Monitoring Activity-Rest Rhythms in Terminal Cancer Using Nonwearable Devices: A Preliminary Observational Study
Abstract
Background: Disruptions in activity-rest rhythms are common in terminal cancer patients, yet continuous monitoring of these changes is challenging. The effects of opioids and psychotropic drugs on sleep quality remain inadequately understood.
Objectives: (i) To objectively evaluate temporal changes in diurnal (8:00 a.m.–8:00 p.m.) and nocturnal (8:00 p.m.–8:00 a.m.) activity-rest rhythms over the final 2 weeks of life in terminal cancer patients using a nonwearable actigraph and (ii) to assess the adjusted impact of opioid and psychotropic drug use on these rhythms.
Design: A longitudinal study.
Settings/Participants: Twenty-six terminal cancer patients in a Japanese palliative care unit.
Measurements: A nonwearable actigraph was placed under the mattress to continuously monitor activity-rest rhythms. Measured parameters included time in bed (TIB: minutes), total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE: 0%–100%), activity score (0–960 counts/min; higher values indicate greater activity), and movement index (MI: percentage of time moving in bed; higher values suggest restlessness).
Results: As death approached, diurnal TIB and SE increased, indicating reduced daytime activity. Nocturnal sleep metrics fluctuated irregularly, with an increase in MI, suggesting deteriorating sleep quality. Opioid users exhibited a higher MI and lower SE both day and night compared to nonusers. Psychotropic drug users showed a decreased nocturnal MI and improved SE.
Conclusion: Terminal cancer patients experience decreased daytime activity and unstable nocturnal sleep as death nears. Opioid use correlates with inadequate rest, while psychotropic drugs may enhance nocturnal sleep quality. Continuous nonwearable monitoring offers valuable insights for optimizing end-of-life care.
1. Introduction
Terminal cancer patients often experience significant physical and psychological distress, making it difficult to achieve adequate rest [1, 2]. Sleep disturbances affect 62%–87% of this population [3, 4], indicating challenges in obtaining sufficient rest [5]. Evidence from broader clinical contexts suggests that improving sleep efficiency (SE) and alleviating restlessness can enhance well-being, which may also apply to terminally ill patients [6].
As the end of life approaches, worsening symptoms such as pain, anxiety, and dyspnea further disrupt rest and sleep [7]. Delirium frequently occurs in the final days [8–10], exacerbating activity-rest rhythm instability. Psychological factors, including depression and anxiety, contribute to these disruptions, destabilizing nighttime sleep and reducing daytime rest [11–13].
Activity-rest rhythms can be assessed using indicators such as time in bed (TIB), total sleep time (TST), wake after sleep onset (WASO), and SE, along with physical activity markers such as the activity score and the movement index (MI). These metrics provide a comprehensive understanding of rest quality and rhythm patterns [5]. Previous research has shown that effective pain management reduces agitation and improves rest [14]. However, the effects of opioids and psychotropic drugs on these rhythms remain unclear, particularly regarding diurnal and nocturnal variations over time.
While over 90% of terminal cancer patients use opioids, psychotropic drugs are also widely prescribed to manage delirium and insomnia [15–17]. However, evidence regarding the temporal effects of these pharmacological treatments on activity-rest rhythms is limited, particularly with respect to diurnal and nocturnal variations. Exploring these patterns could inform tailored interventions to enhance palliative care.
Adequate sleep and rest directly impact the quality of life (QoL) in terminally ill patients [18, 19]. Objective evaluation of activity-rest rhythms, alongside an understanding of the impact of medications, could guide effective pain and symptom management and support nonpharmacological interventions.
2. Objectives
- 1.
To evaluate diurnal and nocturnal activity-rest rhythms (TIB, TST, WASO, SE, activity score, and MI) over the final 2 weeks of life in terminal cancer patients using nonwearable sensing devices.
- 2.
To investigate temporal changes in these rhythms using linear mixed models (LMM), adjusting for opioid and psychotropic drug use.
3. Methods
3.1. Study Design and Participants
A longitudinal study was conducted between April 2018 and October 2019 at a chronic care hospital in Japan. The study was reported in accordance with the STROBE checklist to ensure comprehensive and transparent reporting of observational research. It was part of a larger sensing research project aimed at developing predictive models of patient conditions by integrating biological and behavioral data [14, 20, 21]. The participants were elderly terminal cancer patients admitted to a palliative care unit (PCU). Activity-rest rhythm data were collected using a nonwearable actigraph placed under the mattress, and additional patient demographic and medical information was obtained from medical records.
The inclusion criteria were as follows: (i) terminal cancer patients admitted to the PCU with a prognosis of several weeks, as determined by palliative care physicians using the palliative prognostic index (PPI) and clinical judgment; (ii) patients who participated in the study at least 3 days before death and were not receiving terminal sedation at the time of enrollment. Exclusion criteria included (i) missing data from the nonwearable monitor due to equipment issues, (ii) the presence of neurological conditions (e.g., Parkinson’s disease and dementia) that could affect activity-rest rhythms, and (iii) inability to obtain a continuous series of nighttime recordings throughout the observation period (e.g., sensor detachment).
3.2. Ethical Considerations
The study was approved by the appropriate institutional ethics committee (approval number 1741110) and the ethics committee of the study hospital. All participants or their family members were informed about the study protocol and provided written consent, with adapted consent procedures for patients with impaired consciousness.
3.3. Measurements
Activity-rest rhythms were assessed using a nonwearable actigraph monitor, “Nemuri SCAN” (Paramount Bed Co., Ltd., Tokyo, Japan), placed under the mattress. The device detects patient movements and classifies each 1-min epoch as sleep/wake and in-bed/out-of-bed, thereby evaluating sleep, wakefulness, and out-of-bed states [22]. In a validation study, this sensor achieved an agreement rate of 95.7%, sensitivity of 97.6%, and specificity of 75.8% when compared with wrist actigraphy and showed comparable accuracy to overnight polysomnography [23].
3.3.1. Outcome Variables
The primary outcomes included the following indicators: TIB (minutes), TST (minutes), WASO (minutes), SE (%), activity score (counts/min), and MI (%), which were used to evaluate diurnal and nocturnal activity-rest rhythms.
As a supplementary indicator, time away from bed was recorded to assess the extent to which patients were physically active outside of bed. Time away from bed is visualized in the figures to show its trend but is not the focus of the statistical analysis.
TIB represents the total time spent in bed, while SE is the percentage of actual sleep time within the total TIB. The average SE for older adults typically ranges from 77.5% to 85%, with sleep disturbances leading to reductions to 65%–72% [24–27]. The activity score quantifies the frequency and intensity of activity recorded per minute, while the MI reflects the proportion of movement time during TIB. The MI quantifies restlessness and fragmentation of rest, with higher values indicating poorer sleep quality [6]. No universally accepted clinical cut-offs for the MI exist; therefore, we defined ‘high MI’ as at or above the cohort median (daytime 68.6% and nighttime 58.4%) and ‘low MI’ as below the median. In our previous study using the same device [14], the average MI during daytime was 73.9% and 55.2% during nighttime, supporting the validity of the median thresholds applied in this study.
These indicators were evaluated separately for daytime (8:00 a.m.–8:00 p.m.) and nighttime (8:00 p.m.–8:00 a.m.).
3.3.2. Explanatory Variables
The primary explanatory variable was time (days), defined as the number of days from the measurement date to the day of death. The day of death was labeled as D-0, with preceding days numbered sequentially as D-1, D-2, and so on.
3.3.3. Potential Confounders
Potential confounders included age, sex, body mass index (BMI), and medication use (opioids and psychotropic drugs). Opioid use was classified into continuous-use and nonuse groups, while psychotropic drug use was categorized based on regular usage. Age and BMI were treated as continuous variables, while sex and medication use were treated as categorical variables. These variables were based on the values at the start of the measurement period.
3.4. Statistical Analysis
The study analyzed diurnal and nocturnal sleep indicators over the 14 days, leading up to death in terminal cancer patients. First, mean values of the outcome variables were calculated for each day, and the trends for diurnal and nocturnal outcomes were visualized graphically. Additionally, to explore the impact of medication, trends for opioid and psychotropic drug users and nonusers were depicted in the graphs.
To evaluate the effects of temporal progression and medication use on the primary outcomes, we constructed a linear mixed-effects model (LMM) that included both a random intercept and a random slope for time per subject ((1 + time | id)) to account for interindividual variability. Fixed effects comprised age, sex, BMI, time, opioid use, and psychotropic use. Model fit was assessed using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), both of which indicated good agreement between the model and the data. To verify model assumptions, we generated Q–Q plots and residuals-versus-fitted-values plots; the residuals fell approximately along the reference line in the central region, with only mild deviations at the tails and no evidence of heteroscedasticity or influential outliers. These diagnostics suggest that the assumptions of normality and homoscedasticity were broadly satisfied. In our analysis, we excluded any subjects for whom we could not obtain continuous 14-day monitoring data (e.g., due to sensor detachment) and applied restricted maximum likelihood (REML) estimation to the remaining data, thereby obviating the need for list-wise deletion or sensitivity analyses.
Summary statistics and model analyses were conducted using JMP version 18.0 and the lme4 package in R version 3.6.1.
4. Results
4.1. Characteristics of Participants
Table 1 shows the 26 terminal cancer patients that participated in the study. Patients were monitored for an average of 10.23 days until death, with an average age of 76.8 ± 10.5 years. The distribution of primary cancer sites was as follows: digestive tract (30.8%), pancreas (23.1%), breast (11.5%), hepatobiliary (11.5%), gynecological (7.7%), urological (7.7%), and other (lymphoma and melanoma; 7.7%). Subgroup analyses by cancer type were not performed due to small numbers in each category, and no marked cancer-site–specific differences in primary outcomes were noted (Table 1). Regarding medication use, 13 patients received opioids, 16 received psychotropic drugs (13 on sleeping pills only, 2 on antipsychotics only, and 1 on both an antianxiety agent and an antipsychotic), 8 received both, and 5 received neither (mean age of this latter group was 89 years, and they managed symptoms with nonopioid analgesics). No participants underwent chemotherapy, immunotherapy, or targeted therapy during the observation period; thus, the impact of these modalities on activity–rest rhythms could not be assessed.
| Total | Opioid user | Nonopioid user | |||
|---|---|---|---|---|---|
| n = 26 | Psychotropic use n = 8 | Nonpsychotropic n = 5 | Psychotropic use n = 8 | Nonpsychotropic n = 5 | |
| Age, mean (SD) | 76.8 (10.5) | 71.9 (9.6) | 72.2 (7.8) | 76.9 (9.7) | 89.6 (4.4) |
| Sex | |||||
| Male, n (%) | 14 (53.8) | 4 (50) | 4 (80) | 4 (50) | 2 (40) |
| Female, n (%) | 12 (46.2) | 4 (50) | 1 (20) | 4 (50) | 3 (60) |
| Body mass index, mean (SD) | 18.6 (2.8) | 17.9 (1.9) | 19.1 (3.4) | 19.4 (3.8) | 17.8 (3.2) |
| Use of drug | |||||
| Opioids, n (%) | 13 (50) | 8 (100) | 5 (100) | 0 | — |
| Psychotropic, n (%) | 16 (61.6) | 8 (100) | 0 | 8 (100) | — |
| Primary cancer cite | |||||
| Digestive tract | 8 (30.8) | 3 (37.5) | 2 (40) | 3 (37.5) | — |
| Pancreas | 6 (23.1) | 3 (37.5) | 1 (20) | — | 2 (40) |
| Breast | 3 (11.5) | — | — | 2 (25) | 1 (20) |
| Hepatobiliary | 3 (11.5) | 1 (12.5) | 1 (20) | 1 (12.5) | — |
| Gynecological | 2 (7.7) | 1 (12.5) | — | 1 (12.5) | - |
| Urological | 2 (7.7) | — | 1 (20) | — | 1 (20) |
| Other | 2 (7.7) | — | — | 1 (12.5) | 1 (20) |
4.2. Temporal Trends in Sleep Rhythm Indicators Over the Last 14 Days of Life
Figure 1 illustrates the temporal changes in the primary outcomes (TIB, TST, WASO, SE, activity score, and MI) over the final 14 days of life. During the daytime (Figure 1(a)), TIB and TST increased as death approached, while the activity score showed a decreasing trend. The MI remained consistently elevated at 60%–70%. At nighttime (Figure 1(b)), TST and SE exhibited irregular fluctuations, and the MI remained above 50%, showing a slight upward trend closer to death. As a supplementary indicator, time away from bed showed a decreasing trend during the daytime and a slight reduction at nighttime.
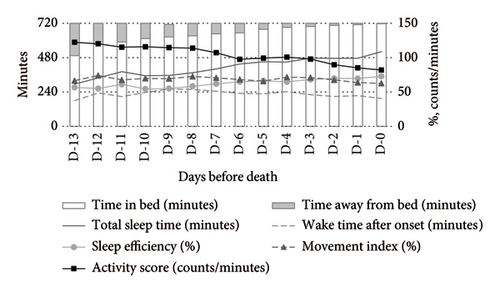
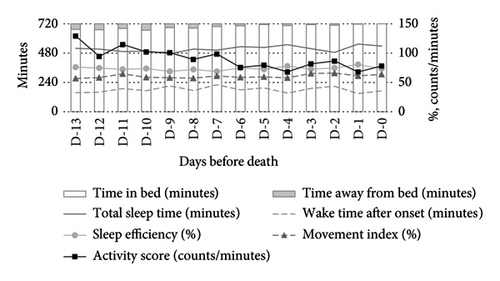
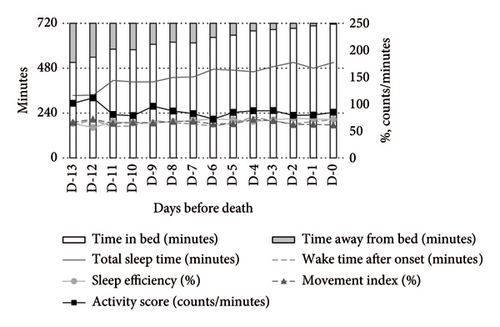
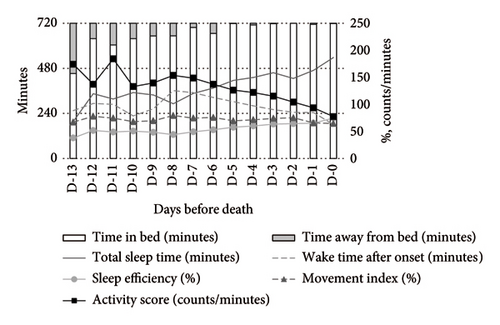
Medication use significantly influenced the activity-rest rhythms. Compared to nonusers, opioid users exhibited a higher MI and activity scores during both daytime and nighttime, alongside lower SE and TST. WASO was shorter among opioid users (Figure 2).
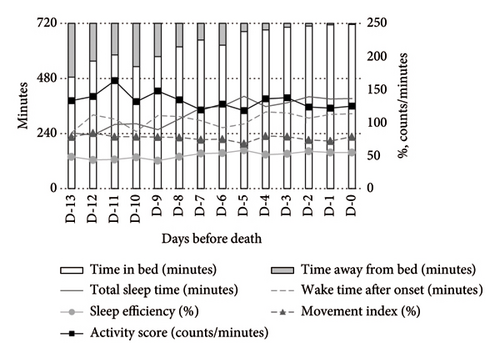
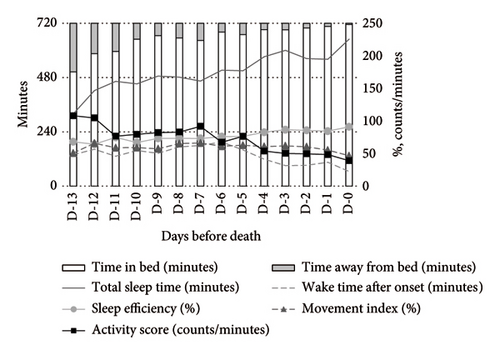
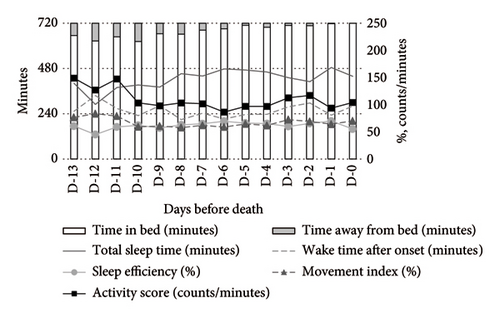

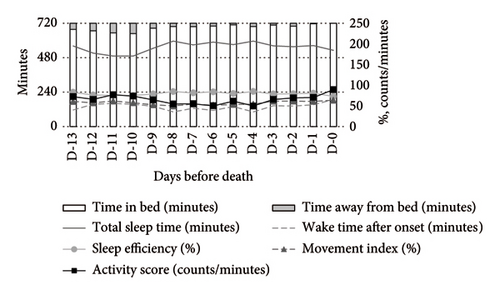
In contrast, psychotropic drug users demonstrated more stable activity scores during both daytime and nighttime. At night, these patients exhibited higher TST and SE compared to nonusers (Figure 3).
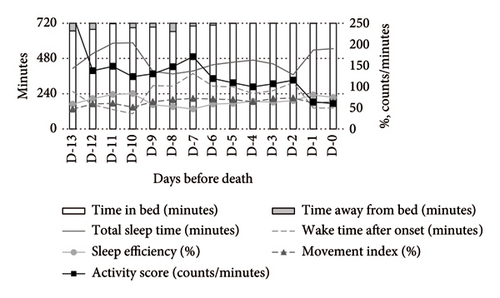
4.3. Changes Over Time in Sleep Patterns Until Death (LMM Analysis)
4.3.1. Daytime (8:00 a.m.–8:00 p.m.)
As death approached, daytime TIB significantly increased (MD: +10.64 min/day, 95% CI: 7.89–13.39), indicating longer periods of rest in bed. TST showed an increasing trend (MD: +10.74 min/day, 95% CI: 5.90–15.59), and SE significantly increased (MD: +0.92%/day, 95% CI: 0.28–1.56). However, there were no significant changes in the activity score or the MI (Table 2).
| TIB (min) | TST (min) | WASO (min) | SE (%) | Activity score (counts/minutes) | MI (%) | |
|---|---|---|---|---|---|---|
| Mean difference | Mean difference | Mean difference | Mean difference | Mean difference | Mean difference | |
| [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] | |
| Age (years old) | 2.38 [−2.25, 7.01] | 4.84 [−2.01, 11.69] | −2.47 [−8.26, 3.31] | 0.44 [−0.40, 2.28] | −1.11 [−3.21, 0.99] | 0.18 [−0.45, 0.82] |
| Sex (male = Ref.) | 5.98 [−69.80, 81.76] | 45.74 [−65.74, 157.2] | −39.19 [−132.9, 54.60] | 7.28 [−6.35, 20.91] | −15.95 [−49.94, 18.04] | 0.27 [−10.01, 10.55] |
|
11.98 [−1.99, 25.96] | 9.55 [−11.06, 30.15] | 2.26 [−15.10, 19.62] | 0.08 [−2.44, 2.60] | 0.07 [−6.23, 6.37] | 1.76 [−0.14, 3.67] |
| Timea (days, time of death = Ref.) | 10.64∗ [7.89, 13.39] | 10.74∗ [5.90, 15.59] | 0.29 [−4.20, 4.78] | 0.92∗∗ [0.28, 1.56] | −1.76 [−3.53, 0.01] | −0.11 [−0.65, 0.42] |
| Opioid use (without opioids = Ref.) | 52.23 [−37.07, 141.52] | −91.29 [−223.6, 40.97] | 144.35∗ [32.58, 256.11] | −19.72∗ [−35.95, −3.49] | 48.75∗ [8.07, 89.43] | 22.37∗ [10.07, 34.68] |
| Psychotropic medications use (without psychotropic medication = Ref.) | −14.81 [−93.70, 64.08] | 74.80 [−40.88, 190.5] | −89.29 [−186.4, 7.81] | 12.52 [−1.60, 26.64] | −38.42∗ [−73.53, −3.30] | −9.46 [−20.08, 1.16] |
- Note: Age, sex, BMI, and opioid/psychotropic use represent values collected at the beginning of the measurement. Mean difference: mean difference of each parameters (TIB: time in bed; TST: total sleep time; WASO: wake time after onset; SE: sleep efficiency; activity score; and MI: movement index) by 1-unit increase of each explanatory variable; 95% CI: 95% confidence interval; Ref.: reference. Significant values:∗p < 0.05 and ∗∗p < 0.01.
- aTime (days) was calculated by subtracting the time of death from the measurement time of parameter measurements.
Opioid users exhibited significantly increased WASO (MD: +144.35 min, 95% CI: 32.58–256.11) and MI (MD: +22.37%, 95% CI: 10.07–34.68), along with a decrease in SE (MD: −19.72%, 95% CI: −35.95 to −3.49). Their activity scores also increased significantly (MD: +48.75 counts/min, 95% CI: 8.07–89.43). Conversely, psychotropic drug users showed a significant reduction in the activity score (MD: −38.42 counts/min, 95% CI: −73.53 to −3.30) but no other significant changes compared to nonusers (Table 2).
4.3.2. Nighttime (8:00 p.m.–8:00 a.m.)
At nighttime, no significant changes were observed in TIB, TST, WASO, or SE as death approached. However, the MI significantly increased (MD: +0.74%/day, 95% CI: 0.10–1.39), while the activity score remained unchanged (Table 3).
| TIB (min) | TST (min) | WASO (min) | SE (%) | Activity score (counts/minutes) | MI (%) | |
|---|---|---|---|---|---|---|
| Mean difference | Mean difference | Mean difference | Mean difference | Mean difference | Mean difference | |
| [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] | |
| Age (years old) | 0.30 [−1.93, 2.52] | 3.39 [−2.70, 9.48] | −3.42 [−8.87, 2.02] | 0.46 [−0.33, 1.25] | −1.31 [−3.63, 1.01] | −0.17 [−0.75, 0.41] |
| Sex (male = Ref.) | −7.19 [−43.08, 28.70] | −1.50 [−101.0, 98.00] | −10.20 [−99.28, 78.87] | 0.49 [−12.49, 13.47] | −0.70 [−38.70, 37.30] | 3.15 [−6.30, 12.59] |
|
3.55 [−3.14, 10.24] | 4.17 [−14.23, 22.58] | 0.60 [−15.87, 17.06] | 0.12 [−2.28, 2.52] | −0.12 [−7.14, 6.90] | 1.39 [−0.36, 3.15] |
| Timea (days, time of death = Ref.) | −1.54 [−4.34, 1.25] | −4.59 [−9.85, 0.67] | 1.12 [−3.40, 5.65] | −0.25 [−0.90, 0.38] | −1.78 [−3.65, 0.10] | 0.74∗ [0.10, 1.39] |
| Opioid use (without opioids = Ref.) | −10.37 [−53.94, 33.20] | −128.62∗ [−246.8, −10.44] | 120.08∗ [14.47, 225.68] | −17.63∗ [−33.00, −2.25] | 31.02 [−13.98, 76.02] | 13.37∗ [2.00, 24.74] |
| Psychotropic medications use (without psychotropic medication = Ref.) | −6.42 [−42.93, 30.09] | 114.56∗ [11.98, 217.13] | −114.38∗ [−206.3, −22.46] | 16.08∗ [2.68, 29.48] | −49.54∗ [−88.78, −10.29] | −12.67∗ [−22.33, −3.01] |
- Note: Age, sex, BMI, and opioid/psychotropic use represent values collected at the beginning of the measurement. Mean difference: mean difference of each parameter (TIB: time in bed; TST: total sleep time; WASO: wake time after onset; SE: sleep efficiency; activity score; and MI: movement index) by 1-unit increase of each explanatory variable; 95% CI: 95% confidence interval; Ref.: reference. Significant values:∗p < 0.05 and ∗∗p < 0.01.
- aTime (days) was calculated by subtracting the time of death from the measurement time of parameter measurements.
Opioid users exhibited significantly reduced TST (MD: −128.62 min, 95% CI: −246.80 to −10.44) and SE (MD: −17.63%, 95% CI: −33.00 to −2.25), along with increased WASO (MD: +120.08 min, 95% CI: 14.47–225.68) and MI (MD: +13.37%, 95% CI: 2.00–24.74).
In contrast, psychotropic drug users demonstrated significant improvements in TST (MD: +114.56 min, 95% CI: 11.98–217.13) and SE (MD: +16.08%, 95% CI: 2.68–29.48), along with reductions in WASO (MD: −114.38min, 95% CI: −206.3 to −22.46), activity score (MD: −49.54 counts/min, 95% CI: −88.78 to −10.29), and MI (MD: −12.67%, 95% CI: −22.33 to −3.01). These results suggest that psychotropic drugs may contribute to improved nighttime sleep quality (Table 3).
5. Discussion
This study objectively evaluated changes in activity-rest rhythms in terminal cancer patients over the final 2 weeks of life. Daytime TIB and SE increased as death approached, while nighttime rhythms remained overall unstable. In particular, the nighttime MI persistently exceeded the cohort median (58.4%), indicating elevated restlessness and disrupted rest as death approached. Opioid use disrupted sleep-wake rhythms, while psychotropic drugs improved nighttime sleep quality.
5.1. Changes in Daytime Activity and Rest
As death approached, patients exhibited significant increases in daytime TIB, reflecting greater time spent resting due to physical fatigue and declining energy levels. The observed increases in TST and SE during the day suggest a shift toward daytime rest as activity levels decreased [12, 28–30]. Although the activity score did not show significant changes, the data suggest that patients likely engaged in minimal activities rather than being completely inactive. Monitoring daytime activity is a valuable approach to understanding patient conditions, providing critical insights for optimizing care strategies and improving QoL in terminal settings.
5.2. Decline in Nighttime Sleep Quality
The nighttime MI showed a slight upward trend, reflecting increasing restlessness and sleep fragmentation. We defined “high MI” as values above the cohort median (58.4%) and observed that the elevated MI persisted closer to death, highlighting clinically meaningful sleep disruption. These disturbances likely stem from terminal symptoms—such as anxiety, pain, and dyspnea—and from daytime somnolence adversely affecting nighttime sleep [18, 30]. The absence of significant improvements in TST, WASO, SE, or activity score suggests that nighttime sleep remains unstable or deteriorates as death approaches. Detecting and understanding these instabilities in nighttime sleep patterns are critical for guiding effective interventions aimed at improving patient comfort and care quality.
5.3. Trends Among Opioid Users
This study revealed that opioid users had significantly higher activity scores and MI compared to nonusers, both during the day and at night, alongside significantly lower SE. Additionally, increased WASO and reduced TST were observed, indicating disruptions in sleep architecture. These findings suggest that while opioids provide pain relief, they may disrupt sleep-wake rhythms through central nervous system effects, particularly contributing to poorer nighttime sleep quality and prolonged wakefulness.
Previous studies have similarly reported that opioids can alter the sleep structure and increase the risk of delirium [31–33], findings that are supported by the present study. Moreover, our previous research demonstrated that effective pain management interventions reduced both activity scores and the MI, highlighting the potential for appropriate pain control to improve sleep-wake rhythms in terminal cancer patients [14].
These findings emphasize the need to carefully balance pain relief with the risk of sleep disturbances when prescribing opioids. The integration of alternative analgesic methods and nonpharmacological approaches, such as environmental adjustments and the promotion of light physical activities, could further enhance sleep quality. This underscores the importance of adopting a multifaceted approach to improve the overall care of terminal cancer patients.
5.4. Trends Among Psychotropic Drug Users
Psychotropic drug users showed a reduced MI and improved SE at night, accompanied by increased TST and reduced WASO, suggesting enhanced nighttime sleep quality. However, the observed reduction in the daytime activity score raises concerns about excessive sedation and diminished daytime functionality. To mitigate these effects, careful selection of psychotropic drug types and dosages is crucial. Combining pharmacological approaches with nonpharmacological interventions may help maintain daytime functionality while improving nighttime sleep quality. Achieving a balance between daytime and nighttime effects remains a key challenge in the use of psychotropic drugs [16, 34, 35].
5.5. Clinical Implications
This study underscores the importance of monitoring activity-rest rhythms to comprehensively understand the health status of terminal cancer patients. Continuous monitoring provides valuable insights for predicting sleep disturbances and the risk of delirium, facilitating timely interventions [36]. These findings support the integration of nonpharmacological approaches, such as environmental adjustments and family involvement, to optimize end-of-life care. Moreover, the study highlights the potential of psychotropic drugs to improve nighttime sleep quality, while emphasizing the need to carefully manage the adverse effects of opioids on sleep. A multidimensional approach incorporating both pharmacological and nonpharmacological strategies is essential to enhance the QoL for terminal patients and their families.
5.6. Study Limitations
This study has several limitations. First, although the MI is a valuable quantitative measure of restlessness and insufficient rest in terminal patients, its normative range and clinical cut-off values have not yet been established. In this study, we defined “high MI” and “low MI” relative to the cohort median (daytime 68.6% and nighttime 58.4%), but this classification remains illustrative. Future large-scale investigations—including healthy older adults and multicenter samples—are needed to establish MI norms and to examine associations with clinical outcomes such as delirium onset and ADL decline. Nonetheless, our work represents a pioneering effort to visualize sustained increases in the MI among terminal cancer patients using a contactless sensor, highlighting continuous restlessness and inadequate rest.
Second, this pilot study was conducted in a single Japanese palliative care ward primarily admitting elderly patients with terminal cancer. Although the facility adheres to standardized palliative care practices under Japan’s universal health insurance system and provides multidisciplinary symptom management, these features may still limit the generalizability of our findings. Factors such as cultural attitudes toward rest, ward routines, and patient age may affect activity-rest patterns. Future multicenter studies across diverse settings - such as home care, hospices, and other regions - are needed to confirm the external validity of our findings.
Third, the two-week observation window up to the time of death may have been insufficient to capture the onset and long-term dynamics of sleep–wake rhythm changes. Moreover, no systemic anticancer therapies (e.g., chemotherapy, immunotherapy, and targeted therapy) were administered during the study period, so the potential impact of these treatments on activity–rest rhythms could not be evaluated. Future study designs should incorporate treatment timing to assess rhythm alterations as potential side effects of specific modalities.
Fourth, subgroup analyses by cancer type were not performed due to limited sample sizes, leaving the question of cancer–site–specific rhythm changes for future investigation.
Taken together, these limitations underscore the need for extended observation periods and larger, multicenter studies to fully elucidate sleep–wake rhythm dynamics in terminal cancer patients. Nevertheless, this study provides important insights into the feasibility of contactless sensing devices for monitoring sleep and rest in palliative care, and it highlights the value of integrating advanced technologies to optimize pharmacologic and nonpharmacologic interventions.
6. Conclusion
Terminal cancer patients experience increased daytime rest and unstable nighttime sleep as death nears. Opioids disrupt sleep quality, whereas psychotropic drugs may improve it. These findings highlight the importance of balanced interventions to enhance end-of-life care.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Osaka University (approval number: 1741110). Written informed consent was obtained from all participants or their family members.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
The conceptualization of this study was carried out by Akari Higuchi, Haruka Tanaka, Yoko Higami, Ayae Kinoshita, and Sakiko Fukui. The methodology was designed by Akari Higuchi, Haruka Tanaka, Yoko Higami, Isseki Maeda, and Sakiko Fukui. Data curation and formal analysis were conducted by Akari Higuchi and Haruka Tanaka. The original draft was written by Akari Higuchi, and the review and editing were carried out by Akari Higuchi, Haruka Tanaka, Yoko Higami, Isseki Maeda, Ayae Kinoshita, and Sakiko Fukui. Supervision was provided by Sakiko Fukui. Every author has approved the final manuscript for submission.
Funding
This study was supported by a Grant-in-Aid for Scientific Research B from the Ministry of Education, Culture, Sports, Science and Technology (grant no. 18H03112) and a research grant from the DAIKIN Industry.
Acknowledgments
The authors thank the patients, the staff of the hospital, and Masanobu Kawazoe, Mamoru Okumoto, Nobuhiro Nakamura, Miyae Yamakawa, and Momoe Utsumi from the research project team.
Open Research
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy and ethical restrictions but can be obtained from the corresponding author upon reasonable request.




