Extracellular Vesicle–Derived microRNAs: A Deep Review of the Latest Literature Investigating Their Role in Drug Resistance, Prognosis, and Microenvironment Interactions in Hematologic Malignancies
Abstract
Extracellular vesicles (EVs) and their molecular content are known as deterministic messengers between tumor-stromal cells involved in cancer development, including hematologic malignancies. Particularly, EVs and exosomes enriched in miRNAs are believed to mediate a wide range of behavioral changes in tumors cells. The objective of this review was to explore the role of miRNAs carried by EVs, including exosomes, in the prognosis, drug resistance, and microenvironmental interactions contributing to the pathogenesis of main hematologic cancers. Our literature review was conducted in PubMed, Web of Knowledge, and Scopus to gather the most recent publications in this area. Exploring the findings of relevant studies highlighted the indispensable contribution of microvesicular and exosomal miRNAs when it comes to evaluating the outcomes of hematologic neoplasms. The strong link between some of exomiRs and survival of patients with leukemias and multiple myeloma deserves to be the subject of future clinical studies. In fact, miRNAs transferred by exosomes derived from various sources, including tumors cells and close and distant cancer-associated stromal and endothelial cells, believed to trigger cellular processes involved in drug resistance and poor clinical outcomes in individuals suffering from hematologic neoplasms. Our review can provide researchers with novel ideas to explore the role of EV- and exosome-derived miRNAs in the clinicopathogenic outcomes of blood cancers.
1. Introduction
Hematologic malignancies refer to neoplasms that affect the hematopoietic system (blood, bone marrow [BM], and related organs) [1]. These malignancies include leukemias, multiple myeloma (MM), lymphomas, myelodysplastic syndromes, and pre-leukemic conditions [2]. These diseases usually recur after treatment [3] and can progress quickly, causing serious complications for the patient. Early diagnosis and appropriate treatment are vital for extending the survival of patients [4].
The role of extracellular vesicles (EVs) is emerging in various clinical aspects pertaining to malignancies, including those originating from hematopoietic cells [5]. EVs vary in size, production, and biogenesis pathways [6]. The nomenclature of EVs is usually based on their biogenesis pathway, where exosomes (30–150 nm in size) and microvesicles (MVs) (100 nm–1 μm in size) release via endocytosis and budding, respectively (Figure 1) [7, 8]. While this terminology (i.e., exosome, MV, ectosome, etc.) is more common, it is sometimes misleading since the biogenesis pathway is difficult to establish. Authors should use operational terms to describe EV subtypes containing physical characteristics, biochemical composition, or cell condition/origin. For instance, for a size-based classification of EVs, the terms “small EVs” and “medium/large EVs” are suggested to describe EVs with < 200 nm and > 200 nm diameter EVs [9, 10].
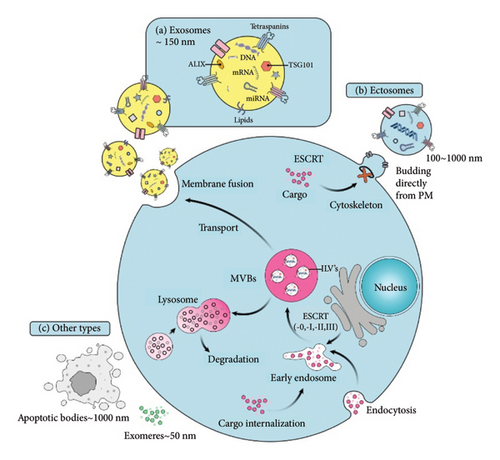
In cancers, EVs and their content appear to play substantial roles in various aspects of carcinogenesis [12]. Exosomes derived from tumor cells interact with the tumor microenvironment (TME) to contribute to tumor development, metastasis, angiogenesis, and drug resistance [13] and mediate intercellular communication by transporting multiple biological molecules such as lipids, proteins, and nucleic acids (DNA, mRNA, miRNA) [12, 14, 15]. The applicability of exosomes and their cargoes as diagnostic and prognostic biomarkers is under intensive investigation [15]. Among important cargoes of EVs with emerging biological roles in hematologic malignancies are miRNAs. These small noncoding RNA molecules play an important role in the post-transcriptional regulation of genes [16] by targeting and degrading mRNAs through binding to their 3′ untranslated regions [17]. Due to their essential role in controlling gene expression, miRNAs are frequently dysregulated in cancers [18]. Research shows that tumor cells can release miRNAs into the bloodstream, and miRNAs in the circulation are protected from nuclease degradation by forming protein complexes or being enclosed in EVs (i.e., microvesicular or exosomal miRNAs) [19, 20]. High stability, easy detection in biological fluids, and resistance to RNAase make microvesicular miRNAs effective biomarkers for diagnostic, prognostic, and therapeutic purposes [20]. The role of miRNAs present in EVs in modulating tumor behavior, intracellular signals, prognostic indicators, and promotion of drug resistance has been noted (Figure S1) [21, 22]. Despite growing evidence on the role of microvesicular and exosomal miRNAs in the pathogenesis of hematologic cancers, our understanding of the potential roles of these molecules in the biology of leukemias and lymphomas is limited. In this review, we aimed to gather evidence that deepens our knowledge about the roles of miRNAs carried by EVs in drug resistance, prognosis, and microenvironmental interactions in hematologic malignancies.
2. Prognostic Role of Microvesicular miRNAs in Hematologic Malignancies
Different expression profiles of microvesicular miRNAs are observed in hematological malignancies, and the importance of this variation in the prognosis of hematologic cancers remains to be fully elucidated. Microvesicular miRNAs can affect cancer progression, treatment response, and relapse rate. Unlike other prognostic biomarkers, miRNAs have significant properties like easy collection from body fluids, secretion by living cells, and variation in different stages of disease, which return them as potent noninvasive biomarkers. The miRNAs content of EVs in combination with profiling of oncomirs can provide unprecedented information about cancer progression mechanisms, and analysis of this information can improve our ability to choose the best therapeutic approaches [23–25]. In leukemias and lymphomas, miRNAs derived from EVs have been shown to correlate with circulating leukocyte count [26], blast percentage [27], and serum prognostic markers such as LDH and β2-MG [28], and can be utilized for their risk stratification [29], bolding their applicability as promising alternative prognostic biomarkers. Table 1 represents current evidence regarding the role of EV-derived miRNAs in the prognosis of hematological malignancies. The following section also addresses the link between EV-derived miRNAs and the most prominent prognostic indicators in hematologic malignancies.
| Study | Type of cancer | Microvesicle source | miRNAs involved | Prognostic implications |
|---|---|---|---|---|
| Yoshizawa et al. [30] | AHSCT recipients | Exosome fractions taken from AHSCT recipients | miR-128, miR-125b | High diagnostic sensitivity and accuracy for late-onset acute GVHD |
| Zhang et al. (2017) [31] | AHSCT recipients | Damaged endothelial cells, plasma, and T lymphocytes | miR-155 | Initiation and progression of acute GVHD and adverse clinical manifestations |
| Rafiee et al. [32] | Lymphoma and MM patients who were candidates for AHSCT | Plasma extracellular vesicles | miR-125b | Prediction of relapse-free survival and risk of recurrence after AHSCT |
| Javanmard et al. [33] | DLBCL patients | Plasma-derived exosomes | miR-146a | Prediction of therapy response and relapse |
| Yan et al. [34] | ALL patients | EVs extracted from peripheral blood | MiR-181b-5p | Association with malignant progression and tumor cell proliferation |
| Rinaldi et al. [35] | Newly diagnosed DLBCL patients | DLBCL cells | miR-22 | Prediction of cancer development, R-CHOP resistance, worse 2-year PFS, high IPI score, overall survival, and poor clinical outcome |
| Ryu et al. [36] | ENKTL patients | Exosomes isolated from ENKTL patients | miR-4454, miR-21-5p, miR-320e | Association with disease progression and relapse, PFS and OS rate, LDH level, stage of cancer, extra-nodal, and BM involvement |
| Moyal et al. [37] | MF patients | Exosomes obtained from CTCL cell lines and patients with MF | miR-155 miR-1246 | Association with increased migration potency (CD81 and PKH26), cutaneous tumor burden, and disease progression |
| Yoon et al. [38] | EBV ± Burkitt’s lymphoma | Plasma exosomes isolated from human Burkitt’s lymphoma cell lines | miR-155 | Induction of neovascularization via the expression of VEGF through the VHL/HIF1a pathway |
| Stamatopoulos et al. [39] | CLL | Cellular and serum RNA obtained from CD19(+) CLL patients | miR-150 | Association with tumor burden (based on Binet stage), disease aggressiveness, IgHV mutational status, ZAP70, LPL, and CD38 expression, cytogenetic abnormalities, TFS (treatment-free survival), and overall survival |
| Garnica et al. [40] | Dogs with MCL | SEVs of liquid biopsy containing miRNAs isolated from canine serum | miR-222, miR-20a, miR-205 miR-93 | Linked with overall survival, response to treatment, and adverse outcome |
| Cao et al. [41] | DLBCL patients | Serum exosomes | miR-451a | Association with disease stage, adverse outcomes, PFS, and OS |
| Drees et al. [42] | cHL patients, HRS cells (KMH2) | Plasma EVs | let-7a-5p miR-24-3p miR-21-5p miR-127-3p | Association with residual disease, response to treatment, and tumor cell growth markers (MYC, SOCS1, BCL6, TNFAIP3, or PRDM1) |
| Forte et al. [43] | MF patients, including those with JAK2V617F mutation | Circulating EVs from MF patients |
|
Correlation with JAK2V617F allele frequency and tumor burden |
| Aguilar-Hernandezet al. [44] | CLL, a review article | EVs extracted miRNAs from CLL patients | miR-21 miR-150 miR-155 miR-223 miR-29c miR148a | Association with prognostic markers such as ZAP70 expression, BCR activation, overall survival, and Rai stage |
| Manier et al. [45] | Newly diagnosed MM patients | Exosomal miRNAs |
|
Prediction of shorter PFS and overall survival, high ISS stage, poor outcomes, and adverse cytogenetic abnormalities |
| Zhang et al. [46] | Newly diagnosed MM patients | Exosomes | let-7d-5p miR-140-3p miR-425-5p | Association with clinical manifestations, kidney damage, tumor burden, and levels of IL-6, creatinine, β-CTX, β2-microglobulin |
| Lee et al. [47] | MM patients | Exosomes released by MM cells in hypoxic condition | miR-1305 | Association with lower overall survival and disease aggressiveness |
| Jiang et al. [48] | Patients with intermediate-risk AML | Exosomal miRNA | miR-125b | Association with elevated risk of relapse and FLT3/MLL mutations |
| Fang et al. [49] | AML patients | Exosomes | miR-10b | Correlation with poor outcome, shorter overall survival, adverse clinical parameters, and prognostically poor cytogenetic abnormalities |
| Egyed et al. [50] | Pediatric ALL | EVs containing miRNAs collected from CSF and BM samples | miR-181a | Association with CNS involvement at diagnosis |
| Saitoh et al. [27] | MDS and AML/MRC patients | EVs carrying miRNAs driven by mesenchymal stromal cells | miR-101 | Linked with progression of disease, high-risk MDS and AML/MRC, negatively related to cell proliferation and blast percentage, downregulation of EZH2, TET2, SUZ12, c-FOS, and c-MYC |
| Hrustincova et al. [51] | MDS and AML/MRC patients | Exosomes |
|
Prediction of response to azacitidine, disease stage, overall survival, and BM blast percentage |
| Zhou et al. [52] | MM patients | Exosomes | miR-21, miR-18a Let-7b | Association with overall survival and prognostic biomarkers such as β2-microglobulin, cystatin C, and serum calcium levels |
| Giudice et al. [53] | MDS | Circulating plasma exosomes |
|
Association with WBC count, LDH, absolute neutrophil count, and response to immunosuppressive therapies |
- Note. CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone; ENKTL = extranodal NK/T-cell lymphoma, HRS = Hodgkin’s Reed-Sternberg cell.
- Abbreviations: AHSCT = autologous hematopoietic stem cell transplantation, ALL = acute lymphoblastic leukemia, AML = acute myeloid leukemia, AML/MRC = acute myeloid leukemia with myelodysplasia-related changes, BM = bone marrow, cHL = classic Hodgkin’s lymphoma, CLL = chronic lymphoblastic leukemia, CNS = central nervous system, DLBCL = diffuse large B cell lymphoma, EBV = Epstein–Bar virus, GVHD = graft-versus-host disease, MCL = mantle cell lymphoma, MDS = myelodysplastic syndrome, MF = mycosis fungoides, MM = multiple myeloma, OS = overall survival, PFS = progression-free survival.
2.1. Disease Progression and Relapse
It has been suggested that miRNAs derived from EVs/exosomes can predict leukemia/lymphoma progression and relapse. In newly diagnosed MM patients, decreased expression of EV-derived let-7d-5p, miR-140-3p, and miR-425-5p correlated with some disease-progression biomarkers, including IL-6, creatinine, and β2-microglobulin [46]. Rafiee et al. also noted that elevated expression of circulatory miR-125b loaded into EVs after AHSCT could be considered as a notable predictor of relapse in MM and lymphoma [32]. The measurement of exosome-derived miRNAs from DLBCL patients suggested that miR-146a could be a notable prognostic marker for predicting relapse [33]. In a study on de novo DLBCL patients, reduced expressions of miR-146a and miR-155, two miRNAs commonly enriched in EVs, were associated with progression markers such as LDH, β2-microglobulin, IPI status, and c-myc expression [54]. Rinaldi et al. asserted that another EV-carried miRNAs, miR-22, derived from DLBCL cells exhibited a role in tumor cell expansion and cancer development [35]. In another report, Yoon et al. noted that exosome-driven from EBV-positive B lymphoma cells containing elevated levels of miR-155 induced the expression of VEGF and promoted angiogenesis [38]. Moreover, the high expression levels of exosomal miRNAs, like miR-4454, miR-21-5p, and miR-320e, were related to disease relapse in NK/T-cell lymphoma [36], and the upregulation of exomiR-155 and exomiR-1246 was found to enhance the migration potency and tumor burden of malignant cells in mycosis fungoides [37]. In addition, the downregulation of EV-miR-101, which has antineoplastic effects and is a suppressor of VEGF-C, in high-risk MDS and AML/MRC patients was negatively correlated with cell proliferation and predicted disease progression [27]. In patients with myelodysplastic syndrome whose exosome-derived miRNA profile were investigated, miR-3200-3p, miR-196b-5p, miR-378i, and miR-1260a were positively correlated to WBC count, and miR-223-3p and miR-19b-3p were observed to positively correlate with LDH level [53]. As noted by Jiang et al., the high expression of serum exosomes carrying miR-125b was found in association with elevated risk of relapse after CR in AML patients [48]. In another study conducted on CNS+ ALL patients, the elevated expression of miR-181a was observed in CNS+ BM samples, suggesting that miR-181a is a good predicting factor for CNS involvement and disease progression in ALL [50]. In a report by Forte et al., investigating the expression of EV-derived miR-34a-5p, − 127-3p, and − 212-3p in JAK2V617F positive and triple negative primary myelofibrosis patients, it was noted that the JAK2V617F allele burden (a dominant marker of disease progression) was positively related to miR-34a-5p and negatively to miR-212-3p. In addition, significantly high expression of miR-361-5p was observed in the TN group versus JAK2V617F mutated [43]. The decreased cellular and elevated serum levels of circulatory miR-150 were related to poor prognosis and aggressiveness of CLL [39]. The dysregulation expression of miR-223, miR-29c, miR-21, miR-148a, miR-150, and miR-155 has been reported as a notable prognostic factor in CLL, particularly miR-155, the most studied miRNA in CLL patients and highly abundant in CLL-derived EVs, has been noted as a valuable prognostic biomarker [44]. Recently, Dubois et al. have reported that the level of EV-derived miR-155 is associated with a poor prognosis in CLL based on Binet stage or IgHV status [55]. In another study on CLL, Paggetti et al. revealed that exosomes can be absorbed by stromal cells, facilitating the transfer of microRNA-150, microRNA-155, and microRNA-146a, which are known to be present in CLL cells. Additionally, leukemic exosomes promote an inflammatory response in stromal cells by enhancing the phosphorylation of AKT and cyclic AMP response element binding protein (CREB) through the NF-κB signaling pathway, leading to a cancer-associated fibroblast phenotype [56]. Conversely, CLL-derived exosomes introduced miR-146a into bone marrow mesenchymal stem cells (BM-MSCs), where miR-146a played a crucial role in transforming BM-MSCs into cancer-associated fibroblasts (CAFs) by targeting USP16 [57]. An exomir, miR-19b, was reported to upregulate Ki67 and suppress p53 in CLL cell lines, suggesting a role for this molecule in the blast phase transformation of CLL [58]. Also, exo-miR-181a enhanced proliferative and survival markers, including Ki-67 and BCL2, and abrogated the expression of pro-apoptotic BAD and BAX in B-ALL cell lines, promoting the proliferation of leukemic cells [59]. The profiling of exo- and EV-derived miRNAs can be a viable option for predicting disease development and recurrence in hematologic neoplasms [60, 61]. Overall, the mechanisms by which EV-derived miRNAs participate in the development, progression, and relapse of various types of hematologic malignancies and the clinical implications of this phenomenon are yet to be elucidated in future efforts.
2.2. Overall Survival
Survival of patients with hematologic malignancies is expected to become prolonged with targeted novel therapies; however, predictors of overall survival can have a significant impact on the selection of the most suitable treatment. The predictive function of EV-derived miRNAs for survival of patients with hematologic neoplasms has been studied in various studies, suggesting these molecules as promising non-invasive biomarkers. In a study by Zhou et al., the downregulation of exosomal miR-21, miR-18a, and Let-7b was reported to predict shorter overall survival in MM patients, demonstrating an inverse correlation with β2-microglobulin, cystatin C, and serum calcium levels [52]. Newly diagnosed MM individuals with downregulated exosomal let-7b and miR-18a endured shorter PFS and OS [45]. In another report, high levels of exosomal miR-1305 were predictors of lower overall survival in MM subjects. Mechanistically, the transduction of miR-1305 into macrophages was shown to induce their M2 phenotype polarization, suggesting a role for immunosuppression in miR-1305-mediated poor outcomes [47]. In individuals with DLBCL, EV-driven miR-155 and miR-146a were reported to be correlated with the PFS rate [54], and in another report, the outcome of DLBCL patients was poorer in those with lower expression of exosomal miR-451a [41]. Moreover, the upregulation of some exomiRNAs, such as miR-4454, miR-21-5p, and miR-320e, predicted poor survival in NK/T-cell lymphoma [36]. In HIV-1-infected patients diagnosed with cHL, the upregulation of miR-20a and miR-21, as EV-derived miRNAs, foresaw adverse outcomes in patients [62]. Moreover, elevated serum levels of EV-miR-10b in AML/CN-AML patients were correlated with poor outcomes and shorter overall survival [49]. Investigating the prognostic role of EV-derived miRNAs, researchers reported that the lower expression of hsa-miR-181b, hsa-miR-143 [29], and exomiRNA-532 [63] and elevated levels of hsa-miR-188 and hsa-miR-501 [29], as well as an exomir, miR-21 [64], predicted an unfavorable prognosis and short survival in AML patients. The results of these studies consistently suggest that the expression profiling of EV-derived miRNAs can be helpful in predicting the survival and outcome of patients with hematologic malignancies; however, a clearer outlook in this field requires more evidence-based clinical data.
2.3. Microvesicular miRNAs and Treatment Response/Outcomes
Nowadays, prediction of response to treatment in cancer is considered a critical issue for picking the most suitable and beneficial therapeutic protocols. The quantity of plasma exosomes has been noted to be associated with the chemotherapy success rate [40]. In canine multicentric lymphoma, the overexpression of exosomal miRNAs (miR-205, miR-222, and miR-20a) was prominent in the complete treatment response group, suggesting a good prognostic index [40], whereas the upregulation of another miRNA derived from exosomes (miR-93) was associated with progressive disease [40]. Drees et al. observed that let-7a-5p, miR-24-3p, miR-21-5p, and miR-127-3p were increased in pretreatment/partial responders versus complete metabolic responders, suggesting that the quantification of these miRNAs, especially let-7a-5p, in combination with serum thymus and activation-regulated chemokine (TARC, a validated biomarker in cHL) could be considered as an acceptable prediction tool for fluorodeoxyglucose (FDG)–positron emission tomography (PET) status in serial therapy monitoring of cHL patients [42]. The measurement of exosome-mediated miRNAs suggested that miR-146a [33], miR-155/miR-146a [54], and miR-22 [35] could be notable prognostic markers for predicting treatment response in DLBCL patients. Moreover, the upregulation of exosome-derived miR-4454, miR-21-5p, and miR-320e was noted as a predictor of treatment failure in patients diagnosed with NK/T-cell lymphoma [36]. In mycosis fungoides, elevated levels of exosomal miRNAs, miR-155, and miR-1246 were associated with the chemoresistance of malignant cells [37]. In MDS patients, EV-derived miRNAs, including miR-126-3p, miR-151a-3p, miR-199a-3p, miR-125a-5p, and miR-423-5p, were closely associated with response to azacitidine [51]. Likewise, EV-derived miRNAs have been noted to have a role in the outcomes of allogenic hematopoietic stem cell transplantation (AHSCT), a definitive and bottom-line curative therapeutic option in many hematologic malignancies. The expression of microvesicular miRNAs has been associated with the initiation and progression of graft-versus-host disease (GVHD) [65], with miR-128 being proposed as a strong predictor of the initial phases of late-onset acute GVHD [30]. Zhang et al. reported that miR-155-enriched microparticles released by damaged endothelial cells following AHSCT could play a role in GVHD development probably by targeting T lymphocytes and altering their functional signaling pathways [31]. In this way, evaluating miRNAs level as a prognostic indicator for monitoring response to treatment and outcomes of AHSCT in hematologic neoplasms can help better manage patients and improve their outcomes. In the following section, we explored possible mechanisms through which microvesicular miRNAs can modulate the drug resistance/sensitivity of hematologic tumoral cells.
2.4. The Potential Therapeutic Application of EV-Derived miRNAs
The inhibition of EV-derived oncomirs has been applied to combat various cancer cells. For instance, Zhang et al. proposed that inhibiting EV-derived miR-125b-5p and concomitant overexpression of TNFAIP3 can enhance rituximab sensitivity in DLBCL patients [66]. In another study, Khalife et al. represented that the EVs-enrichment miR-16 could impair EV-induced M2-macrophage polarization in MM cells and increase bortezomib (BTZ) sensitivity by directly targeting IKKα/β complex [67]. Additionally, Zhang et al. reported that the BM-MSC-derived exosomes containing miR-222-3p can mediate cell apoptosis and inhibit cell proliferation by suppressing IRF2/INPP4B in THP1 cells [68]. In a study by Hu et al., the transfection of miR-34a mimic to AML stem cells suppressed the proliferation of leukemic cells through JAK1/STAT2/p53 pathway in mice model [69]. In a report by Reis et al., an evaluation of the modulatory effect of BM-derived MSC EVs on dendritic cells revealed that highly expressed EV-miR-21-5p could downregulate the CCR7 gene expression, impair DC migration capability toward CCL21, reduce antigen uptake by immature DCs, and decrease proinflammatory cytokine production. These effects further promote the treatment of GVHD and immune dysregulation [70].
Moreover, Chen et al. observed that RBC-derived EVs loaded with antisense oligonucleotide targeting miR-125b inhibited the proliferation of MOLM13 cells, reduced tumor burden in MOLM13-xenografted mice, and suppressed the progression of leukemia in patient-derived AML xenografts [71]. Taverna et al. revealed that curcumin induces the release of exo-miR-21 and consequently upregulated the expression of PTEN in CML cells [72]. On the other hand, Hu et al. demonstrated that inhibition of miR-21 through antisense oligonucleotide could decrease cell migration and proliferation rate, promote cell apoptosis, and induce the expression of tumor suppressor PDCD4 in K562 cells [73]. MicroRNA-21 mediates hematopoietic suppression through SMAD7 inhibition and TGF-β overexpression, making it a potential therapeutic target in MDS [74]. Based on Yuan et al., the knockdown of highly expressed miR-34a-5p in T-ALL cell–derived EV promoted osteogenic differentiation and bone formation of BMSCs by activation of WNT1/β-catenin signaling pathway [75]. Also, Fujii et al. reported that highly expressed mir-125a EVs, derived from BM-MSC, could suppress functional T cell differentiation and induce murine immunomodulation during GVHD [76]. Therefore, EV-derived miRNAs can serve as potential therapeutic targets in hematologic malignancies.
3. Exosomal and Microvesicular miRNAs and Drug Resistance in Hematologic Cancers
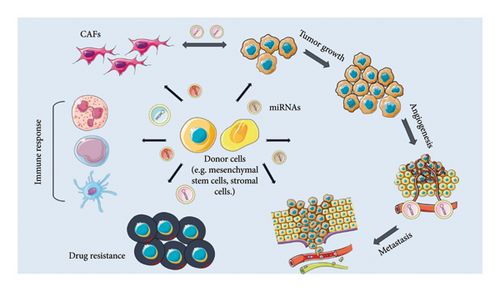
Most hematological cancers recur because tumor cells quickly develop drug resistance, which thereafter significantly promotes tumor proliferation and metastasis. In a number of hematological tumors, exosomes can produce a permissive microenvironment in the BM that promotes angiogenesis and suppresses the immune system, further enhancing the resistance of cancer cells to treatment. In part, drug sensitivity of tumor cells can be modulated via the transport of exosomal and EV cargos, including miRNAs [77, 78], by affecting various subcellular processes such as signaling cascades (e.g., MAPK, PI3K-Akt, Ras) and apoptosis [79, 80] (Figure 2). This section explores the role of exosome- and EV-derived miRNAs and their role in drug resistance in hematological malignancies.
3.1. EV-Derived miRNAs and Chemoresistance in MM
The role of EV-derived miRNAs in drug resistance in MM has received growing attention in recent years. MM is one of the most aggressive hematological malignancies in which exosomal miRNAs have a pivotal role in progression, recurrence, and medication resistance [81–83]. In a recent study, EVs isolated from HEK293T were loaded with miR-1252-5p and then electroporated into MM cell lines (U266 and RPMI-8226), leading to the marked overexpression of miR-1252-5p and a reduction in heparanase (HPSE) expression in these cells, which further correlated with decreased cell survival and greater responsiveness to BTZ [81]. Zhang et al. examined the role of exosomal miR-140–5p and miR-28–3p derived from BM stromal cells in modulating BTZ resistance in MM cell lines and observed that these exosomal miRNAs inhibited SPRED1, a protein that functions as a negative modulator of the MAPK signaling pathway [22, 84]. Moreover, their study has suggested that inhibition of these miRNAs, either by their specific antagonists or inhibitors of hypoxia signaling, mitigated BTZ resistance, indicating their potential as novel strategies for MM therapy [22]. In a study by Gao et al., it was shown that the chemoresistance of MM cells can be conferred by MSC-derived exosomal miR-155 through inducing stemness [85]. In another study, Zhang et al. stated that the lower expressions of exomirs, miR-17-5p, miR-20a-5p, miR-15a-5p, and miR-16-5p could foretell BTZ resistance in MM patients [86]. Hypoxia induction of the release of exosomes containing miR-182 from BM-MSCs was reported to boost carfilzomib resistance in MM cells via targeting SOX6 [21], a member of the SOX family that has been recognized as a tumor suppressor in several malignancies [87].
3.2. Exosomal miRNAs and Drug Resistance in CML
Although the advent of successive generations of tyrosine kinase inhibitors (TKIs) has revolutionized CML treatment, patients still are exposed to resistance to these medications and disease progression. The ability of miRNA-loaded exosomes and EVs derived from imatinib-resistant CML cells to internalize into drug-sensitive CML cells and transmit drug resistance features has been shown in an investigation by Min et al. [88]. Using microarray and qRT-PCR, this study revealed that the level of miR-365 in exosomes from drug-resistant CML cells was noticeably higher than that from sensitive cells. After the transfection of pre-miR-365, imatinib-sensitive CML cells showed reduced apoptosis and chemosensitivity, suggesting that exosomal miR-365 could confer chemoresistance to drug-sensitive CML cells at least partly by suppressing pro-apoptosis signals [88]. In another investigation conducted by Zhong et al. [89], Hsa_circ_0058493 (CircRNA) was shown to be highly overexpressed in the PBMCs of CML individuals and imatinib-resistant leukemic cell exosomes. On the other hand, high levels of circ_0058493 were linked to imatinib unsatisfactory clinical effectiveness, and suppression of circ_0058493 dramatically slowed the growth of imatinib-resistant CML cells, which was in parallel with the overexpression of exosomal miR-548b-3p. According to bioinformatic analyses, circ_0058493 may use miR-548b-3p as a “sponge” to carry out its regulatory role [89]. According to a study by Jiang et al. [90], who evaluated the expression levels of miR-629-5p in imatinib-sensitive and resistant CML cell lines, this miRNA seemed to be enriched in EVs released from K562-resistant cells. Additionally, the SENP2/PI3K/AKT/mTOR pathway seemed to be activated by EVs secreted by imatinib-resistant CML cells. Another study by Karabay et al. [91] found that drug-resistant CML cells expressed higher levels of exosomal miR-125b-5p and miR-99a-5p and lower levels of exosomal miR-210-3p and miR-193b-3p in comparison to drug-sensitive cells.
3.3. Acute Leukemias’ Drug Resistance and Exosomal miRNAs
Acute leukemias are among the deadliest hematologic malignancies, and this grave prognosis mainly comes from drug resistance. The role of EV-derived miRNAs has received much attention as an important mechanism conferring chemoresistance to AML cells [92]. Lei et al., in a study on KG1a AML cell line, found that exosomal miR-4755-5p was involved in 5-aza-2-deoxycytidine (DAC) resistance partly via modulating cyclin-dependent kinase inhibitor 2B (CDKN2B) expression in the target cells [93]. Also, miR-10a internalization by AML cells after being co-cultured with exosomes derived from BMSCs led to the higher resistance of tumor cells to cytarabine mediated by the activation of the Wnt/β-catenin signaling pathway and inhibiting the expression of the Regulation of Nuclear Pre-MRNA Domain Containing 1 (RPRD1A) gene [94]. RPRD1A is a regulator protein involved in cellular development and transcription processes that initiates the Wnt/β-catenin signaling pathway [95]. EVs carry miRNAs to compartmental BM stromal cells and govern their communication with AML cells and other hematopoietic components [96]. When a drug-sensitive promyelocytic leukemia cell line, HL60, was co-cultured with EVs secreted from chemo-resistant HL60 cells overexpressing multidrug resistance protein 1 (MRP-1), the results showed that EVs enriched in miR-19b and miR-20a could transfer the resistance phenotype to sensitive cells and boost the expression of MRP-1 on their surface [97]. The substantial role of miRNAs has been emerged in drug resistance in ALL as well. According to research published by Schotte et al., children with ALL who carried TEL-AML1 fusion had increased levels of miR-125b, miR-99a, and miR-100 (14–25 fold), which was associated with resistance to vincristine and daunorubicin. Also, L-asparaginase resistance was linked to the suppressed expression of miR-454 [98]. In a study, Zamani et al. attempted to determine which miRNAs could be able to control the expression of ABCA3 in pediatric ALL. In comparison to healthy controls, they discovered that pediatric ALL patients had lower expression levels of the miR-324-3p and miR-508-5p miRNAs. Additionally, they discovered that, following a year of treatment with chemotherapy, patients with positive and negative minimal residual disease had substantially high expression levels of miR-324-3p and miR-508-5p, whereas relapsed ALL patients only had underexpressed miR-508-5p. Furthermore, a negative association across these two miRNAs’ expressions and ABCA3 was found, confirming their regulatory role in drug resistance via their interaction with ABCA3 [99]. In another study, Colangelo et al. found that EVs secreted by T-ALL cells are enriched in members of the miR-17-92a cluster, counteracting γ-secretase inhibitor-dependent blocking of ALL proliferation in vitro, underlining the importance of EV-mediated chemoresistance in ALL [100]. Yet in another study by Saffari et al., an exosomal miRNA, miR-326, could predict drug resistance in children with B-ALL [101]. Potentially, these miRNAs can transfer the resistance phenotype through EVs and exosomes, a notion that needs to be evaluated.
3.4. Exosomal miRNAs and Drug Resistance in Non-Hodgkin and Hodgkin Lymphomas
Non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) lymphomas are types of lymphoproliferative cancers characterized by the development of clonal lymphoid tumors originating from B cells, T cells, and NK cells. The substantial role of EV-derived miRNAs has been suggested in the drug resistance of NHLs and HLs. According to Eijndhoven et al., primary and relapsed cHL patients had higher levels of EV-associated miR21-5p, miR127-3p, let7a-5p, miR24-3p, and miR155-5p than healthy controls. Following therapy, miRNA levels dropped, and it was shown that patients who experienced relapse had elevated levels of these miRNAs [102]. In another study, Feng et al. measured the expression of exosomal miR-99a-5p and miR-125b-5p in 116 DLBCL patients and observed that individuals resistant to chemotherapy had much greater expression levels of these miRNAs than those who were responsive to chemotherapy [103]. In another investigation, Zhang et al. reported that DLBCL cells internalizing EV-derived miR-125b-5p showed enhanced resistance to rituximab [66]. MiR-155-5p transported by exosomes derived from ibrutinib-resistant B-cell lymphoma cell line (OCI-Ly1) has been suggested to play a role in ibrutinib resistance in cancerous cells [104]. Likewise, exosomal miR-155-5p was suggested to be involved in R-CHOP resistance in DLBCL patients, evidenced by the elevated levels of this miRNA in patients with refractory/relapsed (R/R) compared to responsive patients [105]. These findings highlight the significant role of miRNA-155-5p in the progression and drug resistance in B cell lymphomas; however, exact mechanisms need to be clarified in future studies. According to Parka et al., exosomes containing miR-155-5p released from ibrutinib-resistant OCI-Ly1 cells could suppress the expression of KDM5B (a lysine demethylase) and DEPTOR (an mTOR interacting protein), ensued by the activation of the PI3K and NF-κB pathways, contributing to ibrutinib resistance in parental cells [104]. Similarly, DLBCL showing rituximab resistance after exposure to EVs carrying miR-125b-5p were reported to have reduced sensitivity to rituximab by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3) [66], an inhibitor of the NF-κB signaling pathway. EV-derived miRNA-mediated drug resistance in NHLs can also be promoted by remodeling the TME [106]. Kunou et al. found that exosomal miR-47175p released by CAFs could contribute to the development of tolerance to anti-pyrimidine medications (gemcitabine and cytarabine) via suppressing equilibrative nucleoside transporter 2 (ENT2) expression in lymphoma microenvironment [107]. In fact, EVs secreted by cells in the TME of hematologic tumors seem to play more prominent roles than thought before, a more detailed discussion about which has been provided in the following sections.
4. Exosomal/Microvesicular miRNAs and Microenvironment Remodeling
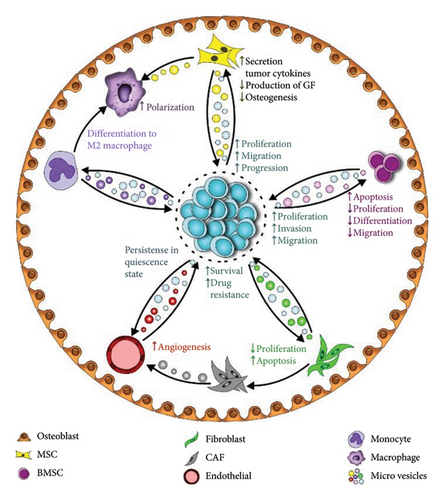
TME is a dynamic place where tumor and surrounding cells receive/send signals to either favor or suppress tumor expansion. Bidirectional communication with the microenvironment is essential for the homing and survival of cancer cells with implications for disease biology and behavior. These interactions are of utmost importance in hematologic neoplasms, where the BM, blood, and secondary lymphoid structures provide a dynamic environment for such interactions (Figure 3). EVs seem to be effective tools for transferring and receiving intercellular messages within the TME, facilitating microenvironment remodeling as an integral part of leukemogenesis. In fact, miRNAs enclosed within EVs are important effectors responsible for conveying messages between TME components. There is a great heterogeneity in the cellular origin and recipients of these EVs in the TME depending on the type and stage of cancer, as well as the specific features of cellular and noncellular components of the environment, leading to a variety of functional and structural changes in the microenvironment (Table 2).
| Study | miRNAs | Source | Target | Functional consequences on target cells |
|---|---|---|---|---|
| Zhang et al. [108] | miR-126 | Endothelial cells | Leukemic stem cells | Persistence of leukemic stem cells in a quiescence state |
| Long et al. [21] | miR-182 | BMSCs | MM cells | Augmented proliferation and invasion of tumor cells |
| Saltarella et al. [109] | miR-214-3p miR-5100 | Fibroblasts | MM cells | Enhanced survival and bortezomib resistance of tumor cells |
| Kunou et al. [107] | miR-4717-5p | Cancer-associated fibroblasts | Lymphoma cells | Longer survival and augmented drug resistance of cancer cells |
| Ji et al. [110] | miR-26a-5p | BMSCs | AML cells | Promotion of the migration, invasion, and proliferation of leukemic cells |
| Khalife et al. [67] | miR-16 | MM cells | Monocytes | Differentiation of monocytes to M2 type immunosuppressive macrophages |
| Tian et al. [111] | miR-let-7c | MSCs | Macrophages and vascular endothelial cells | Favoring M2 macrophage polarization and angiogenesis |
| Gao et al. [112] | miR-320 | Leukemic cells | BMMSC | Suppression of osteogenesis |
| Qi et al. [113] | miR-222 | THP-1 cells (M1-type macrophages) exposed to hypoxia/serum deprivation | BMSCs | Inducing apoptosis in BMSCs, reduction of their viability and migration |
| Zhang et al. [22] | miR-140–5p miR-28–3p | BMSCs exposed to hypoxia | MM cells | Attenuation of bortezomib sensitivity in tumor cells |
| De Veirman et al. [114] | miR-146a | MM cells | MSCs | Promoting the secretion of proinflammatory cytokines/chemokines, facilitating tumor cells’ migration and growth |
| Khalife et al. [67] | miR-16 | MM cells | Monocytes | Facilitating the differentiation of M2-type macrophages |
| Frassanito et al. [115] | miR-27b-3p miR-214-3p | MM cells | Fibroblasts | Triggering the activation, proliferation, and resistance to apoptosis of fibroblasts, facilitating transition from MGUS to overt myeloma |
| Miaomiao et al. [116] | miR-21 | Cancer-associated fibroblasts from MM |
|
Promotion of angiogenesis, transformation of normal fibroblasts to cancer-associated fibroblasts |
| Horiguchi et al. [117] | miR-7977 | AML cells | MSCs | Reducing the production of hematopoietic growth factors by MSCs |
| Tzoran et al. [118] |
|
AML cells | Cord blood stem cells | Influencing the differentiation of stem cells, especially in the presence of MSCs |
| Muntion et al. [119] |
|
MSCs from patients with MDS | CD34+ cells | Increasing the viability and clonogenicity of stem cells. |
| Hornick et al. [120] | miR-150 miR-155 | AML cells | Hematopoietic stem and progenitor cells | Suppressing the proliferation and differentiation of stem and progenitor cells and impairment of their clonogenicity |
| Jiang et al. [121] | miR-711 | K562 cells (a CML cell line) | BMSCs | Reducing the adhesive capabilities of BMSCs, evidenced by the surface downregulation of CD44 |
| El-Saghir et al. [122] |
|
Adult T-cell leukemia/lymphoma cells | BMSCs | Enhancing proliferation, induction of genes involved in migration and angiogenesis, activation of the NF-kB pathway, inducing morphological changes |
| Abdelhamed et al. [123] | miR-1246 | AML cells | Long-term hematopoietic stem cells (LT-HSC) from the BM of murine xenografts | Impairment of protein synthesis in stem cells, diverting them toward quiescence |
| Wu et al. [94] | miR-10a | BMSCs | AML cells | Reduction of the sensitivity of leukemic cells to cytarabine |
4.1. Tumor Cells as Recipients of EV-Derived miRNAs and Functional Consequences
Hematologic tumor cells can be the recipients of miRNA-carrying exosomes and EVs released from a variety of cells in TME. Functional translations of this event can be diverse depending on the tumor/clinical stage and the source of miRNAs, ranging from cancer persistence to regression. In a study, leukemic stems cells (LSCs) in CML receiving miR-126 through exosomes released by endothelial cells were noted to be able to remain in quiescence, enabling the tumor to regenerate after a while [108]. In AML, LSCs transfected with a mimic of miR-34a were observed to undergo apoptosis, a linked process to the stabilization of histone deacetylase 2, activation of JAK-1, and p53 signaling [69]. Additionally, Chen et al. reported that LSCs treated with miR-1246-loaded EVs derived from AML cells showed the activation of the STAT3 pathway, while the transferring of the inhibitor of miR-1246 via EVs suppressed this signaling route, leading to the decreased viability, colony formation, and differentiation capacities of LSCs [124]. In another experiment, the same team showed that exosomal miR-1246 could suppress cell cycle progression by targeting checkpoint regulators such as Cyclin D1 and pRb [79]. Another study showed that LSCs in AML can be prompted to undergo p53-dependent or independent senescence, a process that could be achieved by enforced expression of exosomal miR-34c-5p [125]. Not only LSCs but also tumor cells themselves can be modified in terms of cellular and behavioral features through miRNAs transferred by EVs and exosomes. An EV-derived miRNA, miR-221 was noted to boost AML cells’ proliferative capacity via favoring cell cycle progression and suppressing apoptotic signals, partly by targeting Gbp2 [126]. In another report, Ji et al. reported that EVs carrying miR-26a-5p isolated from AML patients could enhance the proliferative, migration, and invasive capacities of leukemic cells partly by activating the Wnt/β-catenin signaling pathway [110]. In myelodysplastic syndrome, EVs originating from BM-MSCs and containing miR-101 were suggested as a negative regulator of cell proliferation through modulating cellular communication and gene expression [27].
In MM, MSCs seem to be a major source of miRNA-enriched EVs and exosomes that can modify the behavior of cancerous plasma cells. MM cells showed augmented capabilities to proliferate and invade after receiving exosomes carrying miR-182 secreted by BMSCs, mediated via the suppression of SOX6 [21]. Another member of the SOX family, SOX4, was reported to be targeted by miR-335, and EVs containing this miRNA were seen to induce apoptosis in B lymphocytes [127]. Exosomes carrying miR-483-5p released by MSCs also activated the TIMP2 pathway, contributing to the progression of MM [128]. A number of other studies have also indicated that EVs or exosomes released by BMSCs, including those enriched in miR-10a/miR-16 [129], miR-10a [130], and miR-155 [85], were able to facilitate MM progression by various mechanisms such as augmenting cancer cells’ proliferation, suppressing apoptotic mediators, maintaining stemness, and promoting drug resistance. Also, fibroblast-MM-BM-MSC-derived exosomes were noted to upregulate the expression of miR-214-3p and miR-5100 in MM cells, boosting the survival and BTZ resistance of these MM cells through downregulating their target genes, PTEN and DUSP16, respectively, and modulating the MAPK, PI3K/AKT/mTOR, and p53 signaling pathways [109]. The transfection of miR-19b into CLL cell lines upregulated the proliferative marker, Ki67, while downregulated p53 in these cells, suggesting a mechanism contributing to the progression of disease to Richter transformation [58]. Haque and Vaiselbuh isolated exosomes from the sera of children with ALL and the conditioned media of leukemic cell lines and observed that these exosomes were enriched in miR-181a and enabled leukemic B cell lines to proliferate (evidenced by the overexpression of PCNA and Ki-67) and survive (evidenced by the downregulation of Bad and Bax and upregulation of Bcl-2) [59]. Lymphoma cells internalizing exosomes enriched in miR-4717-5p released from CAFs were reported to have longer survival and augmented drug resistance [107]. In DLBL, miR-107 was identified as a potential tumor suppressor marker, abrogating the proliferation and invasion of cancer cells in vitro by targeting genes involved in routes such as PI3K-Akt and MAPK pathways [28]. Overall, tumor cells are a major destination for exosomal and microvesicular miRNAs in hematologic TME, mainly favoring their growth and expansion.
4.2. Impacts on Stromal Cells
A substantial portion of exosomal/microvesicular miRNAs in the TME of hematologic malignancies is released by stromal cells; however, these cells themselves can also receive function-modifying signals from exosomal miRNAs. Among key stromal cells that function as the recipients of exosomal/microvesicular miRNAs in hematologic TME are BMMSCs (mesenchymal/stromal) [75, 112–114], monocyte/macrophages [67, 131, 132], vascular endothelial cells [111, 133], epithelial cells [134], osteoblasts [135], and fibroblasts [115, 116]. The functional and structural TME changes following the internalization of exosomal and microvesicular miRNAs by these cells range from the polarization of M2 macrophages [67] to the generation of new vascular beds for tumor expansion [111], the proliferation of tumor cells [135], apoptosis induction in stromal cells [113], suppression of osteoporosis [112], and production of tumor growth factors [114]. The exposition of BMSCs with EVs derived from T-ALL cells containing miR-34a-5p inhibited the ability of MSCs to differentiate to osteoclasts through suppressing the expression of WNT1 [75]. In two separate studies, it was reported that the treatment of monocytes with miR-16-enriched EVs isolated from MM patients [67] and EVs containing miR-146a-5p from anaplastic large cell lymphoma (ALCL) cells [132] directed these immune cells toward differentiation to macrophages with the immunosuppressive M2 phenotype, and further investigation suggested a role for miR-16 in this phenomenon by targeting the NF-κB pathway [67]. A specific population of monocytes expressing low levels of HLA-DR can transform to myeloid-derived suppressor cells (MDSCs) that facilitate tumor progression by subsiding T cell responses. In CLL, cancerous cells were shown to potentiate MDSCs at least partly via exosomal transferring of miRNAs [131]. Interestingly, CLL exosome-mediated transfer of noncoding RNAs to monocytes contributes to cancer-related inflammation and concurrent immune escape via PD-L1 expression [136]. Concerning the polarization of macrophages to M2 state by “leukemic” EVs, it has been observed by Dubois et al. that CLL-EV obtained from BCR activated CLL cells induced significant modifications in monocytes (shape change, microRNA and gene expression, secretome) suggesting nurse-like cell (NLC) differentiation, the tumor-associated macrophages of CLL [55]. Exosome- or EV-derived miRNA-triggered signals received by endothelial cells generally act toward the expansion of the tumor vascular network. In MM, exosomal miRNAs, miR-let-7c [111], and miR-135b [133] were reported to boost the function of vascular endothelial cells and facilitate angiogenesis in MM BM [111]. Fibroblasts internalizing exosome-derived WWC2 protein were able to endogenously overexpress miR-27b-3p and miR-214-3p, conferring them resistance to apoptosis due to the overexpression of MCL1 [115]. The fibroblasts transforming to a tumor-supportive phenotype (known as CAFs) have a role in promoting angiogenesis. A study provided evidence that exosomes derived from CAFs, enriched in miR-21, could trigger the expansion of MM endothelial cells [116]. An important phenomenon involved in cancer progression is epithelial–mesenchymal transition (EMT), and miR-21-enriched exosomes released by MM cells were noted to promote EMT in renal proximal tubular epithelial cells by targeting the TGF-β/SMAD7 route evidenced by E-cadherin downregulation and vimentin upregulation [134].
4.3. Role of External Stimuli in the Release of Exosomal/Microvesicular miRNAs
The release of EVs by either tumor or stromal cells is believed to be accelerated by environmental stimuli. In TME, hypoxia and starvation are common due to the high proliferation rate of cancer cells. Exosomes enriched in miR-222 released from M1 macrophages exposed to hypoxia and serum deprivation were reported to induce apoptosis in BMSCs through targeting the Bcl-2 pathway [113]. Also, MM cells were reported to prominently enforce the release of exosomes enriched in miR-135b under chronic hypoxic stress, expediting angiogenesis and tumor growth [133]. Hypoxia-induced EVs released from BMSCs containing miR-140–5p and miR-28–3p could increase the BTZ resistance of MM cells through promoting the MAPK pathway [22]. A master transcription factor under hypoxia condition, hypoxia-inducible factor-1α (HIF-1α), has been reported to be involved in regulating the expression of miRNAs and mediators engaged in the release of EVs [21]. Also, a proinflammatory state in TME favors tumor expansion and angiogenesis through favoring the release of miRNA-enriched exosomes and EVs. MSCs receiving MM cell–derived exosomes containing miR-146a were able to facilitate MM progression by releasing a set of proinflammatory cytokines (CXCL1, IL6, IL-8, IP-10, MCP-1, and CCL-5) through activating the Notch signaling pathway [114]. The identification of stimuli involved in the release of functionally and clinically important miRNA-enriched exosomes and EVs can be of special importance in terms of therapeutic and monitoring of hematologic malignancies.
4.4. Suppression of Normal Hematopoiesis
A key feature of TME in hematologic malignancies is the suppression of normal hematopoiesis. Signals precluding normal HSCs from proliferation in TME in hematologic malignancies, ensued with their gradual and full replacement with cancer stem cells, can be triggered via miRNAs carried within exosomes and EVs. These signals can target MSCs, leading to the aversion of a number of their supportive functional roles in hematopoiesis, such as the cessation of secreting hematopoietic growth factors [117] and loss of contact support for HSCs [121]. In a report, miR-7977-containing exosomes released by leukemic cells were suggested to modify MSCs in a way that they lost their ability to produce appropriate amounts of hematopoietic growth factors such as angiopoietin-1, stem cell factor, and Jagged-1 [117]. The removal of cell–cell contacts between MSCs and HSCs has been implicated in cancer progression in hematologic malignancies. This functional defect in MSCs can be conferred to them via miRNAs transferred via exosomes released from leukemic cells, taking adhesive molecules on these cells, as noted by a study by Jiang et al. noting that exosomes derived from K562 cells containing miR-711 attenuated the expression of CD44 on BM-MSCs [121]. Not only in the BM, MSCs can also favor the metastatic niche environment after receiving messages from leukemic cells through paracrine exosomal routes; two miRNAs (miR-21 and miR-155) delivered to MSCs through exosomes generated by ATL leukemic cells induced the genes involved in the migration and proliferation of MSCs and promoted their pro-angiogenic activity [122]. Stem cells can also be directly targeted by exosomal/microvesicular miRNAs. Tzoran et al. reported that AML-derived EVs internalized by CD34+ cord blood stem cells could affect the differentiation of these stem cells, partly mediated through the overexpression of miR-125b and miR-155, particularly in the presence of MSCs [118]. Another report affirmed that hematopoietic stem and progenitor cells exposed to AML-sEV were debilitated in terms of clonogenicity and differentiation [126]. Also, EVs from MDS patients, enriched in some miRNAs, including miR-10a and miR-15a, could increase the expression of MDM2 and P53 genes in CD34+ cells, enhancing their viability and clonogenic capacity [119]. In a study on xenograft mice and in vitro culturing models, AML-derived exosomes, enriched in miR-150 and miR-155, suppressed c-MYB transcripts, inhibiting the proliferation and differentiation of hematopoietic stem and progenitor cells (HSPC) [120].
4.5. Clinical Implications of EV-miRNAs-Induced TME Remodeling
The most important clinical implications of TME modulation by exosomal and EV-derived miRNAs can be resistance to chemotherapy, disease relapse, and disease stage/prognosis [96]. In a study enrolling 204 MM patients, those showing resistance to BTZ have reduced expression of a number of exosomal miRNAs (miR-16-5p, miR-15a-5p and miR-20a-5p, miR-17-5p), revealing a substantial role for exosomes in MM drug resistance [86]. The expression of exosomal miRNAs of miR-151, miR-8908a-3p, and miR-486 could differentiate between vincristine-resistant or sensitive canine lymphoma cell lines [137]. Residual stem cells in the leukemic niche play an important role in disease relapse. The quiescence and persistence of leukemic stem cells in CML following TKI treatment were observed to be mediated by miR-126 so that an inhibitor of this miRNA or its knockdown enhanced the therapeutic efficacy of TKI [108]. In another study, EVs derived from AML cells promoted the quiescence of long-term hematopoietic stem cells (LT-HSC) via suppressing protein synthesis, mediated partly via miR-1246 and targeting the mTOR pathway [123]. Saitoh et al. stated that miR-101 level in EVs derived from BMSCs, but not its cellular or serum levels, was a possible predictor of MDS risk group and blast percentage through negatively regulating cell proliferation via inducing epigenetic modulators [27].
5. Conclusions and Future Perspectives
The role of EVs and exosomes and their biological content in hematological cancers is emerging. Particularly, miRNAs carried by exosomes and EVs can promote a variety of functions in both tumor and stromal cells that can bear clinical importance in terms of diagnostic and therapeutic areas. Hematologic malignancies are among the main causes of mortality and disabilities in the globe, and we need to expand our understanding regarding the role of EV- and exosome-derived communication mechanisms in these cancers. Exosomes and EVs can transport miRNAs from tumor cells to stromal cells and vice versa and play an essential role in cell–cell and cell–matrix communication in the TME, as well as in tumor development, progression, chemoresistance, recurrence, and metastasis. In this review, we explored the latest evidence on the prognostic and role of miRNAs carried by EVs and exosomes and their contribution to drug resistance in hematologic cancers. A robust piece of evidence suggests that miRNAs derived from EVs/exosomes can predict leukemia/lymphoma progression and relapse through various mechanisms, including modulation of interplay between tumor cells and their microenvironment, targeting key cellular adaptors and genes involved in proliferation, invasion, and apoptosis of cancer cells, regulation of neovascularization, transferring drug-resistant phenotype, modulating tumor-directed immune responses, regulating apoptotic and autophagic pathways, and modifying the behavior of stromal cells to favor tumor expansion. It is highly demanded to identify key exosomal and microvesicular miRNAs that can help optimize diagnostic and therapeutic procedures in hematologic malignancies, particularly in patients fail to effectively respond to routine drugs.
Ethics Statement
This study was approved by the ethics committee of Kerman University of Medical Sciences (Ethical Code: IR.KMU.REC.1403.377).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Supporting Information
Figure S1: Extracellular vesicles released by tumor and tumor microenvironment cells carry various bioactive molecules, particularly miRNAs. EV-derived miRNAs contribute to the tumor behavior, hematopoiesis suppression, and drug resistance induction. They may also serve as prognostic biomarkers.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




