Pharmaceutical Management of Rosacea—An Australian/New Zealand Medical Dermatology Consensus Narrative
Abstract
Rosacea, a common chronic, predominantly centro-facial dermatosis, has previously been classified into distinct subtypes with a range of morphological signs that overlap with other inflammatory skin disorders. Recently, there has been a move towards diagnosis of clinical phenotypes, largely driven by a better understanding of the pathophysiology of rosacea and clinical trial endpoints. Despite this, treatment remains a challenge. The Australasian Medical Dermatology Group held a Rosacea workshop in November 2022 to develop a practical narrative. Eighteen recommendations were agreed upon using a modified eDelphi process in the first round, including a rosacea treatment algorithm.
1. Methodology
Twelve senior medical dermatologists from Australia and New Zealand met in November 2022 to develop a practical narrative for the medical management of rosacea. Premeeting, an extensive literature search (to October 2022), was conducted for papers on individual rosacea medications, international and national rosacea guidelines and systematic reviews, including Cochrane treatment reviews, expert opinions, patient outcomes, scoring systems, pathophysiology and the rosacea microbiome, in the English, French and Spanish literature.
Search terms included rosacea (acne rosacea, ocular rosacea and perioral dermatitis), epidemiology, classification, phenotype, skin barrier, quality of life, scoring systems, microbiome, triggers, pathophysiology, skincare and individual topical treatments including artemether, azelaic acid, benzoyl peroxide, brimonidine, ivermectin, metronidazole, oxymetazoline, retinoids, timolol, tranexamic acid and individual systemic therapies including azithromycin, beta-blockers, botulinum toxin A (BTX-A), doxycycline, erythromycin, hydroxychloroquine, isotretinoin, minocycline, sarecycline, sumatriptan, tetracyclines, vitamin B12, inhibitors of Janus kinase (JAKinibs) and monoclonal antibodies. Three hundred and forty-seven full-text manuscripts were made available to the group; the Oxford evidence-based medicine (EBM) levels of evidence were used to grade the various articles. Each member of the Australasian Medical Dermatology Group was a lead discussant for a subtopic, which was followed by an in-depth discussion of the published literature and included the group’s clinical experience.
Based on the major discussions held during the workshop, 18 practice statements covering history, examination and treatment were subsequently developed by the steering committee; these were subjected to an eDelphi process. Consensus was defined as ≥ 80% agreement, with a second eDelphi round planned for any statements not achieving agreement.
1.1. Rosacea Epidemiology
There are limited specific Australian/New Zealand data on prevalence/incidence of rosacea, but internationally the prevalence has been quoted at 2.7%–10%. A recent large meta-analysis of over 26 million individuals from 41 different populations showed a pooled prevalence of 5.5% (95% CI: 4.9–6.0) [1]. Previously, many studies used selected patient cohorts, for example, Irish or Estonian populations, or single-clinic databases, that often lacked detail on how the diagnosis of rosacea was ascertained, making extrapolation to Australia/New Zealand difficult [2–4].
The emphasis on rosacea being more common in populations with Fitzpatrick skin Phototypes I–II, particularly those with a northern European and Celtic heritage, is being challenged. Recent studies that have included non-Caucasian populations show similar rates of rosacea, although diagnosis may be more difficult in skin of colour [1, 5]. Whilst rosacea is most prevalent in middle-aged females, it can be seen at any age, including younger children. The phymatous pattern seems to be more common in older men, but there is anecdotal suggestion that the prevalence of this subtype in Australia/New Zealand may be diminishing.
1.2. Clinical Diagnosis
The diagnosis of rosacea is based on clinical history (Supporting Table 1) and examination. A history of sensitivity to facial skincare products or to other irritants, and to changes in temperature or ultraviolet (UV) exposure, often predates clinical signs of rosacea by many years. Flares with periods of remission are common. As the rosacea progresses, the patient can feel as if the skin is hot or burning, but the skin can also be described as painful. Flushing can be common, although difficult to distinguish from normal physiological blushing.
Previously, rosacea was classified into subtypes displaying a range of morphological signs (Supporting Table 2) [6] which often overlapped with other inflammatory disorders of the midface, including seborrheic dermatitis, acne vulgaris, periorificial dermatitis and sensitive skin syndrome [7]. Subtypes may overlap, which has prompted the use of the predominant clinical phenotype for treatment recommendations, largely driven by clinical study endpoints [8–10]. The primary diagnostic features include persistent centro-facial erythema with periods of increased intensity and phymatous changes. Major features (signs), diagnostic when there are at least two, include flushing/transient erythema, inflammatory papules and pustules, centro-facial telangiectasia and ocular manifestations. The erythema can be challenging to see in people with skin of colour or in patients wearing makeup. Comedones should be absent, but may be present if the patient also has acne. Minor features (symptoms) include burning, stinging, swelling or dry sensation of the skin. Furthermore, each feature should be graded on a severity spectrum independent of concurrent phenotypes.
Investigations are rarely needed, although patch testing may be indicated if there is suspicion of a contact dermatitis. Dermoscopy may be useful to detect Demodex folliculorum, and occasionally, a biopsy may be indicated when clinical features overlap with other inflammatory skin diseases, or in diagnostic uncertainty, for example, cutaneous lupus erythematosus, granuloma faciale and sarcoidosis. Diascopy may also help evaluate erythema in patients with skin of colour.
1.3. Practice Statements—History and Examination (eDelphi Agreement, Supporting Table 9)
- •
Sensitivity to topically applied cosmetic preparations may predate clinical signs of rosacea by many years.
- •
Patients should be asked to bring all their facial skincare products to the consultation; these should be checked for potential irritants, as patient awareness is low.
- •
Screening for cognitive decline (by an appropriate healthcare professional) may be appropriate in older patients with recent onset rosacea.
- •
Advise patients to remove makeup at least 30 min prior to the consultation, as friction can increase erythema.
- •
Dermoscopy may be helpful in identifying Demodex folliculorum.
- •
Erythema and inflammation may be difficult to assess in skin of colour.
- •
Rosacea may coexist in patients with periorificial dermatitis, seborrheic dermatitis, irritant contact dermatitis, xerosis and acne vulgaris, with the clinical pattern changing between clinic visits.
1.3.1. Differential Diagnosis
Whilst rosacea can usually be distinguished from acne vulgaris, periorificial dermatitis and/or seborrheic dermatitis, they can occur in the same patient at the same time. The less common differential diagnoses (Supporting Table 2) also include psoriasis/sebopsoriasis, irritant and allergic contact dermatitis, granuloma faciale and lupus erythematosus.
Several comorbidities have been identified in rosacea, including migraine [11], Parkinson’s disease [12] and dementia [13], such that screening for cognitive decline may be appropriate in older patients with recent onset rosacea. The risk of rosacea is increased threefold in early ulcerative colitis; the role of Heliobacter pylori remains controversial [14].
1.4. Rosacea Pathophysiology and the Microbiome
The pathophysiology of rosacea is complex, involving many triggers, cell types and inflammatory pathways [15] (Supporting Tables 3 and 4). Multiple downstream effectors from various cell types have been associated with neurogenic inflammation in rosacea. These effectors include adenosine triphosphate (ATP), calcitonin gene–related peptide (CGRP), endothelin-1 (ET1), endothelin A receptor (ETAR), kallikrein-5 (KLK-5), cathelicidin (LL-37), matrix metalloproteinase (MMP), inflammasomes (NALP3, NACHT, LRR and PYD domain-containing protein 3), pituitary adenylate cyclase–activating peptide (PACAP), substance P (SP), transforming growth factor-beta (TGF-β), transient receptor potential (TRP), thymic stromal lymphopoietin (TSLP) and vascular endothelial growth factor (VEGF) [16–18]. These may be potential targets for treatment.
Facial erythema of rosacea occurs secondary to a variety of factors, although neurovascular dysregulation and augmented immune detection and response are pivotal components. Whilst inflammatory lesions when present may contribute to the overall facial redness of rosacea by creating perilesional erythema, persistent diffuse central facial erythema of rosacea is caused primarily by fixed vascular changes [19].
An American genome-wide association study of 22,952 individuals, 2618 with rosacea, identified a DNA variation that was intergenic between human leucocyte antigen-DRA (HLA-DRA) and butyrophilin-like 2 (BTNL2), indicating a central role of immune dysregulation in rosacea pathogenesis [20].
The histological changes observed in rosacea include inflammation (perivascular and pilosebaceous infiltrate), vascular changes and lymphatic abnormalities (dilatation), glandular changes (hyperplasia) and fibrosis. Granulomatous rosacea has a specific histology [21].
1.4.1. The Microbiome
Recent studies indicate a potential role of the skin microbiome in the pathophysiology of rosacea [5]. The microbiome is impacted by environmental factors including skin pH and exposure to both sunlight and skin irritants; it is also affected by the skin’s lipid composition, sex and ageing. Microbiome studies in patients with rosacea have shown conflicting results, which may reflect the different phenotypes, the influence of ageing and previous treatments.
- •
Demodex folliculorum: Demodex has been observed on the skin of patients with rosacea at five times the levels seen in controls. A 2017 meta-analysis (23 case–control studies, 1513 patients) showed higher rates of Demodex (OR 9.0; 95% CI, 4.8–16.9) with higher density (standard mean difference, 1.6; 95% CI, 1.1–2.1) [22]. Demodex feeds on epithelial cells and may induce a hypersensitivity response. In addition, Demodex induces an environment that allows bacilli and staphylococci to penetrate deeper into the follicles.
- •
Staphylococcus epidermidis: S. epidermidis has been observed more in rosacea lesions than on normal skin and may be more abundant in areas of more severe inflammation [5, 23].
- •
Bacillus oleronius: B. oleronius has been shown to be antigenic and increase skin temperature by inducing an active inflammatory response.
- •
There may also be roles for various Actinobacteria species, which comprise up to 15.8% of facial skin flora in rosacea, as well as species of Geobacillus and Gordania.
- •
Cutibacterium acnes and Serratia marcescens are also enriched in individuals with rosacea compared to those with acne vulgaris [24].
There is a lack of consistent findings from studies of the gut microbiome in rosacea [5, 17]. Patients with small intestinal bacterial overgrowth (SIBO) have up to a 13-fold increased prevalence of rosacea. In addition, inflammatory bowel disease shares a number of pathways, such as those involving HLA-DRB1 0301, with rosacea. Bifidobacteria BRO3 and Lactobacillus salivarius have similar inflammatory activity in the gut, brain and skin [25]. Helicobacter pylori is inconsistently linked with rosacea.
1.5. Skin Barrier
The skin barrier in patients with rosacea is impaired, with an increase in skin pH, decreased hydration and increased transepidermal water loss (TEWL). It remains unclear whether these barrier changes are a cause or an effect of the rosacea, but irrespective of this, the reduced barrier function influences both the symptoms of rosacea and the tolerability to topical treatments [26, 27].
1.5.1. Rosacea Triggers
When counselling patients, it is important to emphasise triggers that affect one patient may not affect another [18]. Temperature, weather and physical exertion are all known triggers for rosacea (Supporting Table 5); the common threads appear to be direct heat, which leads to vasodilation, reactive heat (leading to intense vasoconstriction) and humidity. Protection from UVA and UVB lights as well as infrared, which can damage collagen and is a known mast cell degranulator, is important. There is a slight decreased risk of rosacea in current smokers, but an increased risk in ex-smokers compared to never-smokers. In Australia and New Zealand, sun damage is a major precipitating factor, including for extrafacial rosacea on the shoulders and scalp.
Patients often comment that various foods, including those high in histamine, histamine liberators or mast cell degranulators, can trigger their rosacea (Supporting Table 6). Interestingly, various mast cell stabilisers, for example, brimonidine tartrate, artemether emulsion, sodium cromoglycate, hydroxychloroquine, artemisinin and tranexamic acid, have been found useful in the management of rosacea [28–30]. A 2018 survey reported that changing diet reduced the frequency of rosacea exacerbation in 73% of patients [31]. Increased alcohol intake is associated with an increased rosacea incidence of 12% (HR 1.12; 95% CI 1.05–1.20) for alcohol intake of 1–4 g/day and by 53% (HR 1.53, 95% CI 1.26–1.84) for more than 30 g/day. Wine and spirits are associated with a significantly higher risk of rosacea [32]. Studies suggest that fatty foods might exacerbate rosacea (OR = 2.49; 1.26–4.92), whilst dairy-based foods may offer some protection (OR = 0.13; 0.03–0.67) [33]. Some evidence exists supporting the use of supplementary omega-3 fatty acids in ocular rosacea [34].
Various medications may aggravate rosacea including calcium channel blockers, beta-blockers and vitamin B3/nicotinic acid [35]. Topical corticosteroids are well recognised to trigger/aggravate rosacea. Frequent physiological flushing, menopause, chronic cough and caffeine withdrawal syndrome can also influence rosacea in some patients.
Skincare products that contain irritants and allergens can disrupt the epidermal barrier, which may trigger/aggravate rosacea [27]. Alcohol and acetone can be particularly problematic, as are any substances that cause redness or stinging. Emotional triggers such as stress and anxiety have also been shown to cause neurovascular volatility and mast cell instability.
2. Management of Rosacea
2.1. General Skincare
General skincare is perhaps the most important adjuvant to pharmacological and energy-based treatments [36]. General skincare, including advice on the use of sunscreens (particularly physical blocking sunscreens), moisturisers, gentle over-the-counter cleansers and avoidance of known triggers, is essential (Supporting Table 7) [27, 37, 38]. Skincare products to avoid include astringents, toners, abrasive exfoliants and cosmetics that contain alcohol, menthol, camphor, witch hazel, fragrance, peppermint, eucalyptus and tea tree oil, waterproof cosmetics and heavy foundations that are difficult to remove without irritating solvents or physical scrubbing, alpha hydroxy acids (AHAs) or topical retinoids.
- •
Those that minimise epidermal barrier dysfunction
- •
Those with anti-inflammatory properties
- •
Those that effect vascular diameter, permeability and growth
- •
Those that modulate TLR-2 receptor expression
2.2. Topical Therapies
A number of effective topical medications are available, although access and funding may vary from country to country. In a systematic review of 152 studies with over 20,000 participants (Oxford EBM Level 1), there was high-certainty evidence that brimonidine temporarily reduced erythema and moderate certainty that topical oxymetazoline did so as well. For reducing papules/pustules, there was high-certainty evidence for the effectiveness of topical azelaic acid and topical ivermectin [9].
Topical brimonidine, an α2-adrenoceptor agonist developed for the treatment of open-angle glaucoma, was associated with two grades of improvement (scale, 0–4) of facial erythema among 114 of 227 (50%) patients compared with 54 of 276 (20%) treated with vehicle (risk ratio [RR], 2.11, 95% CI, 1.60–2.78). Brimonidine’s main indication is for background erythema/persistent facial erythema [9, 39–41] (EBM Level 1).
Oxymetazoline [42], an α1-adrenoceptor agonist, causes cutaneous vasoconstriction. Its main indication is the reduction of background erythema/persistent facial erythema, but it also reduces pro-inflammatory cytokines. In two studies, 99 of 446 patients (22%) treated with oxymetazoline 1% cream showed a two-grade improvement after 3 h compared to 59 of 439 (13%) treated with vehicle (RR 1.65, 95% CI 1.23–2.21 (p < 0.001); number needed to benefit [NNTB] 11, 95% CI 7–27). In the oxymetazoline group, 94 adverse events were reported in 446 participants compared to 70 in 439 participants in the vehicle group (RR 1.32, 95% CI 0.97–1.78), including pruritus and erythema, and worsening of inflammatory lesions which were considered mild or moderate in severity. Fortunately, rebound at the end of the treatment was low (1.2%–2.2%) [9, 43–45] (EBM Level 1).
Azelaic acid is a naturally occurring organic compound found in wheat, barley and rye. It downregulates the cathelicidin pathway [46]. In a controlled study, 15%–20% azelaic acid was associated with marked to excellent improvement in 383 of 607 patients with rosacea (63%) compared with 241 of 572 (42%) applying a placebo (RR, 1.46, 95% CI, 1.30–1.63) [9] (EBM Level 1). In a 12-week study of doxycycline 40 mg with either 15% azelaic acid gel or topical metronidazole 1% gel in patients with papulopustular rosacea, the doxycycline/azelaic combination worked more quickly, but at 3 months, there was no difference between the two treatment arms. Azelaic acid appears to be helpful for papulopustular lesion as well as reducing the background erythema, without having a negative effect on skin barrier function.
Topical ivermectin was associated with good to excellent improvement of rosacea in 615 of 910 participants (68%) versus 169 of 461 participants (37%) applying vehicle alone (RR, 1.84, 95% CI, 1.62–2.09) [9], as well as quality-of-life improvement. Topical ivermectin was associated with greater improvements in rosacea in 409 of 478 participants (86%) than with topical metronidazole in 362 of 484 participants (75%) (RR, 1.14, 95% CI, 1.07–1.22) [9, 47, 48] (EBM Level 1).
Topical metronidazole compared with placebo was associated with improved rosacea among 94 of 195 participants (48%) versus 40 of 139 participants (29%) (RR, 1.98, 95% CI, 1.29–3.02) [9] (EBM Level 1).
Investigator global assessment (IGA) success was achieved in 31% of vehicle control, 39% with minocycline 1% gel and 46% with minocycline 3% gel, in a 12-week, double-blinded study in 270 patients with papulopustular rosacea [9, 49] (EBM Level 1). The cream or foam formulations may be better tolerated than the gel [50–52].
Timolol, a potent nonselective β-blocker that causes a combination of vasoconstriction and inhibition of inflammatory mediators, has been used for rosacea [53, 54]. In one small study, 16 patients with mild-to-moderate erythrotelangiectatic rosacea (ETR) received topical timolol maleate eye drops 0.5% to one side of their face daily for 28 days and normal saline to the other side of the face. Clinician Erythema Assessment (CEA) and Patient Self-Assessment (PSA) at Day 28 improved by at least 1-point (75.0% vs. 37.5%, p < 0.05) compared to saline. Patients reported a significant difference in warmth and burning sensations (EBM Level 4).
Topical retinoids and benzoyl peroxide have generally been considered too irritant for routine use in the management of rosacea, but newer formulations may be better tolerated [55, 56]; they may also be appropriate in patients with concomitant acne (EBM Level 5).
Artemether emulsion [57], derived from the antimalarial plant Artemisia annua (1%), was studied in 130 patients with papulopustular rosacea; patients were randomised to artemether emulsion (1%) or metronidazole emulsion (3%) twice daily for 4 weeks. More patients (87%) achieved a clinical response with artemether compared to metronidazole (80%). In addition, a greater number of patients in the metronidazole group (21.6%) had relapsed 8 weeks after stopping treatment compared to only 2.4% in the artemether group (EBM Level 2).
Tranexamic acid [29] is a lysine derivative that is commonly used as an antifibrinolytic agent to reduce the risk of excessive bleeding. It has a number of anti-inflammatory effects, as well as repressing angiogenesis, by reducing the number of CD31+ cells and downregulating the expression levels of VEGF in rosacea. In an open-label study of 20 patients treated with tranexamic acid solution (either by impregnated dressings or by microneedling and impregnated dressings), IGA of Rosacea Severity Score (IGA-RSS) improved in all patients (by 2 units in the impregnated dressing group and 3 units IGA-RSS in the microneedling group). Interestingly, the improvement lasted for more than 4 months [58] (EBM Level 4).
An increasing number of natural ingredients/botanicals are being studied for the management of rosacea, but are outside the scope of this narrative [59–61].
2.2.1. Combination Therapies
The combinations of ivermectin 1% cream and brimonidine 0.33% gel [62], ivermectin 1% cream and pulsed dye laser (PDL) [63], and oxymetazoline and PDL have been found to be synergistic [64].
2.3. Systemic Therapies
Systemic therapies have become the mainstay treatment for moderate/severe rosacea and for mild rosacea that has failed to respond to topical therapies. A review of 152 studies based on a phenotype-led approach found there was moderate-to-high-certainty evidence for doxycycline 40 mg modified release and isotretinoin for reducing papules/pustules, and moderate-certainty evidence that oral minocycline was equally effective as doxycycline 40 mg modified release (EBM Level 1). There was low-certainty evidence for tetracycline and low-dose minocycline [9, 65–68]. There are a number of international guidelines which have attempted to position the different available therapies [69, 70]. Although treatment guidelines are gradually becoming more patient focussed, they still fail to acknowledge the chronicity and relapsing nature of rosacea, which are important factors in choosing the most appropriate treatment for an individual patient.
2.3.1. Antibiotics
Oral doxycycline (40 mg) compared with placebo was associated with 2 grades of improvement (scale, 0–4) among 90 of 269 participants (33%) versus 55 of 268 (21%) (RR, 1.63 [95%CI, 1.22–2.18) [9] (EBM Level 2). The combination of a modified-release doxycycline (30-mg immediate-release and 10-mg delayed-release beads) and topical ivermectin 1% cream was assessed in a 12-week, multicentre, randomised, investigator-blinded, parallel-group comparative study in 273 patients with severe rosacea. The combination was more effective and worked more quickly than topical ivermectin alone (80.3% vs. 73.6% reduction of inflammatory lesions, respectively). More patients achieved an IGA score of 0 with the combination therapy (11.9% vs. 5.1%) [71] (EBM Level 1).
A noninferiority study compared minocycline 100 mg against doxycycline 40 mg, which demonstrated similar benefits as determined by patients (excellent or good improvement (RR 1.10, 95% CI 0.72–1.67). The rate of an IGA score of 0 or 1 (clear or near clear) was slightly higher in the minocycline group (RR 3.43, 95% CI 1.67–7.04) (p < 0.001); NNTB was 2 (95% CI 2–4) [66]. In another medium-sized study, minocycline 45 mg demonstrated similar effectiveness with or without topical azelaic acid in reducing inflammatory lesions. There was a reduction of 11–12 lesions in both treatment arms [9, 67] (EBM Level 2).
Once-daily sarecycline, a narrow-spectrum tetracycline-derived antibiotic, has recently been shown to be effective in 102 patients with moderate-to-severe papulopustular rosacea, but data remain limited at this time [72] (EBM Level 2).
Nontetracycline antibiotics have also been tried. A randomised trial of 67 patients compared azithromycin 500 mg thrice weekly (on Monday, Wednesday and Saturday) for one month, followed by 250 mg thrice weekly (on Monday, Wednesday and Saturday) for a second month, and then, 250 mg twice weekly (on Tuesday and Saturday) for a third month, against doxycycline 100 mg/day for the three months. Statistically significant improvement was obtained with both drugs, but neither drug was shown to be more effective than the other [73] (EBM Level 2b). Erythromycin has also been used as an alternative to tetracyclines, but the evidence base is largely anecdotal (EBM Level 5).
However, increasing concerns around antibiotic resistance, and the possible negative effect of antibiotics on the microbiome, is slowly shifting mainstay acne and rosacea treatment away from long-term systemic antibiotics. The main place for systemic antibiotics is short-term use (up to 12 weeks of treatment) to gain rapid symptom control, which may then be maintained with topical therapies, or the patient transitioned to long-term, low-dose isotretinoin.
2.3.2. Retinoids
Oral isotretinoin has been used for more than 40 years and has become the standard of care for recalcitrant moderate/severe rosacea. Initially, high-dose isotretinoin (1 mg/kg/day) was used, but over the last 20 years, the daily dose has gradually reduced. The mechanism of action of isotretinoin remains unclear, but it is known to normalise keratinisation, improves interkeratinocyte communications, induces atrophy of sebaceous glands and has various antiangiogenesis and anti-inflammatory effects, including downregulation of Toll-like receptor two expressions and VEGF [74]. Low-dose isotretinoin may also beneficially alter the microbiome and improve barrier function.
A French multicentre trial of 4 months of isotretinoin (0.25 mg/kg/day) in 156 patients with difficult-to-treat “papulopustular” rosacea (cyclin-refractory or frequently relapsing) showed that 57.4% of isotretinoin recipients achieved a 90% reduction of baseline lesions, compared to only 10.4% of placebo (numbers needed to treat: 2.1 (95% CI 1.7–2.9) [75] (EBM Level 1). As expected, 58.3% relapsed over the subsequent 4 months (median 15 weeks). Adverse effects were minimal. Using a visual analogue scale of 0–100, higher score being better, isotretinoin-treated patients showed a median satisfaction score of 80 compared to a score of 9 in the placebo group. The median reduction in lesion count was 13 (92% reduction) in the isotretinoin-treated group and six lesions in the placebo group (36%). In another large study comparing isotretinoin 0.3 mg/kg, with doxycycline 100 mg/day tapered to 50 mg/day after 2 weeks, isotretinoin was associated with good to excellent improvement in 102 of 129 participants (79%) compared with 85 of 132 (64%) taking doxycycline (RR, 1.23 [95% CI, 1.05–1.43] [76] (EBM Level 1).
Twelve patients with very recalcitrant rosacea responded well to individualised “microdoses” of isotretinoin (0.03–0.17 mg/kg/day) [77]. Initially, the patients received 10 or 20 mg isotretinoin daily over a period of 4 or 6 months. In response to the patients’ fear of relapse at the end of therapy, isotretinoin was then reduced to an individual continuous minimal dose, with excellent results (mean DLQI was 1.2 in the isotretinoin group compared to a DLQI of 8.1 in a control group of patients with rosacea) (EBM Level 4).
Another small study compared once-weekly doses of isotretinoin, 20 mg/week (n = 24) or 40 mg/week (n = 28), against minocycline 100 mg/day (n = 24) for 4–7 months. Isotretinoin 40 mg once per week was highly effective for severe rosacea, achieving complete response (over 90% improvement) in 62.5% of patients and partial response (50%–90% improvement) in additional 29.2% of patients. Isotretinoin 20 mg once per week and 100 mg/day minocycline showed comparable efficacy for mild-to-moderate rosacea (complete response of 10.7% vs. 8.3% and partial response of 28.6% vs. 33.3%, respectively) [78] (EBM Level 4). There are a number of other small studies showing benefit from low-dose isotretinoin in the management of rosacea [79–84] (EBM Level 4).
As relapse is common, treatment may need to be continued for many years, allowing the patient to stop and start as needed (expert opinion—EBM Level 4). There is no need to stop low-dose isotretinoin for planned laser or other energy-based treatments [85] (EBM Level 2). Minimal monitoring is required at this low dose, but pregnancy prevention must be maintained.
2.3.3. Other/Novel Systemic Therapies
Beta-blockers can be of benefit in the management of flushing in some patients, particularly if flushing is associated with anxiety (EBM Level 4). Similarly, clonidine has been shown to benefit some patients (EBM Level 4).
Hydroxychloroquine has a subsequent line of treatment role in patients with recalcitrant papulopustular rosacea. A randomised, double-blind, double-dummy, pilot study showed noninferiority of oral hydroxychloroquine (200 mg/day) to doxycycline (100 mg once daily) in 66 patients with rosacea [28] (EBM Level 2).
Sumatriptan, a serotonin 5-HT1B/1D receptor agonist, used for the treatment of migraine, has also been studied in rosacea [86]. An intravenous infusion of 4 mg sumatriptan reduced PACAP-38-induced facial skin blood flow for 50 min (p = 0.023), constricted the superficial temporal artery for 80 min (p = 0.010) and reduced duration of flushing (p = 0.001) and facial oedema (p < 0.001) in 35 patients with rosacea (EBM Level 4).
Hydroxocobalamin/vitamin B12a (1–2 mg) [87] was injected intramuscularly 1 to 4 weekly in 13 patients with ETR. Thirty minutes after the first dose, the mean CEA had reduced significantly from 2.2 ± 0.6 to 1.2 ± 0.4 (p < 0.001), and the average skin surface temperature fell slightly from 36.7 ± 0.70°C to 36.2 ± 0.61°C (p < 0.001) on the cheeks. It is speculated that this may work via inhibition of inducible nitric oxide synthase (NOS) (EBM Level 4).
Another novel treatment might be BTX-A. In a small open-label study, 16 patients with rosacea and erythema/telangiectasia were injected with BTX-A at 1-cm intervals. One month after injection, CEA scores improved from 2.9 ± 0.6 to 1.0 ± 0.4 and mean flushing scores improved from 47.8 ± 8.7 to 18.7 ± 3.9, which lasted for 3–6 months [88] (EBM Level 4).
Newer potential treatments include monoclonal antibodies that target IL-17, CGRP receptor (erenumab), or directed against SIBO, and by microbiome modification, but evidence needs to improve. The JAK inhibitors may also play a role in future treatment of rosacea, particularly in patients who also have other dermatological disorders including atopic dermatitis and alopecia areata.
2.4. Practice Statements—Treatment Implications (eDelphi Agreement, Supporting Table 9)
- •
Treatment should be started when the rosacea affects the patient’s quality of life.
- •
The diverse triggers and pathways of rosacea demonstrate the need to individualise treatment regimens.
- •
Treatment should focus on the patient’s symptoms and phenotype.
- •
The chronicity and the relapsing nature of rosacea are important factors in choosing the most appropriate treatment for an individual patient.
- •
Treatment success is determined by the patient’s satisfaction in the improvement in their quality of life.
- •
Inadequate response is defined by the patient’s dissatisfaction in the improvement of their symptoms.
- •
Most treatments should be trialled for 3 months, before another treatment is added or substituted.
- •
Maintenance treatment should be continued for 12 months in the first instance, but can be intermittent. Some patients may need 10–20 years of maintenance treatment.
- •
The large number of potential pathophysiological/aetiological targets means new and more effective therapies are likely to emerge.
- •
At this time, the skin microbiome is influenced by the use of topical antibiotics, topical ivermectin and systemic antibiotics, but the exact mechanisms of action remain unclear. It is possible that in the future, microbiome therapy may become possible, either directed against the skin or the gut.
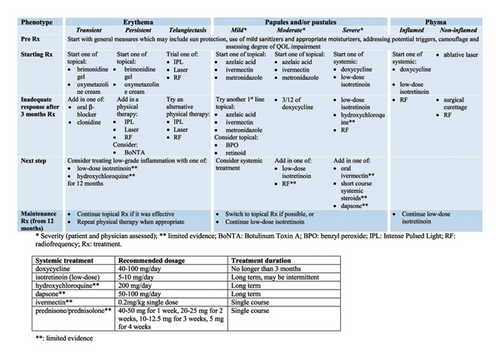
- •
The Australasian Medical Dermatology Group endorses the suggested treatment algorithm for the medical management of adolescents and/or adults with rosacea (Figure 1).
2.5. Physical/Energy-Based Devices
There are an increasing number of studies of the use of energy-based devices in the management of rosacea, either on their own or in combination with topical and/or systemic therapies, but these fall out of the remit of this discussion [89, 90].
3. Discussion
The management of rosacea is complex and needs to be individualised to the patient (Figure 1). Similar to the recent shift in management of atopic eczema and psoriasis to become more patient-focussed, the same is necessary for rosacea. Diagnosis is often delayed, as symptoms may be intermittent, or signs difficult to see, particularly in skin of colour. It is important to recognise that rosacea is a chronic disease and can coexist with acne vulgaris, seborrheic dermatitis and periorificial dermatitis.
Treatment of rosacea should be targeted to patients’ symptoms, recognising that symptoms may vary over the course of the disease. The definition of severity (mild, moderate or severe) should reflect the patients’ interpretation, rather than a specific clinical score. Use of an appropriate validated quality-of-life questionnaire (e.g., DLQI, RosaQoL) should be considered [91].
For mild disease, good skincare and avoidance of irritants/aggravating factors for the specific patient may suffice. As rosacea becomes more symptomatic, topical treatments are indicated. There is no specific recommended algorithm, as the treatment chosen should target the patients’ specific symptoms (flushing, itch/pain) and signs (erythema, papules/pustules and phyma); however, Figure 1 shows a possible approach for patients (note, not all treatments may currently be available or funded). The precise role of physical therapies including intense pulsed light (IPL), vascular laser and radiofrequency (RF) as adjunct treatments of rosacea will benefit from further well-designed studies. IPL and vascular laser have an established role for treating associated telangiectatic vessels and improving diffuse facial erythema. Their role in controlling inflammatory lesions of rosacea remains to be established; however, RF needling is also beginning to show some promise. Ablative lasers remain the current standard of care in phymatous rosacea in combination with medical treatment (low-dose isotretinoin).
If the patients’ symptoms are not adequately controlled with good skincare and topical therapies or they relapse very quickly on stopping, early addition of systemic treatments should be considered. The conventional first-line systemic therapy of antibiotics is being reconsidered because of concerns regarding antibiotic resistance and the potential negative effect on the skin and gut microbiome. The current alternative treatment is low or very low-dose isotretinoin (e.g., 5–10 mg/day or even less), but this has well-known challenges. However, low-dose isotretinoin has the potential for treating all rosacea phenotypes including ocular rosacea. Less evidence-based systemic alternatives are hydroxychloroquine or beta-blockers. Treatment may need to be continued for many years, but it is sensible to allow patients to stop and start at their discretion (but pregnancy avoidance remains crucial even for very low-dose isotretinoin).
The role of the new immune modulators such as JAK inhibitors and monoclonal antibodies has yet to be established, but their significant costs may prove prohibitive; in addition, the consideration of the potential immuno-modulatory adverse effects will need to be balanced against disease severity and impact.
Conflicts of Interest
Marius Rademaker reports acting as an investigator for AbbVie, Akesio, Aslan, Boehringer Ingelheim, Botanix, Douglas, Galderma, Hexima and Suzhou Connect. Peter Foley reports acting as a consultant, investigator, speaker and/or advisor, and/or received travel grants for 3M/iNova/Valeant, Abbott/AbbVie, Amgen, Arcutis, Argenx, Aslan, AstraZeneca, Biogen Idec, BMS, Boehringer Ingelheim, Botanix, Celgene, Celtaxsys, Cutanea, Dermira, Eli Lilly, Evelo, Galderma, GSK/Stiefel, Genentech, GeneSeq, GenesisCare, Hexima, Incyte, Janssen, Kymab, LEO/Peplin, Mayne Pharma, MedImmune, Novartis, Regeneron, Reistone, Roche, Sanofi Genzyme, Schering-Plough/MSD, Sun Pharma, Teva, UCB and Wyeth/Pfizer. John Sullivan reports acting as an investigator, advisory board and speaker for AbbVie, Novartis, Janssen and Candela. Katherine Armour reports acting as a speaker and/or member of advisory board for AbbVie, Janssen-Cilag, Novartis and Sun Pharma. Christopher Baker reports acting as a clinical investigator, speaker and/or advisory board for AbbVie, Janssen, Novartis and Pfizer. Annette Ferguson reports acting as a clinical investigator for AbbVie. Kurt Gebauer reports acting as a consultant, investigator, speaker and/or advisor, and/or received travel grants for 3 M/iNova/Valeant, Abbott/AbbVie, Amgen, Biogen Idec, BMS, Boehringer Ingelheim, Celgene, Celtaxsys, Cutanea, Dermira, Eli Lilly, Galderma, GSK/Stiefel, Janssen, LEO/Peplin, Novartis, Regeneron, Sanofi Genzyme, Schering-Plough/MSD, Sun Pharma, UCB and Wyeth/Pfizer. Gillian Marshman reports acting as clinical investigator for AbbVie and Novartis. Diana Rubel reports acting as clinical investigator and/or advisory board for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis and Sun Pharma. Li-Chuen Wong reports acting as advisory board for Pfizer. Monisha Gupta and Erin McMeniman declare no conflicts of interest.
Author Contributions
M.R. prepared the manuscript. All authors helped design the study, performed the literature search, were involved in the discussion, voted in the eDelphi process and reviewed and approved the final manuscript.
Funding
There was no funding for this study. AbbVie Pty Ltd (Australia) provided an unrestricted educational grant to the Australasian Medical Dermatology Group which covered the costs of holding the workshop. AbbVie Pty Ltd (Australia) had no influence in the Rosacea Workshop, nor in the discussion, eDelphi voting or preparation of this manuscript.
Acknowledgements
We are grateful for Catherine Panwar, independent medical writer, for taking notes during the meeting. She was not otherwise involved in the preparation of this manuscript.
Supporting Information
Supplementary Tables 1–9, Supporting Table 1: Important questions to ask patients with rosacea. Supporting Table 2: Differential diagnosis of rosacea. Supporting Table 3: Potential contributors to the development of rosacea. Supporting Table 4: Common triggers, pathways and current therapeutic regimes. Supporting Table 5: Environmental factors that may aggravate rosacea. Supporting Table 6: Foods and beverages that may aggravate rosacea. Supporting Table 7: Skincare advice for patients living with rosacea. Supporting Table 8: Cosmeceuticals in rosacea. Supporting Table 9: Modified eDelphi Practice Statements.
Open Research
Data Availability Statement
The data supporting this narrative review are from previously reported studies and datasets, which have all been cited.