Turkish Validity and Reliability Study of the Cancer Care Evaluation Scale
Abstract
Purpose: The aim of the study is to adapt the “Cancer Care Evaluation Scale” to Turkish and to test its validity and reliability.
Methods: The research was conducted methodologically. The data of the study were obtained from patients in a university hospital in eastern Türkiye between January and April 2023. The research was carried out with 350 people who met the inclusion criteria. The data were collected by the researchers using the “Patient Information Form” and the “Cancer Care Evaluation Scale.” Content validity index (CVI), Bartlett’s test of sphericity, Kaiser–Meyer–Olkin (KMO) index, and confirmatory factor analysis (CFA) were used to find out the content and construct validity. Cronbach’s alpha coefficient, split-half reliability analysis, and correlation analysis were used to test reliability.
Results: As a result of the evaluations and analyses, the CVI of the scale was found to be as 0.94. The Cronbach’s alpha value of the subdimensions of the scale ranged from 0.843 to 0.975 and the total Cronbach’s alpha value was found to be 0.948. In the split-half reliability analysis, Spearman–Brown’s coefficient was found to be 0.989. The fit index values were found to be as follows: X2/SD = 2.57, RMSEA = 0.067, GFI = 0.83, CFI = 0.955, and SRMR = 0.062. CFA results showed good fit index values.
Conclusion: As a result of the research, the factor structure of 35 items and 12 subdimensional scales was confirmed by CFA. It was determined that the Turkish version of the Cancer Care Evaluation Scale is a valid and reliable measurement tool to evaluate the care of cancer patients. The scale allows the care offered to cancer patients to be evaluated from the patient’s perspective. For this reason, it is thought that the scale will contribute to the identification of deficiencies in care and the improvement of the quality of care.
Summary
- •
Limitations
- ◦
The limitations of the study are that the study was conducted in a single institution, and some of the methods used to assess the reliability of the scale (e.g., split-half reliability analysis) changed over time or gave different results.
- ◦
The scale is a scale with acceptable reliability and validity for patients with nonterminal cancer.
1. Introduction
Cancer is considered one of the leading health problems of our age. It is a large group of diseases that can develop in almost every organ or tissue of the body as a result of the uncontrolled growth of abnormal cells [1, 2]. Cancer is a universal problem that requires long-term treatment and care and includes multiple symptoms [3]. Cancer cases are increasing rapidly due to factors such as aging societies and unhealthy lifestyles [4]. It is stated that in 2022, there were 9.7 million deaths due to cancer, as well as close to 20 million new cancer cases [5]. It is estimated that one in six global deaths is due to cancer [6, 7]. There are differences between countries in terms of cancer types and incidence [4]. It is noted that cancer is the second leading cause of death in the United States [8] In Türkiye, the incidence of cancer is 225.2 per hundred thousand. In 2018, a total of 211,273 people were newly diagnosed with cancer in Türkiye [7]. It is predicted that an average of 13 million people will die every year from cancer-related causes by 2030 [4].
Cancer patients face many physical, psychological, and spiritual difficulties. For this reason, it is stated that these patients should be considered as a special group [9, 10]. A patient-centered approach to care is necessary to achieve better treatment outcomes in cancer care [11]. In light of technological developments and information, the extent to which the care provided increases the expected results of the patients and reduces the undesirable results is stated as an indicator of quality in healthcare. Fully meeting the individual needs of each patient constitutes the quality of care [12]. Considering the increase in the number of cancer patients, it is very important to evaluate the quality of cancer care to make the necessary improvements in care [13].
Taking patient opinion, which is an important indicator of quality of care, as well as evaluating care from the patients’ perspective, is an important element in improving the quality of care [14, 15]. Understanding how individuals perceive the care and support they receive is important for achieving quality care [16]. In the provision of a quality health service, it is important to take into account the patient’s feelings, thoughts, expectations, and needs as well as knowing the details of the disease [17].
The quality and adequacy of health services can be measured by the satisfaction of the individuals served [18]. Patients’ satisfaction has been associated with factors such as the healthcare environment, the physician’s behavior, and the skill of the healthcare personnel [9]. Determining patients’ satisfaction levels with care has a guiding role in identifying and eliminating deficiencies and improving service quality [18].
Quality in healthcare is assessed from various aspects such as the care provided by the nurse, the behavior of the staff, the medical environment, and the quality of medical care [19]. A care model has been created by Avedis Donabedian that includes all these elements to assess the quality of health services. Donabedian stated that information on the quality of care can be obtained from three components. These are structure, process, and outcomes [20]. The structure component evaluates the physical and organizational characteristics of health services. The process component assesses the services provided to patients, such as diagnosis, treatment, and care. The outcome component assesses the impact of health services on the condition of patients and populations [20, 21].
Today, it is necessary to provide better quality care to cancer patients, who are rapidly increasing and face many problems, and to make necessary improvements by evaluating the service provided. However, when the literature was examined, a scale specific to cancer patients, in which care is comprehensively evaluated from the perspective of patients, was not found in our country. In the Cancer Care Evaluation Scale (CCES) developed by Masukawa et al. [13] based on the Donabedian care model, care is handled with the dimensions of “structure, process, and outcome.” The scale enables the evaluation of all processes including the treatment process of patients with cancer who are not in the terminal stage. Twelve factors such as “relationship with physician, relationship with nurse, physical care by physician, physical care by nurse, psychoexistential care, help with decision-making for patients, coordination and consistency, environment, cost, availability, care for the side effects of cancer treatment by a physician, and care for the side effects of cancer treatment by a nurse” are evaluated in this scale. When the literature was examined, it was determined that Turkish validity and reliability studies of this scale were not conducted. Therefore, in this study, the Turkish validity and reliability study of the CCES was conducted. It is thought that the scale will contribute to the evaluation of the quality of cancer care and the improvement of the quality of health services.
1.1. Research Questions
- •
Is the CCES a valid scale for the Turkish community?
- •
Is the CCES a reliable scale for the Turkish community?
2. Methods
2.1. Aim and Type of Study
The study was designed and conducted methodologically to determine the Turkish validity and reliability of the CCES.
2.2. Sample and Population of the Study
The study was conducted in a university hospital located in eastern Türkiye. Cancer patients admitted to the hospital between January and April 2023 formed the population of the study. It is recommended that the sample size in scale adaptation studies should be at least 5–10 times the number of items in the scale [22]. For this reason, it was aimed to reach at least five times the number of items in the scale (175), and no sample calculation was made. The study was completed with 350 cancer patients who met the inclusion criteria within the aforementioned population. The study inclusion criteria were as follows: an admission to a hospital within the last 2 years, a diagnosis of a solid tumor, and age ≥ 20 years.
The exclusion criteria were no history of diagnosis of a solid tumor or outpatient treatment.
2.3. Data Collection Tools
The data were collected by the researchers using the “Patient Information Form” and “CCES.”
2.3.1. Patient Information Form
The form was created by the researchers. The form includes 9 questions designed to assess the sociodemographic characteristics of the participants (such as gender, marital status, age, employment status, time since cancer diagnosis, primary cancer site, time since last cancer treatment, time since last hospital admission, and Eastern Cooperative Oncology Group [ECOG] performance status [PS]). The ECOG PS Scale is used to determine the functional status of cancer patients. PS-0 is able to maintain all preillness performance without restriction. PS-1 can be limited in physically strenuous activities, but not in ambulatory, and should be able to carry out the work of a light or sedentary nature. PS-2 is able to stand and perform all kinds of personal care but cannot perform any work activities; more than 50% of waking hours are awake, while PS-3 has only limited self-care ability and spends more than 50% of the waking hours in bed or chair but can be treated with caution. PS-4 is unable to maintain any personal care, it is completely dependent on the bed or chair, and PS-5 means death [23].
2.3.2. CCES
The scale was developed by Masukawa et al. [13].
It is stated that the scale has acceptable reliability and validity for patients with cancer who are not in the terminal stage. The scale is a 6-point Likert-type scale (from 1: absolutely disagree to 6: absolutely agree). Scoring for this scale is performed by adding up the total score. The increase in the total score shows an improved in the quality of cancer care. The scale consists of 2 domains, 12 subdimensions, and 35 items. There are 10 subdimensions and 29 items in the domain “Common component in cancer care” and 2 subdimensions and 6 items in the domain “Component in cancer treatment.” The subdimensions in the scale are “relationship with physician (1–3 items),” “relationship with nurse (4–6 items),” “physical care by physician (7–9 items),” “physical care by nurse (10–12 items),” “ psychoexistential care (13–15 items),” “help with decision-making for patients (16–18 items),” “coordination and consistency (19–21 items),” “environment (22–24 items),” “cost (25, 26 items),” “availability (27–29 items),” “care for the side effects of cancer treatment by a physician (30–32 items),” and “care for the side effects of cancer treatment by a nurse (33–35). The Cronbach’s alpha values of the subdimensions of the scale are 0.93, 0.94, 0.95, 0.96, 0.94, 0.88, 0.85, 0.86, 0.85, 0.85, 0.85, 0.94, and 0.96, respectively. Cronbach’s alpha values are 0.97 for the “Common component in cancer care” domain and 0.95 for the “Component in cancer treatment” domain. Cronbach’s alpha value of all domains is 0.98.
2.4. Stages in Adaptation of the Scale to Turkish
A certain standard approach is followed in adapting a scale to a different language and culture and ensuring language validity. These include group translation, back translation, expert opinion, and pilot implementation [24, 25].
2.4.1. Language and Content Validity
The original of the scale was first translated into Turkish by two independent language experts as recommended in the literature [24]. The translations were evaluated by the researchers, and the Turkish form was structured. As a result of the revisions, the form that was created was translated into the original language by language experts. The translated form was checked with the original scale, and it was determined that the form was similar to the original form. The resulting Turkish form was then reviewed by 10 experts with at least a Ph.D. degree in the field (two experts who are also experts in scale development) to check the Turkish language, content, and cultural appropriateness of the scale items. These experts were asked to evaluate each item on the scale by scoring between 1 and 4 after choosing from the expressions “4 = completely appropriate,” “3 = very appropriate,” “2 = appropriate but the items need small changes,” and “1 = not appropriate.” A test of content validity was conducted to determine both the linguistic and cultural equivalence of the form. The content validity index (CVI) was calculated according to the Davis technique [26].
2.4.2. Pilot Application
In scale adaptation studies, it is recommended to apply the pilot application to a sample group of approximately 30 individuals [22, 24]. A pilot study was conducted with 30 individuals. As a result of the pilot study, no items were removed from the scale. The data of the pilot study were not included in the research data.
2.4.3. Main Application
The study data were collected from 350 cancer patients who met the inclusion criteria and were admitted to the hospital between January and April 2023. Data were obtained using a face-to-face interview technique after the patients were provided with the required information. The data were collected using the Patient Information Form and CCES. It took 5–10 min for each participant to complete the data collection tools. When a sufficient number of samples was reached, the data collection process was completed.
2.5. Data Analysis
Statistical analyses were performed using the SPSS 22 package program and AMOS software. Descriptive statistics such as arithmetic mean, standard deviation, percentage, and minimum–maximum values were calculated. CVI, Bartlett’s test of sphericity, Kaiser–Meyer–Olkin (KMO) test, and confirmatory factor analysis (CFA) were used to find the validity. Fit indices, such as chi-square/SD ratio, goodness-of-fit index (GFI), comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR), were evaluated. Cronbach’s alpha reliability coefficient, correlation analysis, and Spearman’s correlation analysis were used to find the internal consistency and reliability. The factor structure of the scale is illustrated by the PATH diagram.
2.6. Ethical Considerations
Approval was obtained from the ethics committee of a university (2020/12 numbered) for the research and from the scale owner for the adaptation of the CCES used in the study to Turkish.
Written official permission was taken from the hospital where the study was conducted in. The purpose of the study was explained to the individuals participating, and verbal consent was obtained from them. The study was conducted in accordance with the principles of the Helsinki Declaration of Human Rights.
3. Results
3.1. Participants’ Characteristics
The participants’ characteristics are shown in Table 1. The participants’ mean age was 54.45 ± 12.64 years. It was found that 50.9% of the participants were female, 88.6% were married, 32.3% were employed, 80.9% had an ECOG PS of ≤ 1, 21.1% had breast cancer, 54% had a duration of disease of ≥ 1 year, 41.4% had 3–6 months since their last cancer treatment, and 34.6% of participants had < 3 months since their last hospital admission.
| n | % | ||
|---|---|---|---|
| Age (mean ± SD) − (min–max) | 54.45 ± 12.64 | 21–98 | |
| Gender | Female | 178 | 50.9 |
| Male | 172 | 49.1 | |
| Marital status | Married | 310 | 88.6 |
| Single | 40 | 11.4 | |
| Employment status | Full-time/part-time | 113 | 32.3 |
| None | 237 | 67.7 | |
| Primary cancer site | Lung | 67 | 19.1 |
| Gastric and esophagus | 41 | 11.7 | |
| Colon and rectum | 46 | 13.1 | |
| Hepatobiliary and pancreatic | 43 | 12.3 | |
| Breast | 74 | 21.1 | |
| Uterus and ovary | 37 | 10.6 | |
| Others | 42 | 12.0 | |
| Time since cancer diagnosis | ≤ 5 months | 65 | 18.6 |
| 6–11 months | 96 | 27.4 | |
| 1–2 years | 91 | 26.0 | |
| 2–5 years | 73 | 20.9 | |
| ≥ 5 years | 25 | 7.1 | |
| Time since last cancer treatment | < 3 months | 125 | 35.7 |
| 3–6 months | 145 | 41.4 | |
| 6–12 months | 80 | 22.9 | |
| Time since last hospital admission | < 3 months | 121 | 34.6 |
| 3–6 months | 82 | 23.4 | |
| 6–12 months | 83 | 23.7 | |
| ≥ 1 year | 64 | 18.3 | |
| ECOG performance status | 0 | 155 | 44.3 |
| 1 | 128 | 36.6 | |
| 2 | 54 | 15.4 | |
| 3 | 8 | 2.3 | |
| 4 | 5 | 1.4 | |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; SD, standard deviation.
3.2. Validity
3.2.1. Language and Content Validity
Expert opinions were taken to evaluate the scales in terms of language and culture and to reveal the content validity of the scales, which were evaluated with the CVI. The item-based CVI (I-CVI) was between 0.80 and 1.00, and the scale-based CVI (S-CVI) was found to be 0.94 in the study.
3.2.2. Construct Validity
KMO analysis and Bartlett’s test of sphericity were used to determine the suitability of the sample size and the suitability of the dataset for factor analysis [22, 27]. KMO value was found as 0.909. KMO value obtained from the study shows that an adequate sample was reached. Bartlett’s test of sphericity set was found to be significant (x2 = 17,296,439: p < 0.001).
3.2.3. CFA
The fit indices used to determine the fit adequacy of the obtained model are given in Table 2.
| Index | Normal value | Allowable value | Measurement | Result |
|---|---|---|---|---|
| χ2/SD(CMIN/DF) | < 2 | < 5 | 2.575 | Allowable compatibility |
| RMSEA | < 0.05 | < 0.08 | 0.067 | Acceptable fit |
| GFI | > 0.95 | > 0.90 | 0.832 | Allowable compatibility |
| CFI | > 0.95 | > 0.90 | 0.955 | Perfect compatibility |
| SRMR | < 0.05 | < 0.08 | 0.062 | Perfect compatibility |
- Abbreviations: CFI, comparative fit index; GFI, goodness-of-fit index; RMSEA, root mean square error of approximation; SD, standard deviation; SRMR, standardized root mean square residual.
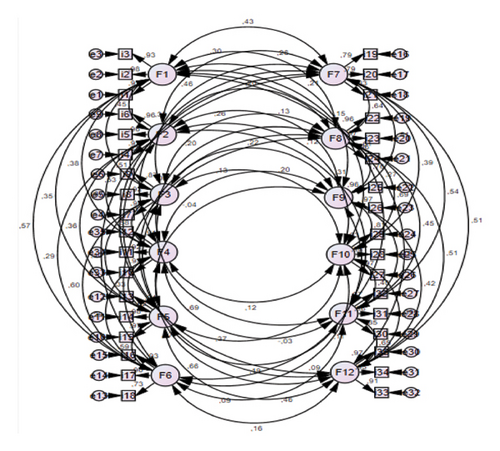
The PATH diagram obtained as a result of the CFA is given in Figure 1.
3.3. Reliability
Cronbach’s alpha coefficients and the results of the correlation analysis conducted to determine the relationship between the scale factors are given in Table 3. Cronbach’s alpha consistency coefficient was measured as 0.948 for the whole scale. The consistency coefficient of the subdimensions of the scale is between 0.843 and 0.975. Intraclass correlation coefficient (ICC) values were found to be between 0.59 and 0.93. Total correlation coefficient values were found to be as 0.84.
| Subdimensions of the scale | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | Number of items | Min–max | X ± SD | Cronbach’s α | ICC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 1.000 | 3 | 3–18 | 15.84 ± 3.04 | 0.97 | 0.88 | |||||||||||
| F2 | 0.447 | 1.000 | 3 | 3–18 | 15.68 ± 3.17 | 0.97 | 0.92 | ||||||||||
| F3 | 0.833 | 0.480 | 1.000 | 3 | 3–18 | 15.70 ± 3.04 | 0.93 | 0.59 | |||||||||
| F4 | 0.369 | 0.835 | 0.547 | 1.000 | 3 | 3–18 | 15.50 ± 3.22 | 0.94 | 0.60 | ||||||||
| F5 | 0.351 | 0.356 | 0.387 | 0.326 | 1.000 | 3 | 3–18 | 14.27 ± 4.17 | 0.97 | 0.92 | |||||||
| F6 | 0.561 | 0.286 | 0.573 | 0.275 | 0.576 | 1.000 | 3 | 3–18 | 14.41 ± 3.90 | 0.89 | 0.74 | ||||||
| F7 | 0.392 | 0.267 | 0.408 | 0.292 | 0.284 | 0.538 | 1.000 | 3 | 3–18 | 14.15 ± 3.79 | 0.84 | 0.64 | |||||
| F8 | 0.250 | 0.194 | 0.240 | 0.189 | −0.004 | 0.162 | 0.575 | 1.000 | 3 | 3–18 | 13.54 ± 5.15 | 0.97 | 0.92 | ||||
| F9 | 0.203 | 0.128 | 0.205 | 0.125 | −0.040 | 0.108 | 0.457 | 0.734 | 1.000 | 2 | 2–12 | 8.39 ± 3.59 | 0.96 | 0.93 | |||
| F10 | 0.146 | 0.114 | 0.194 | 0.122 | −0.037 | 0.088 | 0.349 | 0.676 | 0.778 | 1.000 | 3 | 3–18 | 12.49 ± 5.39 | 0.97 | 0.92 | ||
| F11 | 0.595 | 0.297 | 0.665 | 0.353 | 0.198 | 0.437 | 0.489 | 0.466 | 0.432 | 0.419 | 1.000 | 3 | 3–18 | 14.33 ± 4.06 | 0.94 | 0.85 | |
| F12 | 0.272 | 0.603 | 0.355 | 0.635 | 0.107 | 0.171 | 0.462 | 0.489 | 0.408 | 0.381 | 0.629 | 1.000 | 3 | 3–18 | 14.01 ± 4.16 | 0.96 | 0.88 |
| Total | 35 | 35–210 | 168.30 ± 30.10 | 0.948 | 0.84 | ||||||||||||
- Abbreviations: ICC, intraclass correlation coefficient; SD, standard deviation.
3.4. Split-Half Reliability Analysis of CCES
The results of the equivalent halves analysis, which is one of the reliability indicators of the CCES, are shown in Table 4. The 35-item scale has been split into two equal parts, with the first half containing odd-numbered items and the second half containing even-numbered items. Cronbach’s alpha coefficient for the 17 items in the first half was 0.892; for the 18 items in the second half, Cronbach’s alpha coefficient was calculated as 0.899. In addition, the correlation between the two equivalent halves was found to be as 0.973. The Spearman–Brown coefficient calculated using this correlation was found to be as 0.989.
| Cronbach’s alpha | First half (odd-numbered items) | Value | 0.892 |
|---|---|---|---|
| Number of items | 17 | ||
| Second half (even-numbered items) | Value | 0.899 | |
| Number of items | 18 | ||
| Correlation between equivalent halves | 0.973 | ||
| Spearman–Brown coefficient: r(general)(2r(equivalent halves correlation))/(1 + r(equivalent halves correlation)) | 0.989 | ||
4. Discussion
When the literature was examined, a scale specific to cancer patients, in which care is comprehensively evaluated from the perspective of patients, was not found in our country. Therefore, a Turkish reliability and validity study was conducted for the CCES developed by Masukawa et al. [13]. In scale adaptation studies, the main psycholinguistic features (language adaptation) and psychometric features (reliability and validity) are examined [24]. This section discusses the findings related to the 35-item and 12-subdimension CCES.
4.1. Validity
Validity refers to the appropriateness and adequacy of the use of measurements obtained from a test or measurement tool and the proposed interpretations [28].
4.1.1. Language and Content Validity
In the study, the original scale was translated into Turkish by language experts and then sent to experts in the field. CVI was evaluated in line with expert opinions. Content validity attempts to determine whether a scale contains questions (items) in an appropriate number and quality to represent the construct of interest [29]. CVI value can be computed for each item on a scale (I-CVI) as well as for the overall scale (S-CVI). The I-CVI was computed for each item as the number of experts giving a rating of either 3 or 4 divided by the number of all experts [29]. According to expert opinions, the I-CVI was between 0.80 and 1.00, and the S-CVI was found to be 0.94 in the study. It is recommended that CVI values be between −1 and +1. It is stated that if there are 3 or more experts, an I-CVI value of 0.78 or higher indicates good content validity. Items are considered to be valid in terms of their content if the CVI is > 0.80 [30]. For this reason, the content validity of the scale was found to be statistically significant, and no item was removed from the scale.
4.1.2. Construct Validity
Construct validity is used to determine which characteristics or concepts the scale measures [29]. KMO analysis and Bartlett’s test are used to determine the suitability of the sample size and the suitability of the dataset for factor analysis [22, 27]. KMO value should be above 0.60 and Bartlett’s test of sphericity should be statistically significant (p < 0.05) [31]. A KMO value of 0.90 and above indicates that the sample adequacy is perfect. The KMO value of 0.50 and below indicates that there is an insufficient sample and factor analysis cannot be continued [32]. The KMO value was found to be 0.909 in this study. Bartlett’s test of sphericity was found to be significant (x2 = 17,296,439: p < 0.001). These results show that the data were fit for factor analysis.
4.1.3. CFA
It is stated that CFA can be performed directly instead of exploratory factor analysis in the process of adapting a measurement tool [22]. CFA is performed to determine the accuracy of a predetermined structure [29]. CFA GFIs are given in Table 2. As a result of the analysis, it was determined that the 12-dimensional scale structure was confirmed. It is recommended to use the fit index to determine the fit adequacy of the obtained model [29]. All the goodness-of-fit values of the scale were found to be within acceptable limits [33]. The items were found to be important for the factors in which they are included.
The PATH diagram obtained as a result of the CFA is given in Figure 1, and it was found that the values obtained are appropriate in terms of item factor coherence (Figure 1). It is stated in literature that an acceptable value of factor loads can be > 0.30 [34]. Therefore, no items were deleted because all items had a factor load of > 0.30. As a result of CFA, it was found that the Turkish form of the 35-item CCES with 12 subdimensions was confirmed with no changes in the original scale form. All the results obtained show that the scale has high validity in Turkish culture.
4.2. Reliability
Reliability refers to the consistency or reproducibility of measurements obtained from a test or measurement tool [28]. Cronbach’s alpha (α) analysis was performed for reliability [29, 35]. Cronbach’s alpha value was measured as 0.948 for the whole scale. Cronbach’s alpha coefficients of the subdimensions of the scale are between 0.843 and 0.975 (Table 3). Masukawa et al. [13] found that the total Cronbach’s alpha coefficient of the original scale was 0.98. Cronbach’s alpha coefficients of the subdimensions of the original scale are between 0.85 and 0.96. It is stated that Cronbach’s alpha value is 4 cut-off points. A value of ≤ 0.50 is determined as low reliability, a value between 0.50 and 0.70 is evaluated as moderate reliability, a value between 0.70 and 0.90 is evaluated as high reliability, and a value of ≥ 0.90 is evaluated as perfect reliability [35]. These results show that the reliability of CCES is perfect.
ICC values were found to be between 0.59 and 0.93 in the study (Table 3). Total correlation coefficient values were found to be as 0.84. ICC values of the subdimensions of the original scale were found to be between 0.62 and 0.75. Total correlation coefficient values were found to be as 0.80 [13]. When the correlations were examined, it was determined that there was a positive relationship [29].
4.2.1. Split-Half Reliability Analysis
Split-half reliability coefficients are given in Table 4. As a result of the split-half reliability analysis regarding the final form of the scale consisting of 35 items, it can be said that the Spearman–Brown correlation value (r = 0.989) of the scale has sufficient values, and the Cronbach’s alpha reliability coefficients (0.973) for the two halves are sufficient. Correlation value of ≥ 0.80 and Cronbach’s alpha value of ≥ 0.90 show that the scale is highly reliable [29, 35]. The results show that the scale is a highly reliable scale.
5. Conclusıon and Recommendatıon
The “CCES” has been adapted to Turkish culture. As a result of adapting the original form of the 12-factor and 35-item scale to the Turkish form, a 12-factor and 35-item structure was obtained. The “CCES” is a valid and reliable measurement tool for Turkish culture. The scale has acceptable reliability and validity for patients with nonterminal cancer. It can be said that the scale is a measurement tool that can be easily applied because it has a small number of scale items and short and understandable expressions.
Ethics Statement
Approval was obtained from the ethics committee of a university (2020/12 issue) for the research and from the scale owner for the adaptation of the CCES used in the study to Turkish. The study was conducted in accordance with the principles of the Helsinki Declaration of Human Rights. The purpose of the study was explained to the individuals participating, and verbal consent was obtained from them.
Disclosure
The research was presented as a verbal presentation at the “2nd International Congress on Health Sciences and Multidisciplinary Approaches.”
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sevgi Doğan: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft, writing, and review–editing.
Yasemin Erden: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, validation, visualization, writing–original draft, writing, and review–editing.
Gülçin Avşar: conceptualization, data curation, investigation, methodology, project administration, resources, validation, visualization, writing–original draft, writing, and review–editing.
Funding
No funding was received for this study.
Acknowledgment
The authors would like to thank the participants for taking part in the study.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




