Risk of Autoimmune Skin Diseases Associated With Immune Checkpoint Inhibitors: A Pharmacovigilance Analysis Using the FAERS Database
Abstract
Background: Immune checkpoint inhibitors (ICIs) revolutionize cancer therapy but frequently cause immune-related adverse events (irAEs), with autoimmune skin diseases (ASDs) being significant toxicities requiring careful management.
Objective: This study explores the association between ICIs and 10 common ASDs using the FAERS database, aiming to identify risk profiles, time-to-onset patterns, and clinical implications.
Methods: A retrospective pharmacovigilance analysis of FAERS data (2011Q1–2024Q4) was conducted, focusing on reports involving seven ICIs. Disproportionality algorithms (ROR, PRR, BCPNN, and MGPS) and statistical methods, including Kaplan–Meier and Weibull models, were employed to evaluate ASD risk and onset patterns.
Results: Among 1670 cases, bullous pemphigoid (BP) showed the strongest association, particularly with PD-1/PD-L1 inhibitors, and other prominent ASDs, including vitiligo, psoriasiform dermatitis, lichen planus, and dermatomyositis. Scleroderma, Henoch–Schönlein purpura, pemphigus, alopecia areata, and systemic lupus erythematosus exhibited limited or inconsistent signals across different ICIs. The median time-to-onset was 143 days, with early onset linked to ipilimumab and atezolizumab. Reports predominantly involved males (62.8%) and patients ≥ 65 years old (51.7%), with the United States and Japan contributing most cases.
Conclusions: BP, vitiligo, psoriasiform dermatitis, lichen planus, and dermatomyositis are key irAEs of ICIs, requiring vigilant monitoring and individualized management strategies. Limitations include biases in spontaneous reporting and underrepresentation of newer ICIs.
1. Introduction
Immune checkpoint inhibitors (ICIs), a novel class of anticancer agents, function by blocking critical immune checkpoint pathways, such as programmed cell death receptor-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), thereby restoring the antitumor immune activity of T-cells [1, 2]. The widespread adoption of ICIs has substantially improved survival outcomes in patients with various malignancies [3]. However, the nonspecific activation of T-cells can also lead to immune-related adverse events (irAEs), wherein the immune system attacks normal tissues. These adverse events (AEs) can affect multiple organ systems, including the skin, liver, gastrointestinal tract, and endocrine glands [4, 5]. The skin is one of the most frequently affected organs, presenting with a spectrum of toxicities ranging from mild rashes and pruritus to severe immune-mediated dermatological conditions. These cutaneous toxicities can significantly impair the quality of life and, in severe cases, necessitate discontinuation of anticancer therapy [6].
With the increasing use of ICIs, immune-mediated dermatological diseases have been increasingly reported, including bullous pemphigoid (BP), vitiligo, and alopecia areata [7, 8]. BP is an autoimmune blistering disorder characterized by pruritic erythema and tense bullae, which may require hospitalization in severe cases [9]. Vitiligo, another commonly observed AE, manifests as localized or widespread depigmented macules and is believed to result from immune activation targeting melanocytes [10]. Alopecia areata, characterized by nonscarring hair loss, has also been associated with ICI therapy, likely due to immune-mediated follicular damage [11]. Although current studies suggest that moderate immune-related cutaneous AEs, such as vitiligo and psoriasiform eruptions, may be associated with better prognostic outcomes [12, 13], excessive immune system activation can significantly impact treatment management in affected patients.
The United States Food and Drug Administration AE Reporting System (FAERS) is one of the largest open-access pharmacovigilance databases globally, designed to capture postmarketing adverse drug reactions. Compared to traditional case-control studies or case reports, real-world studies utilizing the FAERS database offer several advantages. First, the large sample size and diverse data sources facilitate the identification of rare or delayed AEs. Second, the broad population coverage across various clinical settings enhances the detection of associations between specific drugs and AEs in diverse patient cohorts. Finally, the FAERS database enables large-scale data mining to detect safety signals early, providing valuable insights for clinical decision-making and pharmacovigilance.
In this study, we employed the FAERS database to systematically evaluate the association between ICIs and 10 common autoimmune skin diseases (ASDs), including pemphigus, BP, systemic lupus erythematosus (SLE), dermatomyositis, scleroderma, Henoch–Schönlein purpura, psoriasiform dermatitis, vitiligo, alopecia areata, and lichen planus (LP). To enhance the robustness and reliability of safety signal detection and reduce the risk of false positives, we utilized four disproportionality analysis algorithms: reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPN), and multigamma Poisson shrinkage (MGPS). This study aims to elucidate the risk profiles of ICI–associated autoimmune skin conditions, providing large-scale real-world evidence to aid clinicians in optimizing ICI therapy safety, facilitating early recognition and promoting individualized management strategies.
2. Methods
2.1. Data Collection and Processing
We conducted a retrospective pharmacovigilance analysis using the FAERS database from the first quarter of 2011 to the fourth quarter of 2024. The FAERS database, an anonymized repository, removes personal identifiers and assigns a unique identification number (ID) to each case for differentiation. It consists of seven key datasets, including demographic information (DEMO); REAC (containing AE information), where AEs are coded using preferred terms (PTs) based on the Medical Dictionary for Regulatory Activities (MedDRA); drug usage records (DRUG); treatment outcomes (OUTC); report sources (RPSR); therapy start and end dates (THER); and indications for drug use (INDI). Following the FDA–recommended deduplication method, we utilized the PRIMARYID, CASEID, and FDA_DT fields from the DEMO file. Reports were sorted by CASEID, FDA_DT, and PRIMARYID, retaining the record with the latest FDA_DT value for duplicate CASEIDs, and selecting the record with the largest PRIMARYID when FDA_DT values were identical [14].
The study focused on the seven most common ICIs approved by the FDA, including pembrolizumab, nivolumab, atezolizumab, durvalumab, ipilimumab, avelumab, and cemiplimab, which target the PD-1/PD-L1 and CTLA-4 pathways. To ensure comprehensive data capture and minimize the risk of missing ICI–related AEs, we searched all generic and brand names for these ICIs through DrugBank (https://go.drugbank.com/) and the Open Targets Platform (https://platform.opentargets.org/). ICIs such as dostarlimab and toripalimab were excluded due to their recent approval and limited AE reports. Within the FAERS database, drugs are categorized as primary suspect (PS) drugs, secondary suspect (SS) drugs, concomitant (C) drugs, and interacting (I) drugs. To precisely evaluate the association between ICIs and ASDs, we included only reports where ICIs were designated as PS drugs.
The target conditions were identified using MedDRA Version 25.0, focusing on 10 common ASDs: pemphigus (PT code 10034280), BP (PT code 10034277), SLE (PT code 10042945), dermatomyositis (PT code 10012503), scleroderma (PT code 10039710), Henweroch–Schönlein purpura (PT code 10019617), psoriasiform dermatitis (PT code 10058675), vitiligo (PT code 10047642), alopecia areata (PT code 10001761), and LP (PT code 10024429). Other ASDs, such as chronic spontaneous urticaria (CSU), were excluded due to challenges in achieving precise definitions within the FAERS database, as many reports originate from spontaneous submissions by nonmedical professionals. To mitigate the risk of classification errors and reporting bias, we implemented stricter inclusion criteria by excluding reports submitted by consumers or legal representatives. Only reports from healthcare professionals (e.g., physicians, pharmacists, and other medical experts) were included for analysis, ensuring the reliability of the data.For detailed information regarding ICIs and ASDs, please refer to Supporting Tables S1-S2.
2.2. Statistical Analysis
Descriptive analysis was performed to comprehensively evaluate reports of ICI–related ASDs, focusing on gender differences, age distribution, indications, AE outcomes, reporting year, and time to onset (TTO). TTO was defined as the time interval between the initiation date of ICIs recorded in the THER file (START_DT) and the onset date of ASDs recorded in the DEMO file (EVENT_DT) [15].
The ROR [16], PRR, BCPN, and MGPS algorithms were employed to evaluate the strength of safety signals for ICI–associated ASDs [17]. Detailed formulas and criteria for positive signal detection are provided in Supporting Table S3. To reduce false-positive signals, a signal was considered positive only if it met the thresholds of at least three out of the four algorithms.
For further TTO analysis, including cumulative distribution curves, Weibull distribution tests, Kaplan–Meier (KM) curves, and log-rank tests, only reports with complete TTO data were included. Cumulative distribution curves were used to visually display the cumulative occurrence probability of AEs. Weibull distribution analysis further explored the temporal patterns and trends of TTO through parameter estimation. KM curves and log-rank tests were applied to compare TTO among different ICI treatment regimens and assess statistical significance. The combination of these methods provided a comprehensive statistical evaluation of TTO distribution characteristics and served as a sensitivity analysis to verify the robustness of the results.
Data mining and statistical analysis were conducted using R software (https://www.r-project.org/, Version 4.3.2). The data visualization was performed using Python (https://www.python.org/, Version 3.9.7). The study adhered to the latest READUS-PV guidelines for signal detection in pharmacovigilance using individual case safety reports, ensuring transparent and clear reporting of scientific findings [18, 19]. The READUS-PV checklist is available in Supporting Table S4.
3. Results
3.1. Basic Information on AE Reports
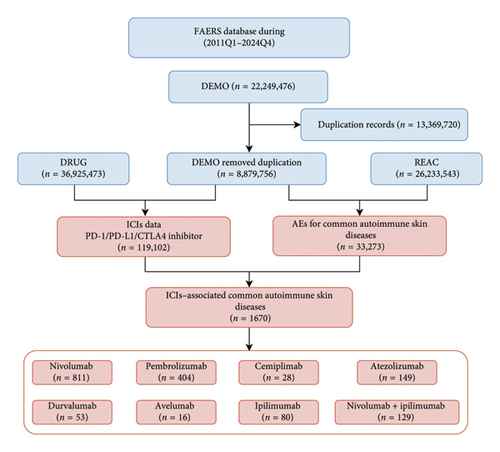
We conducted data mining on ICI–related ASDs reported in the FAERS database from the first quarter of 2011 to the fourth quarter of 2024. Figure 1 illustrates the detailed data mining process. Ultimately, we collected 1670 reports on ICI–related ASDs from 1569 patients, as some patients experienced multiple AEs. Among these, nivolumab accounted for the highest number of cases (811 reports), followed by pembrolizumab (404 reports), atezolizumab (149 reports), ipilimumab (80 reports), durvalumab (53 reports), and avelumab (16 reports). Cemiplimab was associated with 28 reports, while the combination therapy nivolumab + ipilimumab accounted for 129 reports.
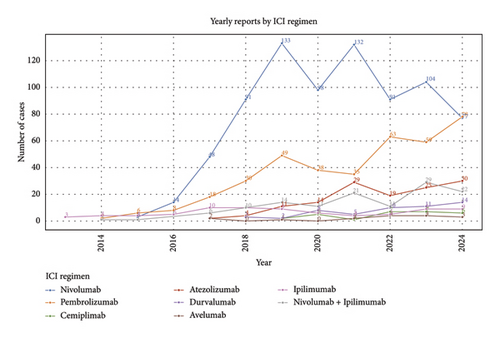
The annual AE report quantity is shown in Figure 2(a). With the widespread clinical use of ICIs, ICI–related ASDs have been progressively identified and reported to the FAERS database. Although fluctuations were observed, the overall trend shows a steady increase in AE reports. The clinical characteristics of patients with ICI–related ASDs in the FAERS database are detailed in Figures 2(b), 2(c), 2(d), 2(e), 2(f), 2(g), and 2(h).
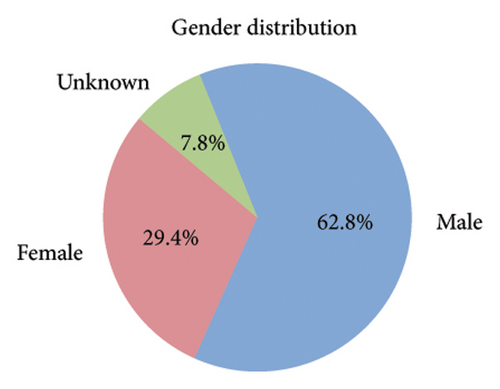
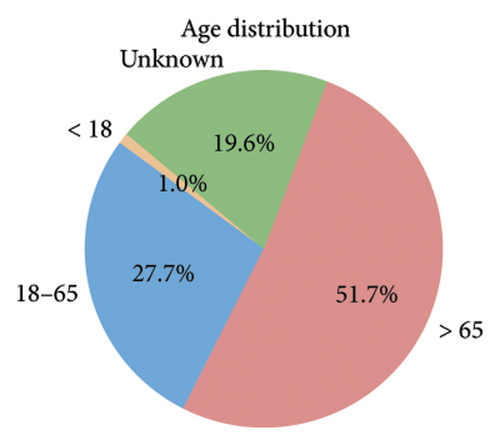
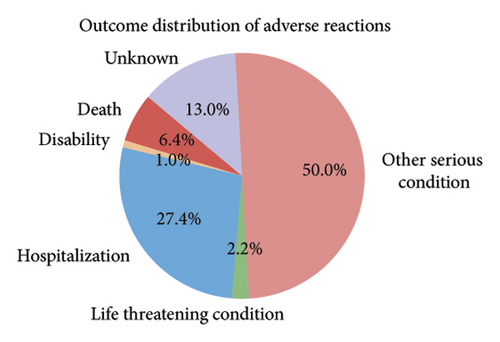
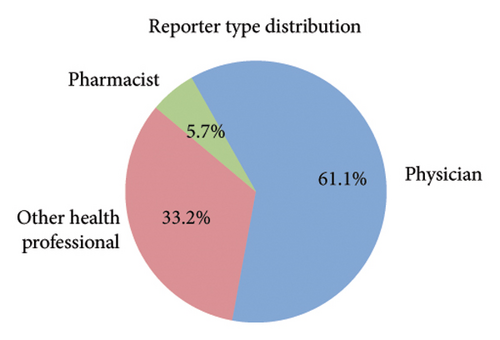
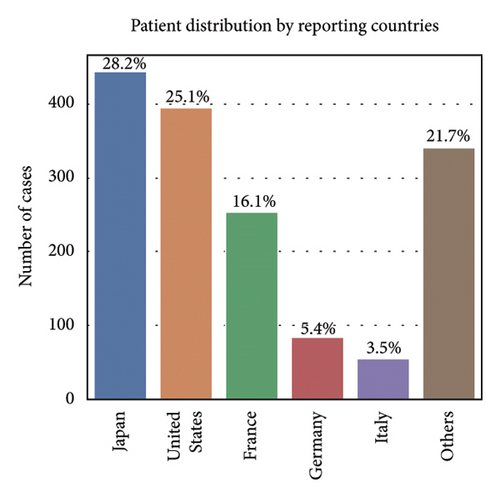
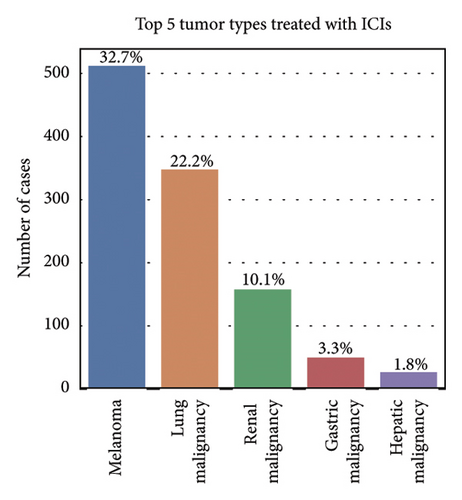
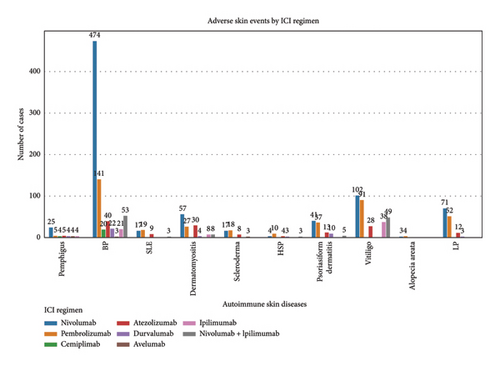
Among the 1670 reports from 1569 patients, 62.8% were male (985 reports), 29.4% were female (462 reports), and 7.8% (122 reports) had an unknown gender (Figure 2(b)). The majority of patients were older than 65 years (51.7%, 811 reports), while 27.7% (434 reports) were aged 18–65 years, and only 1.0% (16 reports) were under 18 years. Age information was missing for 19.6% (308 reports) (Figure 2(c)). The most frequently reported clinical outcomes were “other serious conditions” (50.0%, 784 reports) and hospitalization (27.4%, 430 reports). Other outcomes included death (6.4%, 101 reports), life-threatening conditions (2.2%, 35 reports), and disability (1.0%, 15 reports). Outcomes were unknown for 13.0% (204 reports) (Figure 2(d)). Most reports were submitted by physicians (61.1%, 959 reports), followed by other healthcare professionals (33.2%, 521 reports), and pharmacists (5.7%, 89 reports) (Figure 2(e)). Reports were predominantly from Japan (28.2%, 443 reports), followed by the United States (25.1%, 394 reports) and France (16.1%, 253 reports). Other countries, including Germany (5.4%, 84 reports) and Italy (3.5%, 55 reports), contributed fewer cases. The remaining 21.7% (340 reports) originated from various other countries (Figure 2(f)). Among the tumors treated with ICIs, melanoma was the most commonly reported (32.7%, 513 reports), followed by lung malignancies (22.2%, 349 reports) and renal malignancies (10.1%, 159 reports). Reports involving gastric (3.3%, 51 reports) and hepatic malignancies (1.8%, 28 reports) were less frequent (Figure 2(g)). The distribution of adverse skin events associated with eight ICI regimens is shown in Figure 2(h). Nivolumab had the highest number of cases of BP (474 reports), followed by vitiligo (102 reports), LP (71 reports), and psoriasiform dermatitis (41 reports). Pembrolizumab was also frequently associated with BP (141 reports) and vitiligo (91 reports), with notable reports of LP (52 reports) and psoriasiform dermatitis (37 reports). For cemiplimab, most AEs were reported for BP (20 reports), while other ASDs had fewer than three cases reported. Atezolizumab was linked to BP (40 reports), dermatomyositis (30 reports), and vitiligo (28 reports). Among the PD-L1 inhibitors, durvalumab was primarily associated with BP (22 reports) and psoriasiform dermatitis (10 reports), while Avelumab had few reports across different ASDs. Ipilimumab, a CTLA-4 inhibitor, was predominantly associated with vitiligo (38 reports) and dermatomyositis (8 reports). The combination therapy nivolumab + ipilimumab had notable cases of BP (53 reports), vitiligo (49 reports), and dermatomyositis (8 reports). For other ICIs and ASDs, the number of cases was generally low, with several conditions having fewer than three reports.
3.2. Disproportionality Analysis of ICI–Associated ASDs Under Various Treatment Strategies
To comprehensively evaluate the relationship between ICIs and common ASDs, four disproportionality analysis algorithms were applied: ROR, PRR, BCPN (EBGM), and MGPS (IC). To ensure the robustness of the findings and minimize false-positive results, a signal was considered positive if it met the thresholds of at least three out of the four algorithms. The disproportionality analysis results are visually presented in Figure 3 using a ROR heatmap, which provides an intuitive representation of the association strength between different ICIs and ASDs. Detailed numerical data corresponding to the analysis can be found in Supporting Table S5.
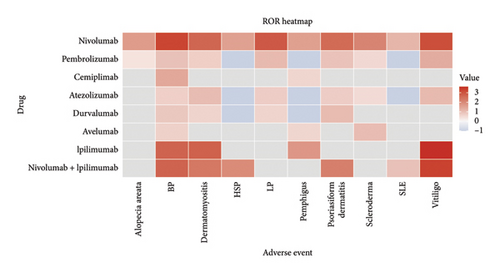
From Figure 3, BP emerged as the most strongly associated ASD with ICIs, exhibiting consistently high ROR values across multiple treatment strategies. Nivolumab (ROR = 1759.43), ipilimumab (ROR = 507.22), and the combination therapy nivolumab + ipilimumab (ROR = 507.31) demonstrated particularly strong signals. These results underscore BP as a well-established irAE induced by ICIs, warranting close clinical monitoring. Vitiligo also exhibited significant positive signals across several treatment strategies, particularly with ipilimumab (ROR = 4208.2), nivolumab (ROR = 1223.57), and nivolumab + ipilimumab (ROR = 1963.36). Pembrolizumab (ROR = 21.41) and atezolizumab (ROR = 11.88) also showed moderate associations, further supporting the hypothesis that ICIs can induce immune-mediated melanocyte destruction. Dermatomyositis showed strong associations with ipilimumab (ROR = 551.86), nivolumab (ROR = 470.37), and nivolumab + ipilimumab (ROR = 212.78), indicating a potential link between ICIs and inflammatory myopathies. Psoriasiform dermatitis was notably associated with nivolumab (ROR = 400.58) and nivolumab + ipilimumab (ROR = 158.51), suggesting a subtype of ICI–induced skin inflammation that mimics psoriasis. LP also showed strong positive signals, particularly for nivolumab (ROR = 765.79) and pembrolizumab (ROR = 11.12), reinforcing the potential immune activation mechanism underlying this condition. In contrast, scleroderma, Henoch–Schönlein purpura, pemphigus, alopecia areata, and SLE exhibited limited or inconsistent signals across different ICIs. Although nivolumab (ROR = 117.5) and nivolumab + ipilimumab (ROR = 90.45) showed notable associations with HSP, the small number of cases suggests the need for further data validation. Other ASDs, such as pemphigus (with the exception of ipilimumab, ROR = 53.23) and alopecia areata, showed no significant associations with most ICIs.
Overall, BP remains the most strongly associated ASD with ICIs, followed by vitiligo, dermatomyositis, LP, and psoriasiform dermatitis. Other ASDs demonstrated weaker or inconsistent signals, suggesting either a lower risk or a need for larger datasets to confirm their association.
3.3. TTO Analysis
The TTO of ICI–associated ASDs was analyzed based on 876 reports with complete TTO data extracted from the database. However, due to the limited number of reports for avelumab and cemiplimab (fewer than 20 cases each), their inclusion in the analysis could lead to considerable statistical bias and unreliable estimations. Given the risk of overinterpreting trends from small sample sizes, these two ICIs were excluded from further TTO analysis. Therefore, the final TTO analysis included six ICI treatment strategies to ensure statistical robustness and reliable estimations: nivolumab, pembrolizumab, atezolizumab, durvalumab, ipilimumab, and the combination therapy nivolumab + ipilimumab.
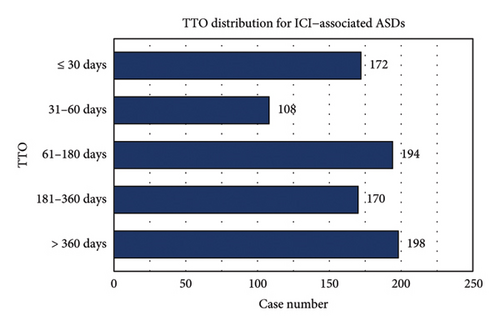
The median TTO of ICI–associated ASDs was 143 days (interquartile range [IQR]: 42–343 days). As detailed in Figure 4(a), the distribution of TTO varied across different time intervals: ≤ 30 days (n = 172, 19.6%), 31–60 days (n = 106, 12.1%), 61–180 days (n = 194, 22.2%), 181–360 days (n = 170, 19.4%), and > 360 days (n = 198, 22.6%). Figures 4(b) and 4(c) illustrate the cumulative distribution and KM survival analysis of TTO, revealing significant differences among ICIs regimens (Kruskal–Wallis p < 0.001; log-rank p = 0.001). Ipilimumab and atezolizumab exhibited the steepest early rises in cumulative incidence, suggesting a higher probability of early-onset ASDs, whereas nivolumab and pembrolizumab displayed more gradual cumulative incidence curves, indicating a delayed onset pattern. These findings highlight the need for early dermatologic monitoring for Ipilimumab and atezolizumab, whereas nivolumab and pembrolizumab require prolonged surveillance due to their potential for late-onset ASDs.
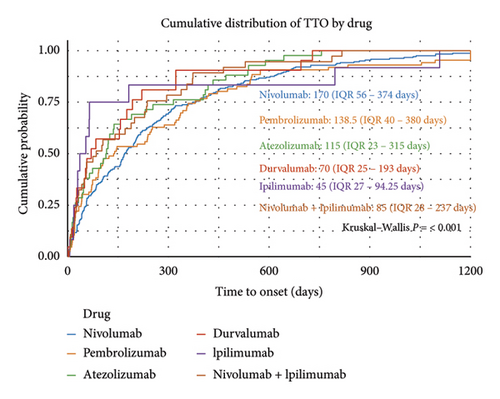

The Weibull distribution analysis further supported these observations. The shape parameter (k) remained below 1 for all ICIs, confirming an early failure pattern where event rates decrease over time. The scale parameter (λ), reflecting the estimated median TTO, varied significantly across different ICIs. Nivolumab (λ = 245.79, 95% CI: 219.67–271.91) and pembrolizumab (λ = 240.68, 95% CI: 193.73–287.63) exhibited the most prolonged onset times, requiring long-term dermatologic monitoring. In contrast, ipilimumab (λ = 131.02, 95% CI: 42.80–219.24) and atezolizumab (λ = 170.55, 95% CI: 123.98–217.13) had earlier onset profiles, necessitating close monitoring in the early treatment phase. The detailed Weibull model parameters, including 95% confidence intervals, are presented in Supporting Table 6.
4. Discussion
This pharmacovigilance study provides a comprehensive evaluation of ICI–associated ASDs, including their risk profiles, TTO patterns, and clinical implications. By leveraging the FAERS database and employing robust disproportionality analysis algorithms, our study offers several distinct contributions compared to previous research. Unlike smaller cohort studies or isolated case reports, the large-scale nature of this analysis enables the identification of rare or delayed AEs with greater statistical power. In addition, the application of four disproportionality algorithms ensures a more robust detection of safety signals, minimizing false-positive associations. Importantly, this study systematically evaluates 10 common ASDs across multiple ICI regimens, providing a broader and more detailed risk profile that extends beyond the scope of most prior investigations.
Our study highlighted notable baseline characteristics among patients experiencing ICI–associated ASDs, focusing on gender, age, and tumor types. Males accounted for 62.8% of the cases, a finding consistent with prior research indicating a higher prevalence of ICI–related AEs in men [20]. This gender disparity may reflect the higher incidence of cancers such as melanoma and lung cancer among middle-aged and elderly males, which are key indications for ICI therapy [21]. Regarding age, 51.7% of cases involved patients over 65 years old, aligning with the typical demographic of cancer patients receiving ICIs. The aging immune system, characterized by immunosenescence, likely increases susceptibility to irAEs [22]. However, the lack of age data in 19.6% of reports highlights a limitation of the dataset and suggests the need for more comprehensive data collection. Analysis of tumor types treated with ICIs revealed that melanoma (32.7%) and lung malignancies (22.2%) were the most common underlying cancers among patients developing ASDs. A relatively high proportion of melanoma cases is expected, as melanoma is a key indicator for ICIs, particularly PD-1 inhibitors, which are known to enhance immune activation [23, 24].
The integration of TTO analysis, including Weibull distribution modeling and KM analysis, adds a temporal dimension to the understanding of ICI–associated ASDs. This approach not only identifies high-risk drugs but also highlights distinct onset patterns, which are crucial for optimizing clinical monitoring and management strategies. Furthermore, the emphasis on rare ASDs, such as dermatomyositis, and the rigorous exclusion of confounding factors, such as SS drugs, enhance the reliability and clinical applicability of the findings.
Our analysis identified BP as the most strongly associated ASD with ICIs, highlighting its role as a significant irAE. This finding is consistent with prior studies reporting BP as a rare but serious cutaneous irAE, particularly associated with PD-1 and PD-L1 inhibitors. For instance, Naidoo et al. [25] documented cases of BP induced by both nivolumab and durvalumab, emphasizing the potential involvement of T-cell and B-cell-mediated immune activation. Liu et al. [26] further characterized nivolumab-induced BP, describing a median onset time of 31 weeks and common clinical manifestations such as tense bullae, pruritus, and urticarial plaques. Their findings highlighted the importance of early recognition and timely intervention, especially in older male patients. Chadha et al. [27] also underscored BP as a well-established irAE, with clinical and histological features mirroring idiopathic BP. They noted that ICI–induced BP often presents with subepidermal blistering, linear deposition of IgG and C3 at the dermoepidermal junction, and elevated anti-BP180 antibody levels, which can serve as diagnostic markers. Together, these findings reinforce BP’s significance as a key dermatologic toxicity associated with ICIs, necessitating vigilant monitoring and tailored management strategies.
Vitiligo, another prominent ASD, was frequently observed across most regimens. Previous research has described vitiligo as a marker of immune activation, particularly in melanoma patients undergoing PD-1 blockade therapy [12, 13]. Research has demonstrated that anti-PD-1 therapy induces a de novo immune response, composed of various T-cell subtypes, which may trigger both vitiligo and antitumor effects [28]. A meta-analysis revealed that vitiligo occurrence correlated with prolonged progression-free survival (PFS) and overall survival (OS) in patients with advanced melanoma [29]. In addition, a prospective observational study found that melanoma patients treated with pembrolizumab who developed vitiligo had a higher objective response rate (ORR) [30]. This suggests that the immune mechanisms leading to vitiligo are closely linked to those mediating tumor regression.
Another finding of this study is the significant association between psoriasiform dermatitis and ICIs, consistent with previous reports [31]. A substantial proportion of patients with ICI–related psoriasiform dermatitis have a personal or family history of psoriasis, indicating that ICIs may initiate or aggravate the condition in genetically predisposed individuals [32]. Research has demonstrated that ICIs activate Th1 and Th17 cells, leading to increased keratinocyte proliferation and an amplified inflammatory cascade, which contribute to the onset and progression of psoriasiform dermatitis [33]. Moreover, serum IL-6 levels are significantly elevated in patients with ICI–related psoriasiform dermatitis [34]. As a key proinflammatory cytokine, IL-6 binds to the cell surfaces or soluble receptors, triggering downstream signaling pathways that regulate immune cell proliferation, differentiation, and function. It plays a crucial role in inflammatory skin diseases. Notably, higher IL-6 levels have been linked to shorter survival [35], poorer treatment response [36], and an increased risk of irAEs [37]. These findings suggest that IL-6 may be a central driver of ICI–induced psoriasis and could influence both prognosis and treatment safety. Therefore, regular IL-6 monitoring and a thorough assessment of family history in patients undergoing ICI therapy may help mitigate potential risks.
ICI–associated LP also showed a strong statistical correlation, particularly in patients treated with nivolumab and pembrolizumab. Compared to spontaneous LP, ICI–induced LP (irLP) displays a more pronounced inflammatory gene expression profile, with elevated interferon-gamma (IFN-γ) levels and activation of the phagosome signaling pathway [38]. This mechanism may explain how ICIs intensify local skin inflammation, contributing to LP development. In addition, the reduced expression of PD-1 and PD-L1 in inflammatory infiltrates within LP lesions further supports the role of ICIs in LP pathogenesis [39].
Besides, we identified dermatomyositis as another notable irAE associated with ICIs. In our study, dermatomyositis demonstrated predominantly positive signals across the majority of disproportionality analysis algorithms, suggesting that, while rare, it remains a significant irAE linked to ICIs. Previous studies have also reported isolated cases of ICI–induced dermatomyositis, particularly in patients receiving CTLA-4 inhibitors or combination therapies. For example, Sheik et al. [40] first described a case of ipilimumab-induced dermatomyositis, characterized by classic cutaneous manifestations and proximal muscle weakness. Similarly, Marano et al. [41] documented a case in which a patient developed dermatomyositis after progressing from subacute cutaneous lupus erythematosus during PD-1 inhibitor therapy, highlighting the potential for multiple autoimmune events in certain individuals. Guerra et al. further demonstrated that ICI–associated dermatomyositis patients exhibited significantly elevated anti-TIF1γ autoantibody levels, suggesting a key role for immune dysregulation in the disease’s pathogenesis [42]. Mechanistically, ICI–induced dermatomyositis may involve enhanced immune activation and cytokine dysregulation, particularly with dual inhibition of CTLA-4 and PD-1/PD-L1 pathways. Clinically, early recognition and timely intervention are crucial, as severe cases often require systemic corticosteroids and additional immunosuppressive therapies to control symptoms and prevent complications.
The study found that scleroderma, Henoch–Schönlein purpura, pemphigus, alopecia areata, and SLE exhibited limited or inconsistent signals across different ICIs, and some of these conditions have been subject to potential debate in previous studies. For instance, Schoenberg et al. [43] reported a case of pemphigus triggered during ipilimumab treatment for melanoma, suggesting a possible link between ICIs and pemphigus. In contrast, Maeda et al. [44] described a melanoma patient with pemphigus foliaceus who underwent ICI therapy without experiencing an exacerbation, implying that ICIs may not universally worsen preexisting pemphigus. It is worth noting that some of the negative findings in our analysis may have been influenced by the limited number of cases available, which could restrict the ability to detect potential associations. Future studies with larger sample sizes may be needed to confirm these results and explore the nuanced relationship between ICIs and these autoimmune conditions.
Our analysis of the TTO for ICI–associated ASDs revealed a median onset of 143 days, with an IQR of 42–343 days. This variability underscores the necessity for ongoing vigilance throughout the treatment course.
Notably, agents such as ipilimumab and atezolizumab demonstrated a higher incidence of early-onset ASDs, as evidenced by steeper initial increases in cumulative incidence curves. This suggests that patients receiving these therapies may require more intensive monitoring during the early phases of treatment. Conversely, nivolumab and pembrolizumab were associated with more gradual onset patterns, indicating that the risk of ASDs persists over a more extended period. These findings are consistent with previous reports highlighting the early appearance of cutaneous irAEs with certain ICIs [45]. The Weibull distribution analysis further corroborated these observations, with shape parameters (k) below 1 across all ICIs, indicative of an early failure pattern where event rates decline over time. The scale parameters (λ) varied among different ICIs, aligning with the observed differences in TTO across agents. These insights emphasize the importance of tailoring monitoring strategies to the specific ICI regimen employed, ensuring prompt identification and management of ASDs to optimize patient outcomes.
This study leveraged the FAERS database to provide large-scale real-world evidence on ICIs associated with ASDs, employing four disproportionality analysis algorithms to minimize false-positive signals and enhance reliability. However, the inherent limitations of spontaneous reporting systems, including underreporting and the inability to establish causality, must be acknowledged. In addition, the exclusion of newer ICls, such as toripalimab, due to limited data may have underrepresented emerging safety signals. Future research should integrate pharmacovigilance data with clinical and genomic datasets to elucidate the mechanisms underlying ICI–associated ASDs. Stratified analyses based on cancer type, patient demographics, and treatment regimens could further refine risk profiles and guide personalized therapeutic strategies.
Ethics Statement
Ethics approval was deemed unnecessary for this study as it involved a retrospective analysis of reports registered in the FAERS.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zi-Yue Dong and Ming-Jie He contributed equally.
Funding
There was no funding for this study.
Acknowledgments
We express our gratitude for the accessibility of the FAERS database, which is made available by the FDA.
Supporting Information
Supporting Table S1: FDA–approved Common ICIs: classes, generic names, and brand names.
Supporting Table S2: Classification of common ASDs.
Supporting Table S3: Detail formulas and criteria for disproportionality analysis algorithms.
Supporting Table S4: The READUS-PV checklist.
Supporting Table S5: Detailed results of the disproportionality analysis.
Supporting Table S6: Weibull Distribution parameters for TTO analysis of ICI–related ASDs.
Open Research
Data Availability Statement
Data are available from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).




