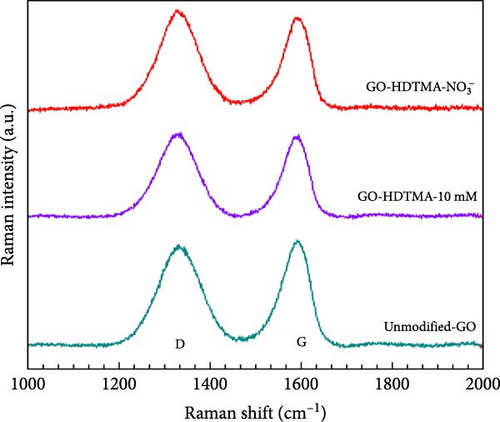
The GO-HDTMA-NO3− on the other hand, has the highest intensity at the D and G peaks, suggesting that the addition of nitrate has intensified the interactions and led to increased disorder on the GO surface. Adsorption of the HDTMA leads to moderate changes in the GO structure, increasing disorder and inducing charge transfer effects. The adsorption of nitrate ions enhances the structural disruptions due to further interactions between the nitrate and GO/HDTMA system.
The ID/IG ratio serves as an accurate measure of the disorder and crystallite sizes within the graphitic layers. ID/IG ratio of 1, 1.01, and 1.03 were calculated for GO, GO-HDTMA, and GO-HDTMA-NO3−, respectively. These values indicate the presence of GO in all samples while a slight increase in ID/IG ratio further confirms the adsorption of HDTMA, and nitrates on the GO surface. These findings (peak values and ID/IG ratio) are in close agreement with cited literature for functionalized GO [48].
5. Adsorption Studies
5.1. Adsorption of Nitrate by Unmodified GO
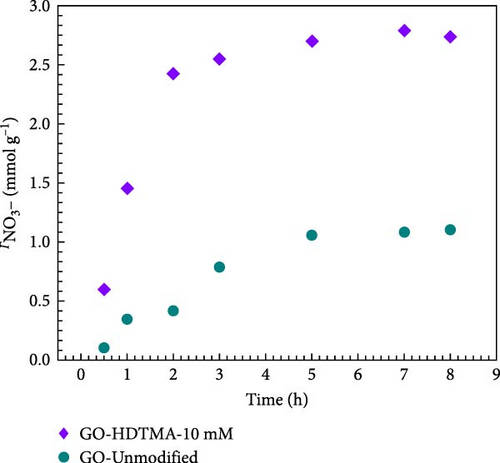
To assess the nitrate adsorption capacity of unmodified GO, 45 mL of 10 mM sod. nitrate aqueous solution is added to 20 mg of unmodified GO. Unmodified GO rapidly adsorbs nitrates during the first 3 h, as evidenced by Supporting Information data S1, and attains equilibrium after 7 h. Therefore, 8 h of adsorption time is set in order to ensure adsorption equilibrium in all of the subsequent experiments. Unmodified GO exhibits poor nitrates adsorption capacity (1.11 mmol g−1) which can be ascribed to the weak interaction forces between unmodified GO and nitrates. Literature reveals that surfactants, such as dodecyltrimethylammonium (DDTMA) [49], hexyltrimethylammonium (HTMA), decyltrimethylammonium (DCTMA), and HDTMA [50] can effectively modify the surface adsorption capacity of solid adsorbents (graphene, GO, activated carbon) [50, 51]. Amongst aforementioned surfactants, HDTMA has been extensively reported for effective removal of perchlorate, ammonium, and nitrates from water [50, 52, 53]. The interaction between a surfactant and an adsorbent is influenced by various parameters, such as the type of surfactant (ionic or nonionic), chain length (linear or branched), structure (hydrophobicity or hydrophilicity), and solution conditions (pH, temperature, and polarity) [51, 54]. Therefore, during this study HDTMA, a cationic surfactant with the longest carbon chain was used to functionalize the GO surface for enhanced nitrate removal.
5.2. HDTMA Loading on GO
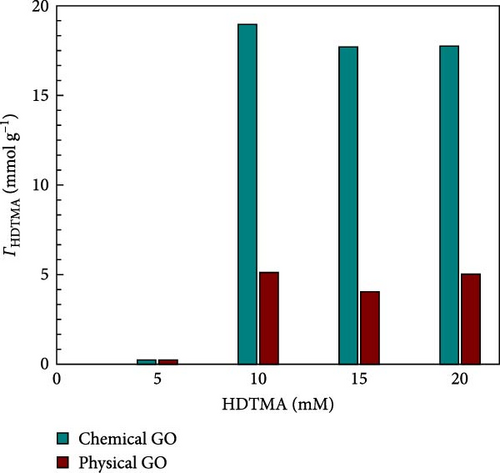
Figure 9a shows the loading of HDTMA on physical and chemical GO. Physical GO exhibits lower HDTMA (~5.0 mM g−1) compared to chemical GO (16 mM g−1). Four different concentrations (5, 10, 15, 20 mM) of HDTMA are used to determine the optimal loading of HDTMA on GO. It can be observed from Figure 9a as the concentration of HDTMA increases, the GO surface loading also increases. The adsorption reaches equilibrium, as the HDTMA concentration approaches 20 mM and no further adsorption can be observed. However, maximum adsorption was measured at 10 mM HDTMA concentration. Therefore, 10 mM was chosen as the optimal HDTMA loading for subsequent experiments. Additionally, the effect of nitrate adsorption on both unmodified GO and HDTMA-modified GO is illustrated in Figure 9b. The data show that the nitrate adsorption rate increases steadily for both adsorbents up to the 4th h, after which it stabilizes. Despite this plateau, the adsorbents exhibit good adsorption capacity throughout the remainder of the reaction, indicating their effectiveness in maintaining nitrate removal.
5.3. Adsorption Equilibrium Time for HDTMA Modified and Unmodified GO
Figure 10a presents the isothermal adsorption of nitrates on unmodified physical and chemical GO. Seven different concentrations of sod. nitrate solution (0.5–20 mmol g−1) were used to measure the adsorption capacity of nitrate at pH = 7. Results indicate that chemical GO shows threefold increase in nitrate adsorption efficiency (1.02 mmol g−1) compared to physical GO (0.32 mmol g−1). Therefore, it can be concluded that chemical GO is more effective in removing nitrate from nitrate solution and further used for subsequent experiments.
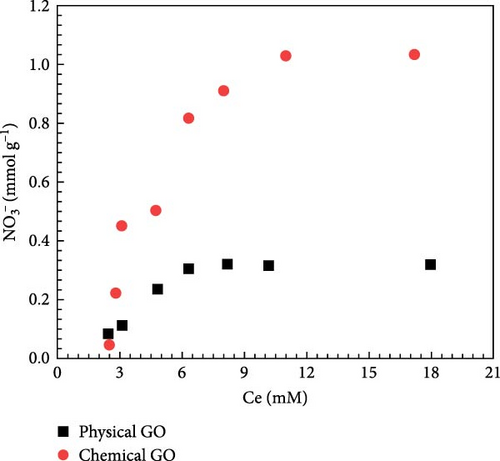
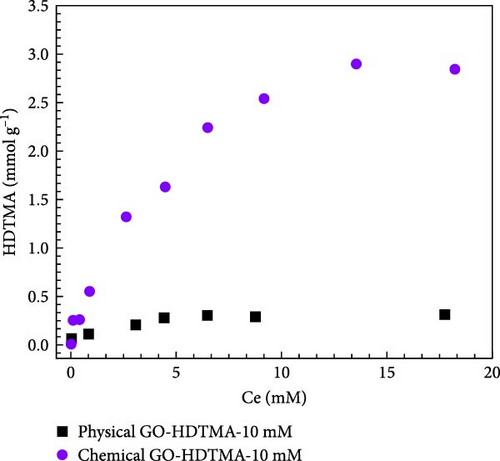

Figure 10b illustrates the isothermal adsorption for physical and chemical GO modified with 10 mM HDTMA for nitrate adsorption at pH = 7. The results indicate that chemical GO exhibited a higher nitrate adsorption efficiency (2.89 mmol g−1) compared to physical GO. Which is approximately twofold increase in adsorption capacity compared to physical GO. Unmodified GO achieves equilibrium in ~ 4 h, while modified GO reaches adsorption equilibrium in 3 h, demonstrating significantly improved adsorption performance. The nitrate adsorption experiment was carried out for 8 h to allow ample time to achieve equilibrium. GO-HDTMA-10 mM shows nitrate adsorption capacity of 2.74 mmol g−1 which is approximately 2.5 times higher than that of unmodified GO (1.11 mmol g−1). The improved nitrate adsorption on GO-HDTMA-10 mM revealed a faster and more effective nitrate adsorption rate due to availability of functional groups (─CHO, ─COOH, and ─OH) which enhance the nitrate interaction both electrostatically and via weak hydrogen bonding as shown in XPS analysis.
The adsorption data for nitrate is fitted using Langmuir and Freundlich model. Solid lines represents the fitting data while symbolic lines represents the original adsorption isotherms. Fitting results indicate that the Langmuir model provides the best fit for the adsorption data. Adsorption isotherms fitted with Langmuir model are shown in Figure 10c. It can be seen from Table 2 that GO-HDTMA-10 mM exhibited a higher nitrate adsorption efficiency (Qmax = 4.19 mmol g−1) compared to GO modified with lower concentrations of HDTMA.
| Adsorbents | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| Qmax (mmol g−1) | KL (L mmol−1) | R2 | N2 | KF (mmol h−1) (L mmol−1)1/n |
R2 | |
| GO-HDTMA-1 mM | 1.96 | 0.04 | 0.985 | 2.62 | 0.16 | 0.866 |
| GO-HDTMA-5 mM | 2.62 | 0.13 | 0.963 | 3.34 | 0.73 | 0.865 |
| GO-HDTMA-10 mM | 4.19 | 0.17 | 0.993 | 4.19 | 0.76 | 0.973 |
5.4. Effect of pH on Nitrate Adsorption of GO
The effect of pH on various nitrate concentrations (0.1, 1, 10, and 30 mM) on adsorption capacity of GO-HDTMA-10 m is shown in Figure 11a. It can be observed that at 0.1 mM nitrate concentration, the influence of pH on the nitrate adsorption is almost negligible (i.e., pH independent). At lower pH value (pH = 3), excess chloride ions (Cl-) can inhibit nitrate adsorption, leading to a reduction in the adsorption capacity for nitrate over modified GO [55]. However, with an increase in nitrate concentration from 1 to 30 mM, nitrate adsorption becomes more pH dependent. At pH = 6, modified GO exhibits the highest nitrate adsorption capacity of 0.53, 2.41, and 2.08 mmol g−1 for 1, 10, and 30 mM respectively. However, at pH > 6, a negative trend in nitrate adsorption is shown. This is likely due to the high concentration of hydroxide ions (OH−) on GO active sites at alkaline pH, which compete with nitrate ions and reduce GO capacity to adsorb nitrate.
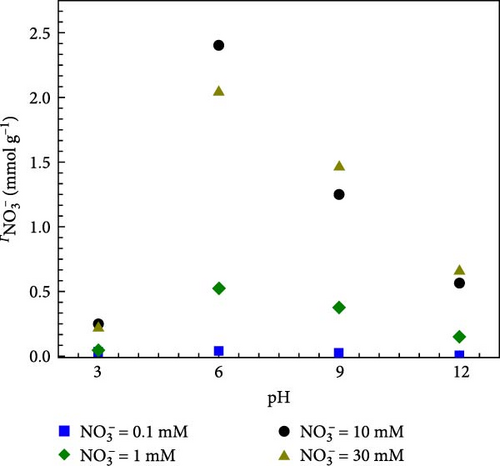
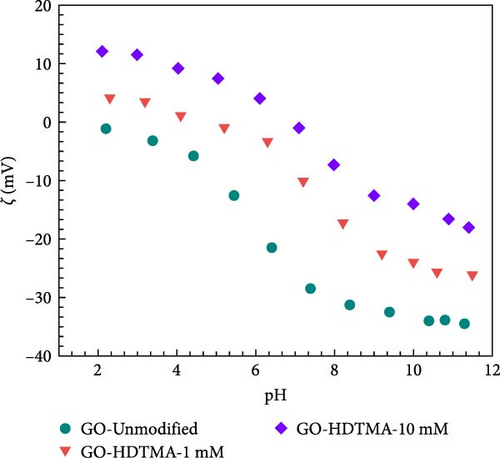
Since pH changes directly affect nitrate adsorption, zeta potentials of unmodified GO, 1 mM and 10 mM HDTMA modified GO were measured at different pH levels whilst results are displayed in Figure 9b. The unmodified GO exhibits a negative charge across the pH range of 2–12, which substantially lowered the adsorption effectiveness of GO due to electrostatic repulsion between negatively charged nitrates (NO3−) and GO. It can also be observed that unmodified GO show zeta potential values more negative than −30 mV (−31.18–34.49 mV) over pH range of 8–12. Zeta potential values more negative than −30 mV are generally considered to represent sufficient mutual repulsion to ensure the stability of a dispersion [56]. Li et al., [57, 58] and Si et al. [59] found that the zeta potential of GO is more negative due to the ionization of oxygen-containing functional groups (carboxylic acid, phenolic, and hydroxyl groups) when dispersed in water. This could also be attributed to the excess of chloride ions (Cl−) inhibiting nitrate adsorption.
However, GO-HDTMA-1 mM, and HDTMA-10 mM maintained positive surface charge till pH = 4 and pH = 6, respectively. However, surface charges become negative over pH > 6. This reversal in surface charge on HDTMA modified GO confirms the adsorption of HDTMA on GO surface. Since the surfaces of both (1 and 10 mM HDTMA-GO) are positively charged at pH < 6, there will be strong adsorption of nitrates on GO surface through electrostatic interaction. Conversely, the electrostatic repulsion occurred between the GO and nitrates at high pH. Therefore, an optimal pH = 6 is selected for this study.
As shown in Figure 11b, when HDTMA concentration increased from 1 to 10 mM, zeta potential increased from approximately 4.10 to approximately 12.08 mM, respectively at pH = 2. While zeta potential of unmodified GO remained unchanged. These results are in good agreement with data in Figure 11a, representing the high nitrate adsorption for 10 mM nitrate concentration at pH = 6. Since the critical micelle concentration (CMC) of HDTMA is 0.92–1.0 mM (water) the formation of micelles can structurally hinder the nitrates ions electrostatic interaction with modified GO.
6. Effect of Coexisting Anions on Modified GO
The competitive adsorption of sulfate ions (SO42−), phosphate ions (PO43−), and nitrate ions (NO3−) in water is shown in Supporting Information Figure S2. These anions are commonly found in wastewater which affect the nitrate adsorption. The concentrations of all ions are set at 0.1, 10, and 20 mM to observe their impact on nitrate adsorption. The results indicate that the presence of coexisting ions reduces the adsorption of nitrate. Compared to the excellent adsorption of nitrate in the presence of a single nitrate ion (2.54 mmol g−1), the adsorption is significantly reduced when other coexisting ions are present, with the adsorption amount decreasing by about half (1.03 mmol g−1).
Additionally, as the contamination concentration increases, the adsorption amount also increases, reaching equilibrium at 10 mmol g−1 without a significant increase at 20 mmol g−1. Supporting Information Figure S2 shows that sulfate ions (SO42−) and phosphate ions (PO43−) exhibit stronger adsorption effects, which can be attributed to the higher valency of these ions and competitive adsorption of PO43−, SO42−, and NO3− [60]. It is observed that a higher valency of anions, such as PO43− and SO42−, promotes the adsorption of specific anions on the adsorbent surface, leading to interference with NO3− adsorption [61, 62]. Therefore, the nitrate wastewater containing a large amount of phosphate ions should be pretreated before denitrification.
7. Reusability Study
The adsorbent reusability across multiple adsorption cycles serves as a critical parameter for evaluating the stability, economic feasibility, and resistance to reaction intermediates. Therefore, investigating the reusability of adsorbent materials is essential. Figure 12 shows the adsorption capacity of the GO-HDTMA-10 mM (adsorbent) decreased with each of the four regeneration. After completing each cycle, the GO-HDTMA surface was washed with NaOH solution to desorb the nitrate from the GO-HDTMA surface and then reused for the next cycle. Adsorption capacity in each cycle is measured in comparison to actual capacity (2.74 mmol g−1) before reusing.
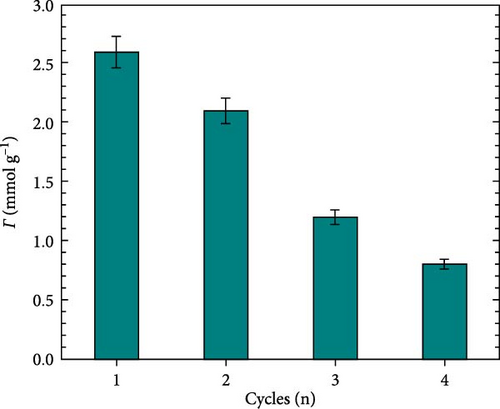
As can be seen in Figure 12, GO-HDTMA nitrate adsorption capacity was approximately 95% after the first reuse cycle. The high adsorption rate is attributable to surface functional groups (─CHO, ─COOH, and ─OH) which enhance the nitrate interaction with GO. In the second reuse cycle, a reduction in adsorption capacity of ~77% is observed; nonetheless, the GO-HDTMA demonstrates effective nitrate adsorption capability. A pronounced decrease in activity was observed in proceeding third and fourth cycles, with drop in efficiency up to approximately 48% and approximately 29%, respectively. Loss in activity can be described to the degradation, saturation and structural changes that hinder the ability to adsorb and remove nitrates. Despite the lowest adsorption efficiency in fourth cycle, GO-HDTMA demonstrates high activity up to three cycles maintaining the removal capacity up to approximately 50%. However, beyond fourth cycle, regeneration is necessary to restore the optimal performance. In addition, level of nitrate concentration in water, as well as the presence of coexisting anions, such as sulfates and phosphate ions, also affect reusability. Investigation on the impact of coexisting anions on HDTMA-modified GO was detailed in the preceding section 6.5.
The evaluation of the performance and enhanced efficiency of HDTMA-modified graphene oxide (GO) compared to other studies is presented in Table 3. The data in Table 3 demonstrate that HDTMA-modified GO achieves a significantly higher nitrate adsorption capacity (2.89 mmol g−1) relative to unmodified GO and other reported materials. Secondly, unlike many alternative materials, the modified GO retains its adsorption efficiency across three consecutive reuse cycles, highlighting its practical usability. Thirdly, the optimal nitrate adsorption occurs at pH 6, which corresponds to environmentally relevant conditions, further supporting its suitability for real-world applications. These findings emphasize the superior performance and potential utility of HDTMA-modified GO in the effective removal of nitrate from aqueous systems.
| Material | Functionalization method | Adsorption capacity (mmol g−1) | Optimal pH | Reusability efficiency |
Reference |
|---|---|---|---|---|---|
| Chitosan-based composite | Metal oxide modification | 0.50–1.20 | 5–7 | Not reported | [63] |
| Zeolite | NaCl-treated | 1.75 | 6.5 | Partially reusable | [64] |
| Quaternary ammonium functionalized cellulose | Quaternary ammonium groups | 3.87 | 5–7 | Not reported | [65] |
| Biochar–zeolite composite | Functionalized biochar with modified zeolite | 0.39 | 6.5 | Maintained for five cycles | [64] |
| Almond shell biochar | Pyrolyzed at 900°C | 0.32 | 7 | Not reported | [66] |
| Carbon-based nanoadsorbent | Nanostructured carbon materials | 0.48 | 7 | Not reported | [67] |
| GO-HDTMA (10 mM) | HDTMA functionalization | 2.89 | 6 | Maintained for three cycles | This study |
8. Proposed Mechanism
Considering the structural and chemical changes in GO-HDTMA interacting with nitrate NO3−, an adsorption mechanism can be proposed. Physical and electrochemical investigations suggested that HDTMA adsorbs onto and intercalates within the GO structure. This interaction leads to a reduction in the interlayer spacing of GO, which can be attributed to the adsorption or binding of NO3− ions at the active sites on the GO surface. Surface modifications observed indicate that HDTMA adsorption enhances the hydrophobicity of the GO surface, facilitating interactions with nitrate ions. The adsorption of HDTMA induces a hydrophobic environment that promotes electrostatic attraction of NO3− to the positively charged sites of HDTMA, as illustrated in Equation (1). Nitrate ions may either adsorb onto or intercalate within the expanded interlayer regions of GO-HDTMA. Furthermore, the conductive properties of GO contribute to electron transfer processes during adsorption, supporting the overall adsorption and nitrate removal mechanism. Overall nitrate adsorption mechanism is depicted in Figure 13.

Although, thermodynamic studies on HDTMA-modified GO for NRR are limited. However, research on similar materials provides insights into their potential mechanisms. A recent research nZVI@rGO nanocomposites showed promising activity for nitrate removal from water solutions. It was found that rGO served as a stabilizing agent, preventing nZVI aggregation and enhancing electron transfer during the reduction process. Further, thermodynamic analyses indicated that nitrate reduction by nZVI@rGO is spontaneous under specific conditions, with the reaction favorability influenced by factors, such as pH and temperature [60]. In another study by Zabierowski et al., [68 ] reported the solvothermal functionalization of graphite and thermally reduced graphene oxide (TRGO) using a bis-hydrazone copper (I) complex. A significant increase of 328% in the graphite BET specific surface area was observed, achieving a value of up to 374 m2 g−1, whereas the surface area of TRGO exhibited no change [68]. Additionally, research on HDTMA-modified natural zeolite combined with GO-based covalent organic frameworks has shown potential in desalination applications. While this study focused on ion removal from saline water, the functionalization with HDTMA improved the material’s adsorption capacity and selectivity. Thermodynamic parameters suggested that the adsorption processes were spontaneous and endothermic, implying increased efficiency at higher temperatures [69].
Although direct thermodynamic studies on HDTMA-modified GO for NRR are limited, the combination of HDTMA’s surfactant properties with GO’s high surface area could enhance nitrate adsorption and reduction. Thermodynamic analyses would likely reveal that such modifications improve the spontaneity and efficiency of nitrate reduction processes. Further experimental investigations are essential to fully understand the thermodynamics of nitrate reduction using HDTMA-modified GO.
9. Conclusion
The structural modifications induced by surfactants on GO play a critical role in facilitating the NRR process. This study demonstrates the significant impact of HDTMA functionalization on the structural and functional properties of GO for efficient nitrate removal from water. HDTMA-modified GO exhibited a remarkable twofold increase in nitrate adsorption capacity compared to unmodified GO, with optimal performance at a pH of 6. The adsorption mechanism, driven by a combination of chemical interactions and electrostatic forces, was supported by XPS, Raman, and XRD analyses, which revealed critical structural modifications contributing to enhanced efficiency. Furthermore, the reusability of the modified GO over multiple cycles underscores its potential for practical applications. These findings establish HDTMA-modified GO as a promising material for water treatment, particularly for nitrate remediation. Future efforts could focus on optimizing functionalization strategies, exploring environmental variables, and integrating this material into scalable, hybrid systems for comprehensive water purification solutions.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yu-Hsuan Huang and Muhammad Sheraz Ahmad contributed equally to this work.
Funding
Research supported by: National Science and Technology Council of Taiwan (Grants 112-2221-E-131-007 and 112-2221-E-182-070-MY3) and Formosa Center and the Center for Sustainability and Energy Technologies at Chang Gung University (Grant URRPD2N0041).
Acknowledgments
This work was supported by the National Science and Technology Council of Taiwan with grants 112-2221-E-131-007 and 112-2221-E-182-070-MY3. The authors also acknowledge the support from the MCUT Formosa Center and Center for Sustainability and Energy Technologies at Chang Gung University (Grant URRPD2N0041).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data can be provided on a proper request to the corresponding author.




