Effect of Lactated Ringer Administration on Survival Outcomes in Critically Ill Patients With Acute Kidney Injury: A Retrospective Cohort Study
Abstract
Background: Although lactated Ringer’s (LR) solution is widely used in managing patients with acute kidney injury (AKI), its specific impact on mortality remains unclear. This retrospective cohort study aimed to evaluate the effects of LR administration on survival outcomes in severely ill patients with AKI.
Methods: Critically ill patients with AKI were identified using data from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database. Propensity score matching (PSM) was employed to address baseline discrepancies between patients who received LR and those who did not. The association of LR administration with survival, duration of hospitalization and intensive care unit (ICU) stay, requirement for renal replacement therapy (RRT), renal function recovery, and hyperkalemia was analyzed using restricted mean survival time (RMST), logistic regression, and linear regression models.
Results: A total of 5620 patients with AKI were included. Following PSM, LR administration was associated with prolonged survival at 28 and 90 days compared to non-LR use (28-day survival increase: 1.12 days, 95% confidence interval [CI] 0.62–1.63, p < 0.001; 90-day survival increase: 3.73 days, 95% CI 1.70–5.76, p < 0.001). The survival benefit became more pronounced, with higher LR use linked to more remarkable 90-day survival. However, LR administration did not significantly affect renal function recovery or hyperkalemia incidence.
Conclusion: Administering LR to critically ill patients with AKI was associated with improved survival at both 28 and 90 days.
1. Introduction
The global prevalence of acute kidney injury (AKI) varies substantially, influenced by factors such as whether it is hospital-acquired (h-AKI) or community-acquired (c-AKI). AKI affects approximately 7%–18% of hospitalized patients [1] and occurs at a rate of 20–200 cases per million in the general population [2]. In ICUs, AKI substantially contributes to morbidity and mortality [3, 4], with a mean prevalence ranging from 30% to 50% [5]. The primary causes of AKI in patients hospitalized in the ICU are sepsis and hypovolemia [6, 7]. Timely fluid administration stabilizes hemodynamics in critically ill patients with anuric AKI by restoring intravascular volume, increasing stroke volume, and improving perfusion pressure. These effects improve renal perfusion and glomerular function [8]. However, uncertainties remain regarding the advantages of fluid therapy in individuals with AKI [9, 10]. Legrand et al. reported that active fluid resuscitation did not significantly impact renal microvascular blood flow or oxygen delivery in a hemorrhagic shock rat model [9]. Current evidence does not support fluid resuscitation as a means to improve AKI prognosis [11–13]. A multicenter observational cohort study suggested that a positive fluid balance might increase AKI-related mortality [12]. Moreover, fluid administration beyond the correction of hypovolemia can lead to adverse outcomes. For decades, 0.9% sodium chloride (saline) has been the most commonly used intravenous fluid. However, the chloride present in saline has been associated with renal vasoconstriction [14], inflammation [15], AKI [16], hyperchloremic metabolic acidosis [17], and eventually, increased mortality [18]. As a balanced crystalloid fluid, LR mimics the plasma’s electrolyte composition and has recently gained popularity as a substitute for saline. Current studies among critically ill adults suggest that, compared to saline, balanced crystalloids (with LR being the primary formulation) are associated with lower mortality rates, reduced need for RRT, and a decreased incidence of renal dysfunction [19, 20]. However, the benefits of LR in improving ICU patient outcomes remain uncertain. Two large, single-center randomized controlled trials (RCTs)—the Saline versus Albumin for the Treatment of Patients with Decompensated Cirrhosis and Acute Kidney Injury: An Evaluation of Diuretic Response and Renal Outcome (SALT-ED) and Sepsis Management with Activated Recombinant Thrombomodulin (SMART) trials—investigated the use of balanced crystalloids, primarily LR, compared to saline in adults. Both trials reported no significant difference in mortality rates between the two treatment options [21, 22]. Previous research on balanced solutions has focused on specific patient groups, such as those with systemic inflammatory response syndrome (SIRS) [23], trauma [24], sepsis [20], or patients undergoing surgical procedures [25, 26]. However, studies specifically focusing on patients with AKI remain limited. Therefore, this study aimed to evaluate the impact of LR administration on mortality outcomes in patients experiencing AKI using data from the MIMIC-IV database.
2. Methodology
2.1. Data Source
This retrospective cohort study utilized data from the MIMIC-IV (version 1.0) database, which includes over 200,000 emergency ICU admissions at Beth Israel Deaconess Medical Center between 2008 and 2019 [27]. The lead author, Shengling Huang, completed the Collaborative Institutional Training Initiative Examination and obtained approval for database access (record ID: 42251770). The data extraction code is publicly available on GitHub (https://github.com/MIT-LCP/mimic-iv). Per the Privacy Rule of the Health Insurance Portability and Accountability Act, individual patient consent was waived since all data were anonymized to ensure privacy.
2.2. Participants
Patients were diagnosed with AKI based on the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [28]. The baseline serum creatinine (SCr) level was defined as either the lowest SCr value recorded within 7 days before admission or, if unavailable, the initial SCr measurement obtained upon admission [29]. According to KDIGO clinical practice guidelines, AKI staging was determined by evaluating urine output and creatinine levels within the first 48 h of ICU admission [28].
Inclusion criteria included: (1) analysis of data from the initial ICU admission for individuals with multiple admissions; (2) age ≥ 18 years; and (3) clinical diagnosis of AKI within 48 h of ICU admission.
Exclusion criteria included: (1) hospital discharge within 48 h of ICU admission, (2) mortality within 48 h of ICU admission, (3) pre-existing diagnosis of chronic kidney disease stage 5 (CKD5), (4) receipt of RRT before screening, (5) history of renal transplantation, (6) administration of crystalloid fluids before AKI diagnosis, and (7) total crystalloid infusion volume of < 2 L within 24 h after AKI diagnosis.
2.3. Data Collection
Data for relevant variables on the first day of ICU admission were extracted from the MIMIC-IV database. These included demographic characteristics, sequential organ failure assessment (SOFA) score, simplified acute physiology score II (SAPS II), systolic and diastolic blood pressure, heart rate, mean arterial pressure (MAP), vasopressor use, respiratory rate, body temperature, body weight, oxygen saturation (SpO2), SCr, baseline creatinine, chloride, potassium, bicarbonate, anion gap, mechanical ventilation status, RRT use, and anchor year group.
AKI patients were categorized into two groups: the “LR” group, individuals administered with LR solution (with or without saline or other crystalloid solutions, and the “non-LR” group), and patients who were administered only non-LR crystalloids, including saline and other crystalloid solutions. The proportion of LR solution administered was calculated by dividing the daily volume of LR solution by the total daily volume of all crystalloids. Missing data points were excluded from the analysis (Additional file 1: Table S1).
2.4. Primary and Secondary Outcomes
This study primarily aimed to evaluate the 28-day survival rate, with secondary endpoints including the 90-day survival rate, length of stay (LOS) in the ICU and hospital, peak potassium concentration (hyperkalemia) during the ICU stay, recovery of renal function, and initiation of new RRT. The term “28-day survival” refers to patients who remained alive 28 days after ICU admission, while “90-day survival” denotes survival up to 90 days post-ICU admission. Recovery of kidney function was defined as a significant reduction in creatinine levels to less than 1.5 times the baseline values, along with normalized urinary output exceeding 0.5 mL/kg/h over a continuous 24-h period at discharge [30].
2.5. Restricted Mean Survival Time (RMST) Analysis
The RMST is a measure of the average survival time within a specified observation period (t), offering a meaningful alternative for survival analysis in studies with limited follow-up or right-censored data. By focusing on survival time up to a pre-determined timepoint, RMST circumvents the need to evaluate the entire survival curve and enhances the interpretability of survival outcomes. This method provides a reliable framework for analyzing survival outcomes in scenarios where complete long-term data are unavailable, thereby improving the interpretability of survival studies under practical constraints [31, 32]. RMST represents an absolute measure of survival time, offering a more intuitive alternative to hazard ratios (HRs) for clinicians and patients. RMST does not depend on the proportional hazards (PH) assumption, unlike Cox PH models. Concentrating on survival time within a predefined interval enables meaningful comparisons even when the proportionality of hazards is violated [33, 34]. RMST is calculated as the cumulative survival probability represented by the Kaplan–Meier (KM) curve over a specified time frame [35]. This approach reduced the impact of data from a small number of patients during later follow-up periods, thereby strengthening the robustness and reliability of the analysis.
2.6. Statistical Analysis
Categorical variables were presented as frequencies (percentages), and intergroup comparisons were conducted using appropriate statistical tests, such as the chi-square (χ2) test or Fisher’s exact test. Continuous variables were expressed as either the mean with standard deviation or the median with its interquartile range, depending on the distribution. Group comparisons utilized the t-test or the Mann–Whitney U test as appropriate.
Propensity score matching (PSM) was performed at a 1:1 ratio to minimize baseline characteristic discrepancies between the LR and non-LR cohorts. Propensity scores were calculated using a logistic regression model, with a caliper width set to 0.2 standard deviations to optimize matching.
The association between LR use and extended survival time in patients with AKI was assessed using RMST analysis. The RMST values, their differences, ratios, and corresponding 95% CIs were calculated for 28- and 90-day intervals following ICU admission using the “survRM2” package in R software.
A logistic regression model examined the relationship between LR administration and outcomes associated with renal function recovery, hyperkalemia, and new RRT initiation. Results were reported as HRs alongside their respective 95% CIs. The effect of LR use on ICU and hospital LOS was assessed using a linear regression model, with HRs calculated applying the formula HR = eβi.
Subgroup analyses and sensitivity analysis were conducted to assess the robustness of results and explore potential dose–response relationships between LR administration rates and survival outcomes. The analyses were stratified by age, gender, daily fluid intake, AKI stage, sepsis-3 status, and the proportion of LR administration. All statistical analyses were conducted using the R package (version 4.2.1), with statistical significance defined as a p-value < 0.05.
3. Results
The MIMIC-IV database identified 32,828 patients diagnosed with AKI. After applying exclusion criteria, a final cohort of 5620 individuals was chosen for this study. Of these, 3140 individuals received LR during their ICU stays, while 2480 did not (Figure 1). We conducted the Anderson–Darling (A–D) test for key continuous variables, and the results are shown in Table S4.

Table 1 summarizes the baseline characteristics of the LR and non-LR groups. Before PSM, considerable differences were observed between the two cohorts concerning gender, admission categories, anchor year group, and AKI stage. Compared to the non-LR group, the LR group exhibited the following characteristics: (1) higher heart rate, SCr, baseline creatinine, chloride, and potassium levels, along with elevated SAPS II and SOFA scores; (2) greater likelihood of receiving more significant volumes of pre-AKI fluids, total fluid input, colloid input, and mechanical ventilation (72.5% vs. 67.3%); (3) lower MAP, respiratory rate, fluid output, bicarbonate levels, and vasopressor use (40.5% vs. 56.2%); and (4) reduced incidence of heart failure (21.4% vs. 25.0%) and liver disease (11.8% vs. 14.4%). After PSM, the standardized mean difference (SMD) was less than 0.1, indicating a significant reduction in the baseline variables in both groups (Figure 1S).
| Variables | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-LR (n = 2480) | LR (n = 3140) | p | SMD | Non-LR (n = 1804) | LR (n = 1804) | p | SMD | |
| Age, median (IQR), yr | 66.0 (55.0, 77.0) | 67.0 (55.0, 78.0) | 0.450 | 0.008 | 66.0 (55.0, 77.0) | 68.0 (55.0, 79.0) | 0.484 | 0.012 |
| Gender, male, n (%) | 1437 (57.9) | 1730 (55.1) | 0.035 | 0.057 | 1024 (56.8) | 1027 (56.9) | 0.946 | 0.003 |
| Ethnicity, white, n (%) | 1593 (64.2) | 2094 (66.7) | 0.149 | 0.052 | 1179 (65.4) | 1156 (64.1) | 0.707 | 0.028 |
| Admission type, emergency, n (%) | 1570 (63.3) | 1755 (55.9) | < 0.001 | 0.218 | 1084 (60.1) | 1098 (60.9) | 0.961 | 0.018 |
| Weight, median (IQR), kg | 83.8 (70.1, 99.5) | 82.1 (69.1, 98.4) | 0.395 | 0.003 | 83.0 (70.0, 99.1) | 81.9 (68.5, 97.9) | 0.352 | 0.003 |
| Heart rate, median (IQR), bpm | 84.4 (73.5, 97.3) | 88.4 (77.2, 100.8) | < 0.001 | 0.204 | 86.1 (75.0, 98.5) | 86.5 (75.4, 98.2) | 0.910 | 0.007 |
| MAP, median (IQR), mmHg | 78.0 (71.6, 85.8) | 75.6 (70.3, 82.6) | < 0.001 | 0.198 | 77.2 (71.1, 84.4) | 76.5 (70.9, 84.3) | 0.605 | 0.012 |
| Respiratory rate, median (IQR), bpm | 19.2 (16.9, 22.0) | 18.9 (16.7, 21.8) | 0.021 | 0.051 | 19.0 (16.7, 21.9) | 19.0 (16.7, 21.8) | 0.739 | 0.009 |
| Temperature, median (IQR), °C | 37.0 (36.7, 37.4) | 37.0 (36.7, 37.3) | 0.415 | 0.005 | 36.9 (36.7, 37.4) | 37.0 (36.7, 37.3) | 0.467 | 0.018 |
| SpO2, median (IQR), % | 97.5 (96.0, 98.8) | 97.5 (96.1, 98.8) | 0.546 | 0.010 | 97.4 (96.0, 98.8) | 97.4 (96.0, 98.7) | 0.968 | 0.005 |
| Before AKI input, median (IQR), mla | 1770.0 (600.0, 3547.8) | 2298.0 (812.8, 4577.0) | < 0.001 | 0.232 | 1959.5 (670.3, 3908.8) | 1959.5 (700.0, 3814.8) | 0.806 | 0.003 |
| Before AKI colloid input, median (IQR), mlb | 1213.5 (350.0, 2429.8.0) | 1244.0 (393.8, 2541.3) | 0.140 | 0.050 | 1299.5 (365.5, 2535.8) | 1209.0 (407.0, 2450.5) | 0.854 | 0.001 |
| Bicarbonate, median (IQR), mmol/L | 23.0 (21.0, 26.0) | 22.0 (19.0, 25.0) | < 0.001 | 0.271 | 23.0 (20.0, 25.0) | 23.0 (20.0, 25.0) | 0.693 | 0.010 |
| Creatinine, median (IQR), mg/dL | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | 0.006 | 0.077 | 0.9 (0.7, 1.3) | 0.9 (0.70, 1.3) | 0.540 | 0.018 |
| Baseline creatinine, median (IQR), mg/dL | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | 0.017 | 0.067 | 0.9 (0.7, 1.2) | 0.9 (0.70, 1.2) | 0.496 | 0.027 |
| Chloride, median (IQR), mg/dL | 104.0 (101.0, 107.0) | 105.0 (102.0, 109.0) | < 0.001 | 0.263 | 105.0 (101.0, 108.0) | 104.0 (101.0, 108.0) | 0.865 | 0.008 |
| Aniongap, median (IQR), mg/dL | 15.0 (12.0, 17.0) | 14.0 (12.0, 17.0) | 0.131 | 0.046 | 14.0 (12.0, 17.0) | 14.0 (12.0, 17.0) | 0.392 | 0.015 |
| Potassium, median (IQR), mg/dL | 4.0 (3.70, 4.5) | 4.1 (3.70, 4.6) | < 0.001 | 0.133 | 4.1 (3.7, 4.5) | 4.1 (3.7, 4.5) | 0.648 | 0.012 |
| SAPS II score | 37.0 (29.0, 47.0) | 40.0 (31.0, 49.3) | < 0.001 | 0.169 | 38.0 (30.0, 48.0) | 38.0 (30.0, 48.0) | 0.750 | 0.002 |
| SOFA score | 6.0 (4.0, 8.0) | 6.0 (4.0, 9.0) | < 0.001 | 0.133 | 6.0 (4.0, 9.0) | 6.0 (4.0, 9.0) | 0.780 | 0.002 |
| Fluid input, median (IQR), mlc | 3401.0 (2746.0, 4305.0) | 4221.0 (3277.0, 5644.8) | < 0.001 | 0.588 | 3629.0 (2941.5, 4572.0) | 3611.0 (2989.5, 4594.3) | 0.708 | 0.005 |
| Colloid input, median (IQR), mld | 0.0 (0.0, 0.0) | 0.0 (0.0, 350.0) | < 0.001 | 0.346 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.072 | 0.028 |
| Fluid output, median (IQR), mle | 1289.5 (827.8, 1946.0) | 1125.0 (740.0, 1675.5) | < 0.001 | 0.192 | 1223.5 (793.0, 1855.0) | 1222.0 (776.8, 1770.0) | 0.455 | 0.017 |
| Anchor year group, n (%) | < 0.001 | 0.214 | 0.860 | 0.029 | ||||
| 2008–2010 | 883 (35.6) | 943 (30.0) | 611 (33.9) | 594 (32.9) | ||||
| 2011–2013 | 629 (25.4) | 726 (23.1) | 422 (23.4) | 432 (23.9) | ||||
| 2014–2016 | 583 (23.5) | 728 (23.2) | 429 (23.8) | 421 (23.3) | ||||
| 2017–2019 | 385 (15.5) | 743 (23.7) | 342 (19.0) | 357 (19.8) | ||||
| AKI stage, n (%) | 0.014 | 0.078 | 0.358 | 0.048 | ||||
| 1 | 1869 (75.4) | 2271 (72.3) | 1333 (73.9) | 1358 (75.3) | ||||
| 2 | 587 (23.7) | 846 (26.9) | 458 (25.4) | 428 (23.7) | ||||
| 3 | 24 (1.0) | 23 (0.7) | 13 (0.7) | 18 (1.0) | ||||
| Diabetes, n (%) | 699 (28.2) | 839 (26.7) | 0.233 | 0.033 | 494 (27.4) | 486 (26.9) | 0.793 | 0.010 |
| Sepsis3, n (%) | 1746 (70.4) | 2378 (75.7) | < 0.001 | 0.120 | 1317 (73.0) | 1311 (72.7) | 0.852 | 0.007 |
| Heart failure, n (%) | 621 (25.0) | 672 (21.4) | < 0.001 | 0.086 | 424 (23.5) | 424 (23.5) | 1.000 | 0.001 |
| Renal disease, n (%) | 333 (13.4) | 437 (13.9) | 0.623 | 0.014 | 234 (13.0) | 244 (13.5) | 0.659 | 0.016 |
| Liver disease, n (%) | 356 (14.4) | 371 (11.8) | 0.005 | 0.075 | 251 (13.9) | 249 (13.8) | 0.962 | 0.003 |
| Chronic pulmonary disease, n (%) | 596 (24.0) | 781 (24.9) | 0.486 | 0.020 | 425 (23.6) | 445 (24.7) | 0.460 | 0.026 |
| Cancer, n (%) | 325 (13.1) | 473 (15.1) | 0.040 | 0.056 | 255 (14.1) | 261 (14.5) | 0.812 | 0.010 |
| Rheumatic disease, n (%) | 76 (3.1) | 120 (3.8) | 0.143 | 0.042 | 63 (3.5) | 64 (3.5) | 1.000 | 0.003 |
| Vasopressors use, n (%) | 1394 (56.2) | 1271 (40.5) | < 0.001 | 0.319 | 909 (50.4) | 922 (51.1) | 0.689 | 0.014 |
| Mechanical ventilation | ||||||||
| Invasive, n (%) | 1668 (67.3) | 2276 (72.5) | < 0.001 | 0.114 | 1257 (69.7) | 1254 (69.5) | 0.942 | 0.004 |
| Noninvasive, n (%) | 85 (3.4) | 148 (4.7) | 0.020 | 0.065 | 69 (3.8) | 62 (3.4) | 0.593 | 0.021 |
- Abbreviations: AKI = acute kidney injury, MAP = mean arterial pressure, RRT = renal replacement therapy, SAPS II = simplified acute physiology score II, SMD = standardized mean difference, SOFA = sequential organ failure assessment.
- aBefore AKI fluid input volume was recorded prior to diagnosis of AKI after ICU admission.
- bBefore AKI colloid input volume was recorded prior to diagnosis of AKI after ICU admission.
- cFluid input volume was recorded within the first 24 h after AKI diagnosis after ICU admission.
- dFluid output volume was recorded within the first 24 h after AKI diagnosis after ICU admission.
- eColloid input volume was recorded within the first 24 h after AKI diagnosis after ICU admission.
3.1. Primary Outcomes
The RMST analysis was employed because it violated the PH assumption and the relatively low mortality rates in the LR and non-LR groups. In the pre-matched cohort, the expected survival times for the LR and non-LR groups during the initial 28 days before ICU admission were 25.25 days (95% CI 25.00–25.49) and 24.10 days (95% CI 23.76–24.42), respectively (Table 2). Over the 28-day period, the LR cohort had a mean survival advantage of 1.15 days (95% CI: 0.74–1.56) compared to the non-LR cohort. In the post-matched cohort, the LR group demonstrated a survival benefit of 1.12 days (95% CI 0.62–1.63; p < 0.001; Table 2). RMST analysis revealed a significant increase in 28-day survival time in the LR cohort. The KM curves for 28-day survival post-PSM are presented in Figure 2(a).
| Before PSM | Non-LR n = 2480 | LR n = 3140 | RMSTdb/HRc | 95% CI | p |
|---|---|---|---|---|---|
| RMSTa for 28 days (days) | 24.10 (23.76, 24.42) | 25.25 (25.00, 25.49) | 1.15 | 0.74, 1.56 | < 0.001 |
| RMST for 90 days (days) | 73.74 (72.45, 75.02) | 77.52 (76.50, 78.53) | 3.78 | 2.15, 5.42 | < 0.001 |
| LOS ICU (days) | 4.6 (2.9, 8.2) | 5.0 (3.1, 9.7) | 0.81 | 0.46, 1.15 | < 0.001 |
| LOS hospital (days) | 10.2 (6.5, 16.6) | 12.4 (7.76, 19.9) | 1.89 | 1.21, 2.56 | < 0.001 |
| Recovery of renal function (%) | 1901 (44.7%) | 2354 (55.3%) | 0.91 | 0.81, 1.03 | 0.144 |
| Hyperkalemia (%) | 316 (36.5%) | 550 (63.5%) | 1.02 | 1.01, 1.04 | 0.009 |
| New RRT (%) | 93 (41.0%) | 134 (59.0%) | 1.14 | 0.87, 1.50 | 0.328 |
| After PSM (1:1) | 1804 | 1804 | |||
| RMST for 28 days (days) | 24.04 (23.65, 24.43) | 25.16 (24.84, 25.49) | 1.12 | 0.62, 1.63 | < 0.001 |
| RMST for 90 days (days) | 73.54 (72.03, 75.06) | 77.28 (75.93, 78.63) | 3.73 | 1.70, 5.76 | < 0.001 |
| LOS ICU (days) | 4.6 (3.0, 8.6) | 4.8 (3.0, 8.6) | 0.72 | 0.32, 1.11 | < 0.001 |
| LOS hospital (days) | 10.6 (6.7, 17.1) | 11.1 (7.3, 17.7) | 1.40 | 0.63, 2.17 | < 0.001 |
| Recovery of renal function (%) | 1345 (49.6%) | 1368 (50.4%) | 1.07 | 0.92, 1.25 | 0.375 |
| Hyperkalemia (%) | 242 (46.4%) | 279 (53.6%) | 1.18 | 0.98, 1.40 | 0.080 |
| New RRT (%) | 85 (61.6%) | 53 (38.4%) | 0.56 | 0.37, 0.86 | 0.008 |
- Abbreviations: AKI = acute kidney injury, HR = hazard ratio, ICU = intensive care unit, LOS = lengths of stay, PSM = propensity score matching, RMST = restricted mean survival time, RRT = renal replacement therapy.
- aRMST represents the restricted mean survival time for each group within the first 28 and 90 days after admission to the ICU.
- bRMSTd means the difference of restricted mean survival time between the two groups (RMSTLR-RMSTNon-LR).
- cHR was calculated using the formula HR = eβi.
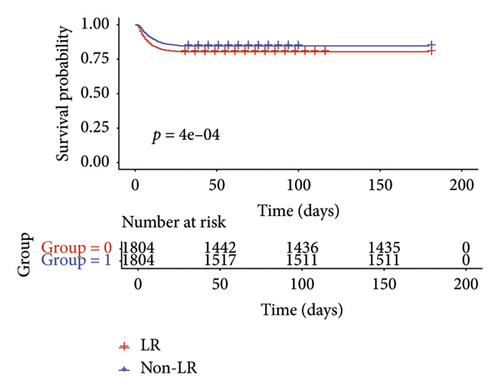
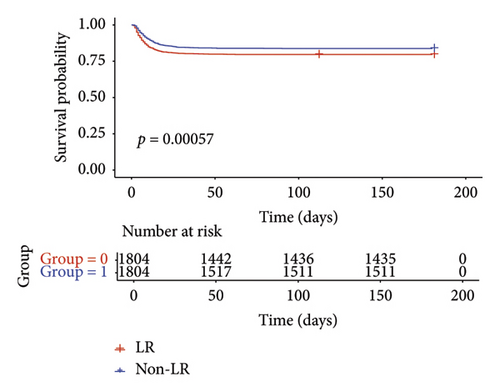
3.2. Secondary Outcomes
During the 90-day follow-up period, patients in the LR cohort had an RMST of 77.52 days (95% CI: 76.50–78.53), which was substantially longer than the 73.74 days (95% CI: 72.45–75.02) observed in the non-LR cohort (Table 2). This outcome indicated a significant survival improvement of 3.78 days (95% CI: 2.15–5.42). Following matching, the KM curve, shown in Figure 2(b), confirms that LR was associated with a 90-day survival advantage of 3.73 days (95% CI: 1.70–5.76). The RMST analysis demonstrated that the survival benefit of LR became more pronounced with longer follow-up durations.
Logistic regression analysis was used to assess the differences in renal function recovery, hyperkalemia, and new RRT outcomes between the two groups. Clinically considerable differences were observed in hyperkalemia and new RRT outcomes after PSM (Table 2). The impact of LR use on ICU and hospital LOS was assessed using a linear regression model. Before PSM (Table 2), the LOS in the LR group had a longer LOS compared to the non-LR group for both the ICU (5.0 vs. 4.6 days) and hospital (12.40 vs. 10.2 days). Following PSM, the LR group showed a more pronounced difference in ICU (4.8 vs. 4.6 days) and hospital (11.1 vs. 10.6 days) LOS.
3.3. Subgroup Analysis
Analysis based on the proportion of LR solution administered indicated a significant correlation between higher proportions of LR administration and improved 90-day survival. However, no significant association was observed between LR proportion and 28-day survival (Figure 3 and Additional file 1: Table S2). Subgroup analysis indicated a positive correlation between LR use and survival time, particularly in patients with AKI (Table 3). Patients aged 36–55 and older than 65 showed significantly longer survival. Furthermore, the RMST difference was more significant in men than women (1.47 vs. 0.80 days and 5.09 vs. 2.79 days, respectively).
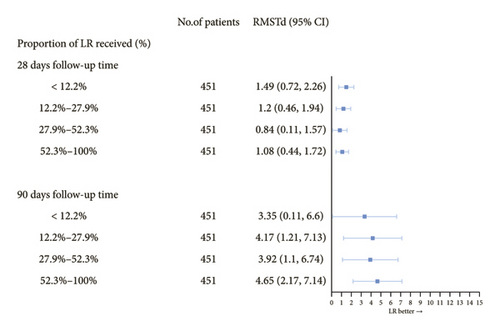
| Subgroup | N | Difference in RMSTa among 28 days | Difference in RMST among 90 days | ||
|---|---|---|---|---|---|
| RMSTdb (95% CI) | p | RMSTd (95% CI) | p | ||
| Age (yr) | |||||
| 18–35 | 224 | 0.15 (−1.05, 1.35) | 0.803 | 0.67 (−4.24, 5.58) | 0.789 |
| 36–55 | 706 | 1.33 (0.48, 2.17) | 0.002 | 5.66 (2.29, 9.03) | 0.001 |
| 56–65 | 736 | 0.73 (−0.21, 1.67) | 0.129 | 1.17 (−2.54, 4.87) | 0.539 |
| > 65 | 1942 | 1.44 (0.72, 2.16) | < 0.001 | 4.94 (2.11, 7.78) | 0.001 |
| Gender (%) | |||||
| Female | 1557 | 0.80 (0.10, 1.51) | 0.026 | 2.79 (0.00, 5.57) | 0.050 |
| Male | 2051 | 1.47 (0.90, 2.05) | < 0.001 | 5.09 (2.79, 7.39) | < 0.001 |
| AKI stage (%) | |||||
| 1 | 2691 | 0.97 (0.44, 1.4) | < 0.001 | 3.25 (1.17, 5.32) | < 0.001 |
| ≥ 2 | 917 | 1.94 (1.06, 2.82) | < 0.001 | 6.80 (3.26, 10.35) | < 0.001 |
| Sepsis3 (%) | |||||
| Yes | 2628 | 1.59 (1.08, 2.10) | < 0.001 | 5.49 (3.46, 7.53) | < 0.001 |
| No | 980 | 1.01 (0.25, 1.76) | 0.009 | 3.80 (1.01, 6.59) | 0.008 |
| Daily fluid input (ml)c | |||||
| (632, 1930] | 902 | 0.48 (−0.44, 1.40) | 0.306 | 1.47 (−2.29, 4.58) | 0.512 |
| (1930, 2430] | 902 | 1.14 (0.28, 2.01) | 0.009 | 4.26 (0.71, 7.81) | 0.019 |
| (2430, 3020] | 902 | 1.97 (1.08, 2.85) | < 0.001 | 7.32 (3.82, 10.83) | < 0.001 |
| (3020, 6840] | 902 | 1.51 (0.55, 2.48) | 0.002 | 5.01 (1.22, 8.80) | 0.010 |
- Abbreviations: AKI = acute kidney injury, PSM = propensity score matching, RMST = restricted mean survival time.
- aRMST represents the restricted mean survival time for each group within the first 28 and 90 days after admission to the ICU.
- bRMSTd means the difference of restricted mean survival time between the two groups (RMSTLR-RMSTNon-LR).
- cDaily fluid input was recorded as every 24 h after AKI diagnosis during the ICU stay.
Table 3 Subgroup analysis of LR use and clinical outcomes after PSM. RMST = restricted mean survival time. RMSTd = the difference of restricted mean survival time between the two groups (RMSTLR − RMSTNon-LR).
Stratification by daily fluid input volume revealed improved outcomes in patients receiving medium to high daily fluid input volumes (> 1930 mL), with no significant benefit seen in those with low fluid volumes (≤ 1930 mL). The most substantial survival difference was observed in patients with a daily fluid intake of 2430–3020 mL (Table 3). Patients meeting the Sepsis-3 criteria exhibited significantly enhanced survival compared to those who did not. Subgroup analyses before PSM yielded consistent results (Additional file 1: Table S3).
3.4. Sensitivity Analysis
Sensitivity analysis included 10,591 patients who died within 48 h of ICU admission, 231 who received crystalloid infusion before AKI diagnosis, and 8466 patients who received < 2 L of crystalloid fluid within 24 h of AKI diagnosis. The results showed that LR administration was significantly associated with improved survival outcomes in patients with AKI (mean survival increase: 0.57 days, 95% CI 0.31–0.83, p < 0.001). In the sensitivity analysis, the Cox PH model demonstrated a similar trend (HR = 0.63, 95% CI: 0.56–0.72).
4. Discussion
This cohort study established a substantial correlation between LR administration and improved survival at both 28 days and 90 days in critically ill patients with AKI. Moreover, the benefits of LR became more evident with extended follow-up periods. Subgroup analysis revealed that patients receiving higher proportions of LR experienced better 90-day survival outcomes. Moreover, there was a substantial difference in new RRT incidence between the two cohorts, while no considerable differences were observed in renal function recovery and hyperkalemia. Furthermore, patients treated with LR had slightly longer hospital LOS and ICU than those receiving non-LR solutions.
The correlation between LR fluid resuscitation and survival in critically ill patients with AKI is novel. This study provides new evidence supporting LR as a potentially more effective fluid choice for these patients, aligning with the findings from a meta-analysis [36] and supporting data from a retrospective study [19]. A meta-analysis of 13 high-quality clinical RCTs found that the impact of balanced crystalloids on patient outcomes varied, showing a relative reduction in 90-day mortality by 9% and an increase of 1% compared to saline [36]. A retrospective cohort study indicated that higher LR use was associated with reduced mortality, influenced by the total volume of fluid volume administered [19]. The SMART trial, which involved 15,802 critically ill adults, showed a mortality rate of 10.3% in the balanced crystalloids versus 11.1% in the saline group (p = 0.06) [37]. Similarly, the SALT trial, including 974 adults admitted to the ICU, reported no significant difference in in-hospital mortality between the two groups [38]. Conversely, our study demonstrated a significant survival difference. Several factors may explain this discrepancy: First, previous studies predominantly focused on critically ill and surgical patients in the ICU when investigating the association between crystalloid resuscitation fluids and survival outcomes [37–39]. Unlike the postoperative cohorts in the SPLIT trial [39] and patients with sepsis or respiratory failure in the SALT trial [38], our study targets explicitly severely ill patients with AKI. Second, the choice of balanced crystalloid solutions varied across studies. For example, the Balanced Solutions in Intensive Care Study (BASICS) [37] and 0.9% Saline vs Plasma-Lyte 148 (PL-148) for ICU Fluid Therapy (SPLIT) trials [39] compared balanced crystalloids, such as Plasma-Lyte 148. Third, our study’s large, retrospective cohort drawn from the MIMIC-IV database may also account for the observed differences.
Saline administration has been linked to higher mortality rates and increased RRT requirements in critically ill adults compared to balanced crystalloids, as evidenced by previous studies [20, 40, 41]. Moreover, results from retrospective research made in the United States revealed that patients who received saline infusion on the day of abdominal surgery exhibited an elevated risk of mortality, acidosis, and need for RRT [42]. Moreover, a supporting analysis of the SMART trial found that balanced crystalloids were not associated with severe hyperkalemia but were significantly correlated with a reduced incidence of new RRT in individuals diagnosed with AKI [43]. This study corroborates previous findings, showing significant differences in outcomes related to new RRT. The current study proposed that the higher RRT rates in the saline group might be attributed to reduced renal blood flow caused by elevated chloride levels [44] and delayed urination associated with saline use [45]. However, chloride in both patient groups in this study contradicts these hypotheses. Treatment decisions regarding RRT initiation may have been influenced by clinician bias. Moreover, the small number of new RRT recipient cases likely introduced assessment bias due to an insufficient sample size.
Balanced crystalloids contain small amounts of potassium, which raises concerns about hyperkalemia, discouraging their use by clinicians. A recent retrospective observational clinical study found no independent association between balanced fluid use and hyperkalemia in patients with impaired kidney function [46]. Similarly, another cohort study revealed that balanced crystalloids were associated with lower incidences of severe hyperkalemia and acidosis compared to saline solutions [47]. The findings of these studies align with our analysis, indicating that LR may be safer than saline in managing potassium levels and minimizing electrolyte disturbances. Furthermore, our study found no evidence to suggest that LR use improved the likelihood of renal function recovery compared to non-LR solutions. Potential explanations for this observation include (1) the absence of a universally accepted definition of AKI recovery, leading to inconsistent criteria and possible underestimation of patients who regained renal function [48], and (2) inappropriate fluid administration, including fluids through medications and nutritional support, which may result in fluid overload, venous congestion, and direct renal parenchymal injury [49]. This finding is significant as it suggests that LR does not have a detrimental effect on kidney recovery in AKI patients, addressing concerns about the potential adverse effects of fluid therapy on renal function.
One of the key strengths of this study lies in its use of RMST analysis. In the sensitivity analysis, the Cox PH model demonstrated a similar trend. However, the PH assumption was violated (p < 0.05), further supporting our choice of RMST as the primary analytical method. This method has demonstrated utility in fields, such as cardiovascular medicine, diabetology [35], and oncology [34], offering a robust and interpretable approach to assessing treatment effects in clinical research where HR is unsuitable. Furthermore, the study provides critical insights into fluid management for critically ill patients with AKI, a population that has historically been underrepresented in fluid therapy research.
However, this study has several limitations. First, as a nonrandomized retrospective analysis, residual confounding could not be eliminated despite employing PSM. Second, clinical decisions regarding the timing of RRT initiation may have been subject to treatment bias. Third, the study could not determine the underlying causes or etiologies of AKI, potentially leaving confounding factors unaddressed. Fourth, the database lacked information on pre-admission fluid administration in the ICU, which may impact the validity of the findings. Lastly, sensitivity analyses revealed differences in secondary outcomes (hyperkalemia and the incidence of new RRT) compared to the primary analysis. This discrepancy may stem from including special populations, increasing patient heterogeneity and clinical complexity. Future clinical trials are necessary to explore the sources of fluid exposure and further validate these findings.
5. Conclusion
In summary, our study suggests that LR administration improves survival in ICU-admitted patients with AKI based on retrospective data. However, LR did not enhance renal function recovery. These findings hold significant clinical implications for choosing crystalloid infusion solutions for critically ill patients with AKI. Additional multicenter RCTs are required to validate these findings.
Nomenclature
-
- AKI
-
- Acute kidney injury
-
- MIMIC-IV
-
- Medical information mart for intensive care-IV
-
- PSM
-
- Propensity score matching
-
- RRT
-
- Renal replacement therapy
-
- ICU
-
- Intensive care unit
-
- RCT
-
- Randomized controlled trial
-
- KDIGO
-
- Kidney disease: improving global outcomes
-
- SCr
-
- Serum creatinine
-
- CKD5
-
- Chronic kidney disease stage 5
-
- SOFA
-
- Sequential organ failure assessment
-
- SAPS II
-
- Simplified acute physiology score II
-
- MAP
-
- Mean arterial pressure
-
- LOS
-
- Length of stay
-
- RMST
-
- Restricted mean survival time
-
- SMDs
-
- Standardized mean differences
-
- PH
-
- Proportional hazards
-
- A-D
-
- Anderson–Darling
Ethics Statement
This study was conducted in accordance with the guiding principles of the Declaration of Helsinki. MIMIC-IV is a publicly available database of patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, USA. The collection of patient information and creation of the research resource was reviewed by the Institutional Review Board at the BIDMC, who granted a waiver of informed consent and approved the data sharing initiative. All data for this study are from MIMIC-IV. One author, Shengling Huang, carried out the Collaborative Institutional Training Initiative Examination and was granted permission to use the database (record ID: 42251770).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Shengling Huang, Wenxue Liang, and Yingxue Zhong designed the study, collected data, and wrote manuscript. Shangjia Huang, Liangmei Chen, Donge Tang, Yunyi Li, Shuang Cui, Lingjun Shen, and Bing Yan extracted data from MIMIC-IV database and helped to analyze data. Lianghong Yin and Fanna Liu designed and guided the study. All authors read and approved the final manuscript.
Shengling Huang, Wenxue Liang, and Yingxue Zhong contributed equally.
Funding
This study was supported by Science and Technology Projects in Guangzhou (2023A03J1011).
Acknowledgments
We thank Bullet Edits Limited for professional linguistic editing and proofreading of the manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The MIMIC IV database (version 1.0) is publicly available from https://mimic-iv.mit.edu/. The codes used in the manuscript are available from https://github.com/MIT-LCP/mimic-IV.




