A Retrospective Study to Investigate Safety of Receiving Interleukin-17 Monoclonal Antibody for Psoriasis Patients With Hepatitis B Virus
Abstract
Background: The increasing utilization of interleukin-17 (IL-17) monoclonal antibody (MA) for psoriasis treatment, coupled with the high prevalence of hepatitis B virus (HBV), underscores the need for comprehensive safety data. This retrospective study aims to evaluate the safety of IL-17 MA treatment in psoriasis patients with concurrent HBV infection.
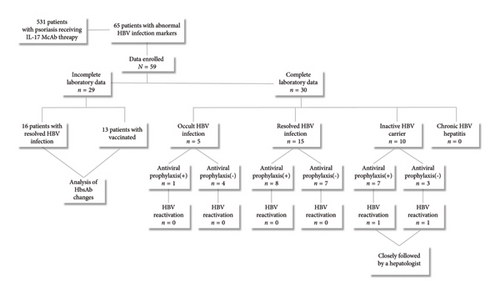
Research Design and Methods: The study screened 531 psoriasis patients treated with IL-17 MA, ultimately enrolling 59 patients with abnormal HBV serological data. Outcomes assessed include HBV virological reactivation and changes in HBsAb serum quantification post-IL-17 MA therapy.
Results: Laboratory data revealed HBV virological reactivation in two psoriasis patients classified as inactive HBV carriers (IBCs). Patients who had previously received HBV vaccination exhibited a significant decrease in HBsAb serum quantification following IL-17 MA therapy.
Conclusion: IL-17 MA therapy presents a potential risk of HBV reactivation in psoriasis patients with HBV infection. Furthermore, IL-17 MA appears to weaken HBV resistance in vaccinated patients.
1. Introduction
Psoriasis is a common chronic dermatological condition characterized by erythematous, squamous plaques on various body areas. This disorder is influenced by significant genetic predisposition and aberrant immune activation, which are key pathogenic features. Approximately 60 million individuals worldwide are affected by psoriasis [1, 2]. As our understanding of the pathophysiology of psoriasis deepens, an array of cytokines have been identified as crucial players in the disease’s etiology, functioning as both mediators and immunomodulatory agents. These include tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12), interleukin-23 (IL-23), and interleukin-17 (IL-17). These cytokines have emerged as promising therapeutic targets, and monoclonal antibodies (MA) directed against them have been developed over the past decade [2, 3]. Secukinumab (SEC), Ixekizumab (IXE), Brodalumab, and Bimekizumab are IL-17 inhibitors currently utilized in the clinical management of psoriasis. Their efficacy has been substantiated through numerous clinical trials [3, 4]. Notably, SEC and IXE were approved for the treatment of psoriasis in China in May 2019 and August 2019, respectively, and have been incorporated into the National Basic Medical Insurance Drug List.
The role of IL-17 in the mechanism of host defense involves stimulating monocytes, dendritic cells, Kupffer cells, and biliary epithelial cells to produce proinflammatory cytokines and chemokines. Some research has revealed that intrahepatic and peripheral IL-17 was overexpressed in patients with HBV infection, suggesting that Th17/IL-17 may play a significant role in suppressing HBV activity [5]. Furthermore, Gao et al. analyzed the ratio of regulatory T cells to T helper 17 cells in liver transplant patients with HBV infection, concluding that changes in this ratio were related to HBV elimination and could contribute to the chronicity of the infection [6]. HBV–deoxyribonucleic acid (DNA) can persist within the hepatocyte nucleus, even during clinical remission of HBV infection. This persistent HBV–DNA can serve as a potential source for HBV reactivation when IL-17 monoclonal antibody (MA) is employed. There may be multiple factors contributing to HBV reactivation. Recently, a meta-analysis conducted by Jamaly et al. aimed to determine the prevalence of HBV reactivation with TNF-α and IL-12/23 inhibitors, finding that the prevalence could differ based on HBV infection state, prophylactic antiviral therapy, and biologic regimen [7]. The serum quantification of hepatitis B surface antibody (HBsAb) has been considered protective against HBV infection, with previous research suggesting that low HBsAb serum quantification contributes to an elevated risk for HBV reactivation [8, 9]. Pe´rez-Alvarez et al. found that patients with HBsAb + showed a 7-fold lower reactivation possibility compared to hepatitis B surface antigen (HBsAg) + carriers [10].
According to the latest report from the World Health Organization, 228–423 million people are living with chronic hepatitis B virus (HBV) infection worldwide. New estimates indicate that approximately 1.5 million people newly acquire hepatitis B infection annually [11]. However, there is limited safety data for the treatment of IL-17 MA in psoriasis patients with concurrent HBV infection, as these patients are typically excluded from pivotal clinical trials [11]. Consequently, we conducted this single-center retrospective study to assess the safety of IL-17 MA therapy in psoriasis patients with HBV infection in a real-life scenario.
2. Methods
2.1. Patients
This retrospective study included psoriasis patients aged 18–70 years who were treated with IL-17 MA from September 2019 to December 2023 at a tertiary medical center in China. Prior to IL-17 MA treatment, all patients underwent comprehensive screening, including blood cell count, urinalysis, liver and renal function tests, albumin levels, fasting blood glucose (FBG), electrolyte analysis, autoantibody detection, T-spot tuberculosis (TB) test, and screening for syphilis, human immunodeficiency virus (HIV), HBV, and hepatitis C virus (HCV). In addition, chest computed tomography (CT) scans were performed. Exclusion criteria encompassed autoimmune diseases (such as rheumatoid arthritis and ankylosing spondylitis), HIV infection, HCV infection, TB infection, syphilis infection, decompensated liver disease, kidney failure, pregnancy, and malignancies. Notably, patients with abnormal HBV serological data were included in this study.
All patients received at least one dose of IL-17 MA. SEC was administered subcutaneously at 300 mg or 150 mg doses at Weeks 0, 1, 2, 3, and 4, followed by every four weeks thereafter. IXE was administered subcutaneously as a 160 mg loading dose, followed by 80 mg doses at Weeks 2, 4, 6, 8, 10, and 12, and then every four weeks thereafter.
2.2. Screening of HBV Infection
All patients initiating IL-17 MA underwent prescreening for HBV infection markers, including HBsAg, HBsAb, hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb), and hepatitis B core antibody (HBcAb). All patients were tested for hepatitis markers, including alanine transaminase (ALT) and aspartate transaminase (AST). We categorized HBV infection into four groups based on previous studies [12–15]: (i) occult HBV infection (OBI), (ii) resolved HBV infection (RBI), (iii) inactive HBV carrier (IBC), and (iv) chronic HBV hepatitis (CBH). In addition, we classified patients with solely HBsAb–positive results as “HBV vaccinated” (Table 1). Patients with positive HBsAg underwent further screening for HBV–DNA serum load.
| Infection category | HBsAga | HBsAb | HBeAg | HBeAb | HBcAb | HBV–DNAb | ALT/ASTc |
|---|---|---|---|---|---|---|---|
| Vaccinated | − | + | − | − | − | − | Normal |
| OBI | − | − | − | ± | + | − | Normal |
| RBI | − | + | − | ± | + | − | Normal |
| IBC | + | − | − | ± | + | − or < 2 × 104 IU/mL | Normal |
| CBH | + | − | ± | ± | + | > 2 × 107 IU/mL | Elevate |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CBH, chronic HBV hepatitis; HBcAb, hepatitis B core antibody; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; IBC, inactive HBV carrier; OBI, occult HBV infection; RBI, resolved HBV infection.
- aReference value range of HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb: 0∼1 COI, 0∼10 IU/L, 0∼1 COI, > 1 COI, and > 1 COI, respectively.
- bThe lowest detection limit value is 20 IU/mL.
- cReference value range is > 42 IU/L.
2.3. Prophylactic Antiviral
According to the domestic “Psoriasis Biologics Treatment Guidelines” and “Guidelines for the prevention and treatment of chronic hepatitis B” in mainland China [14, 16], patients with HBV infection are referred to hepatologists and advised to undergo prophylactic antiviral therapy: either entecavir (ETV) 0.5 mg or tenofovir disoproxil fumarate (TDF) 300 mg orally daily. In this study, CBH is considered a contraindication for IL-17 MA treatment. For cases of IBC with “detectable” HBV–DNA load, patients receive antiviral drugs until the HBV–DNA load becomes “undetectable,” after which IL-17 MA is initiated. As per China’s reimbursement policy in 2019, ETV and TDF were limited to active HBV infection and prevention of mother-to-child HBV transmission. Although the medical insurance policy improved in 2022, some patients still decline prophylactic antiviral therapy for personal reasons. These patients undergo regular review by hepatologists.
2.4. Follow-Up Strategy and Outcome
All psoriasis patients with concurrent HBV infection are recommended to undergo monitoring of HBV antigen/antibody, serum HBV–DNA load, and transaminase levels during IL-17 MA therapy. The electronic medical records of eligible patients were reviewed for baseline and follow-up laboratory data. The primary outcome is the occurrence of HBV virological reactivation, defined as an increase of at least 10 folds or a change from “undetectable” to “detectable” status in the HBV–DNA load. Hepatitis was defined as an ALT or AST increase of ≥ 3 times from baseline, or an absolute increase of ≥ 100 IU/L [12, 17, 18]. The secondary outcome is the difference in HBsAb serum quantification before and after IL-17 MA therapy. The analysis of the results depends on the availability of data; however, follow-up laboratory data were not always available due to patient compliance issues.
2.5. Statistical Analysis
Data integration and statistical analysis were conducted using Excel (MS Office, 2016) and Prism 9 (GraphPad Software, 2022). Measurement data were presented as mean ± standard deviation (SD) and analyzed using t-tests to assess differences between groups. Counting data were expressed as percentages, and comparisons of distributions were performed using the chi-square test or Fisher’s exact test, as appropriate. A significance level of p = 0.05 was established for all statistical analyses performed.
3. Results
Following the evaluation, 531 psoriasis patients who received IL-17 MA were screened for this study. None of the patients exhibited autoimmune diseases, decompensated liver disease, kidney failure, syphilis infection, HIV infection, pregnancy, or tumors. Seven patients presented with positive HCV–IgM, and 31 patients with TB were excluded. Among the remaining 493 patients, 65 were found to have abnormal HBV serological markers. Ultimately, data from 46 patients with HBV infection and 13 patients with HBV vaccination were included (Figure 1). The demographic data, prior systemic treatments, and IL-17 MA therapy details are summarized in Table 2.
| Clinical feature | OBIs n = 5 | RBIs n = 31 | IBCs n = 10 | CHBs n = 0 | Vaccinated n = 13 |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, year | 48.40 ± 16.41 | 41.23 ± 10.24 | 47.60 ± 14.08 | — | 34.62 ± 14.13 |
| Sex ratio (male/female) | 4/1 | 19/12 | 8/2 | — | 10/3 |
| Duration of psoriasis, year | 24.00 ± 13.58 | 10.45 ± 6.97 | 12.20 ± 12.21 | — | 7.92 ± 5.36 |
| Prior systemic treatment, n (%) | |||||
| Acitretin | 5 (100.00) | 11 (35.48) | 3 (30.00) | — | 2 (15.38) |
| Methotrexate | 0.00 | 5 (16.13) | 3 (30.00) | — | 4 (30.77) |
| TNF-α inhibitor | 0.00 | 4 (12.90) | 1 (10.00) | — | 1 (7.69) |
| Traditional Chinese medicine | 0.00 | 11 (35.48) | 3 (30.00) | — | 6 (46.15) |
| Treatment of IL-17 MA∫ | |||||
| IXE, n (%) | 1 (20.00) | 5 (16.13) | 2 (20.00) | — | 0.00 |
| SEC, n (%) | 4 (80.00) | 26 (83.87) | 8 (80.00) | — | 13 (100.00) |
- Note: “—” not available.
- Abbreviations: CBH, chronic HBV hepatitis; IBC, inactive HBV carrier; IXE, ixekizumab; OBI, occult HBV infection; RBI, resolved HBV infection; SEC, secukinumab.
- ∫: None of the patients received additional immunosuppressants.
3.1. Follow-Up Outcome of HBV Reactivation
Among the 5 patients with OBI, the mean duration of IL-17 MA therapy was 14 ± 6 months. For the 15 patients with RBI, the mean duration of IL-17 MA therapy was 11 ± 11 months. Overall, after a mean duration of IL-17 MA therapy of 11 ± 10 months, none of the 20 patients with OBI/RBI (HBsAg- and HBcAb+) experienced HBV reactivation. Of these patients, 9 used antiviral drugs (ETV, 4 ± 2 months), while 11 did not (Figure 1). Of the 10 IBC patients, 3 had “detectable” HBV–DNA loads at baseline but seroconverted to negative after antiviral treatment (ETV for 1 month) before IL-17 MA initiation. Seven of these patients used antiviral drugs (ETV or TDF, 3 ± 1 months), while 3 did not. After a mean duration of IL-17 MA therapy of 9 ± 7 months, 2 of the 10 IBC patients experienced HBV reactivation: One male patient with a baseline HBV–DNA load of 1.29 × 104 IU/mL received ETV therapy for 1 month, resulting in “undetectable” HBV–DNA load. He then discontinued ETV therapy independently, and his HBV–DNA load was found to be 2.64 × 103 IU/mL after 25 months of IL-17 MA treatment. Another female IBC patient with a baseline HBV–DNA load of 4.66 × 103 IU/mL received ETV therapy for 1 month, achieving “undetectable” HBV–DNA load. She continued ETV therapy for 3 months concurrently with IL-17 MA therapy but discontinued ETV independently. Her HBV–DNA load was detected at 3.07 × 103 IU/mL after 5 months of IL-17 MA treatment. Neither patient exhibited elevated transaminase levels or hepatitis symptoms. Nevertheless, both were referred to hepatologists for further management: they discontinued IL-17 MA treatment, with the male patient resuming ETV and the female patient initiating TDF. One month later, both patients’ HBV–DNA loads were seronegative.
3.2. Changes in HBsAb Serum Concentration in Patients With RBI or HBV Vaccination
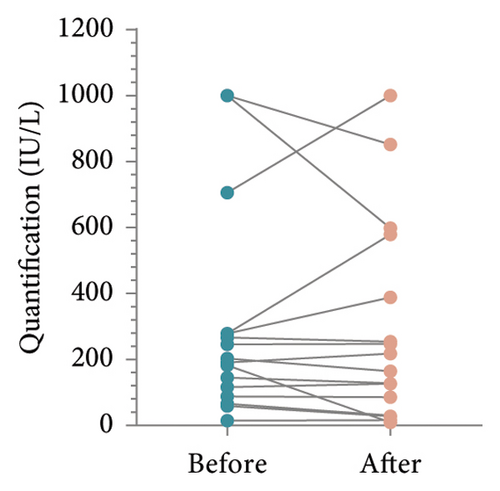
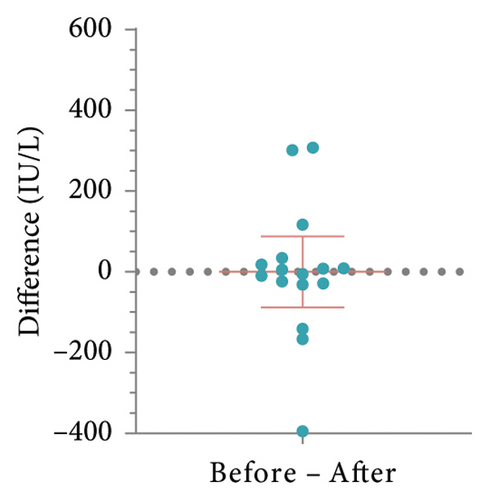
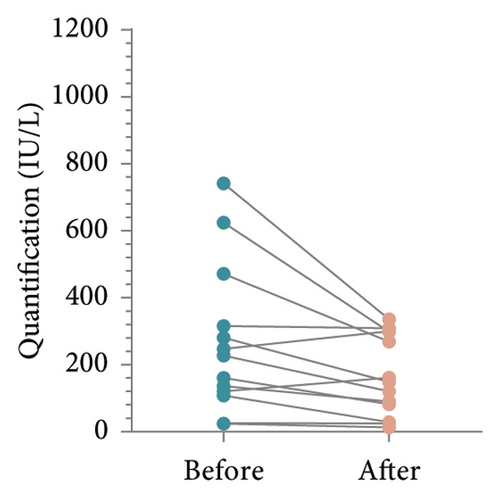
To determine whether patient’s HBsAb serum concentration was significantly reduced after using IL-17 MA, we analyzed HBsAb serum concentration in patients with RBI and those who received HBV vaccination (Figure 1). For 16 patients with RBI, the mean HBsAb serum concentration before and after IL-17 MA therapy was 302.4 ± 314.4 IU/L and 295.2 ± 307.3 IU/L, respectively. The mean difference between these values was 7.15 IU/L (95% CI: −80.58–94.88, p = 0.86) (Figures 2(a) and 2(b)). In 13 patients who received HBV vaccination, the mean HBsAb serum concentration before and after IL-17 MA therapy was 267.9 ± 221.9 IU/L and 167.7 ± 120.0 IU/L, respectively. The mean difference between these values was 110.1 IU/L (95% CI: 16.64–183.70, p = 0.02) (Figures 2(c) and 2(d)).
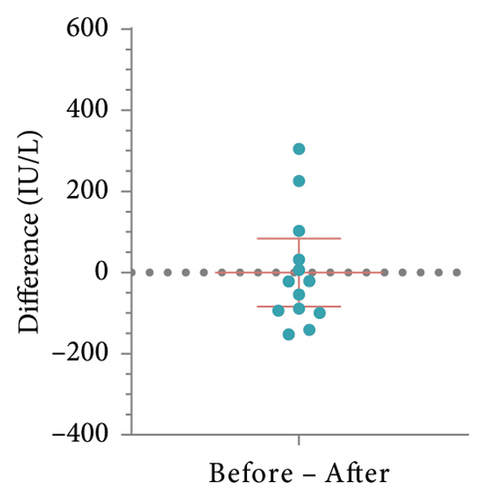
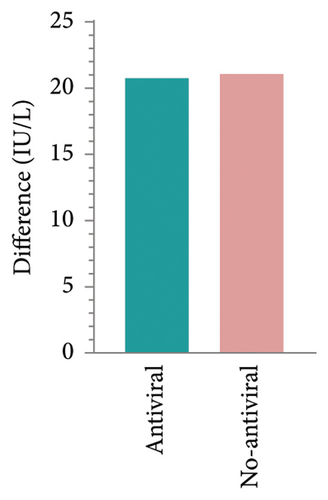
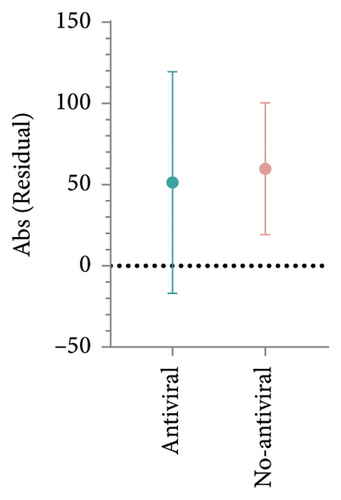
HBsAb serum concentration data from an additional 15 RBI patients were collected to analyze the effects of antiviral prophylaxis on HBsAb changes induced by IL-17 inhibitors, with antiviral or no-antiviral treatment as the grouping factor. The mean change in HBsAb among 8 patients receiving antiviral treatment was 20.76 IU/L (95% CI: −52.46–93.97), while for the 7 patients without antiviral treatment, it was 21.06 IU/L (95% CI: −52.46–93.97). No significant difference was observed between the two groups (p = 0.99, Figures 2(e) and 2(f)).
4. Conclusion
After a mean duration of IL-17 MA therapy for 15 ± 2 months, two patients with IBC experienced HBV reactivation, possibly due to poor compliance with antiviral therapy. IL-17 MA therapy presents a certain risk for psoriasis patients with HBV infection, particularly in patients with IBC. Consequently, appropriate screening, prophylactic antiviral treatment, and monitoring strategies are necessary when administering treatment to psoriasis patients with HBV infection. Based on the changes in HBsAb serum concentration in patients with RBI and those vaccinated, the resistance to HBV in RBI patients appears unaffected by IL-17 MA. However, IL-17 MA therapy weakened HBV resistance in vaccinated patients. In addition, prophylactic antiviral treatment did not significantly impact HBsAb changes induced by IL-17 inhibitors.
5. Discussion
HBV is a hepatotropic DNA virus. Its genome encodes HBsAg, HBcAg, HBeAg, viral polymerase, and HBx protein. The natural history of HBV infection is largely determined by virus-host interactions. After acute infection, the majority of patients eliminate the virus (OBI/RBI). However, a subset of patients progresses to chronic HBV infection (RBI/CBH) [10, 14]. The European Association for the Study of the Liver (EASL) recommends that HBsAg–positive patients preparing for immunosuppressive therapy should receive ETV, TDF, or TAF as prophylaxis or treatment. This should continue for at least 12 months after discontinuation of immunosuppressive therapy. For HBsAg–negative and anti-HBc–positive patients, the administration is based on detailed clinical context [15]. Similarly, the American Gastroenterological Association (AGA) recommends antiviral prophylaxis for HBsAg–positive patients considering immunosuppressive therapy, continuing for at least 6 months after cessation of immunosuppressive treatment. For HBsAg–negative and anti-HBc–positive patients, the same administration as HBsAg–positive patients is recommended under certain conditions, such as the use of > 20 mg of prednisone daily or anthracycline derivatives [19]. Regarding monitoring during immunosuppressive therapy, the AGA emphasizes monitoring HBV–DNA levels for early detection, while the Asia Pacific Association for the Study of the Liver (APASL) considers monitoring ALT/AST sufficient, with antigen and HBV–DNA checks only if ALT > 2 × baseline [20]. Current guidelines emphasize the necessity of screening and risk stratification for HBV infection prior to initiating biologic therapy, with management strategies tailored according to identified risk categories. However, there is a notable absence of specific guidance for IL-17 MA users.
The AGA categorized the risk of HBV reactivation as low (< 1%), moderate (1%–10%), or high (> 10%) [19]. Recent studies have investigated the risk of HBV reactivation in patients with psoriasis undergoing treatment with IL-17 MA. A well-designed prospective cohort study reported that 6 patients with IBC who did not receive antiviral treatment experienced viral reactivation, suggesting a reactivation rate of 27% (6/22) for IBC. Only one case of viral reactivation was observed among OBI and RBI patients without prophylaxis antiviral [12]. Qin et al. reported no HBV reactivation in 4 IBC patients, 2 OBI patients, and 14 RBI patients receiving SEC, with or without prophylaxis antiviral [17]. Another retrospective study observed one case of viral reactivation and hepatitis in an RBI patient who did not receive prophylaxis antiviral [18]. Relatively few studies have assessed the prevalence of HBV reactivation among different biologics in psoriasis. Chiu et al. suggested that HBV reactivation occurred more frequently in patients receiving TNF-α inhibitors (42.9%) compared to IL-12/23 (29%) or IL-17 (27%) MA [21]. Gargiulo et al. reported no HBVr in 20 patients receiving IL-23 inhibitors without prophylaxis antiviral [20]. However, a meta-analysis revealed similar prevalence of HBV reactivation between TNF-α and IL-12/23 inhibitors [7]. In our study, 20 patients with HbsAg–negative and HBcAb–positive status, with or without prophylaxis antiviral, showed no HBV reactivation. Two patients with HbsAg–positive status and high baseline HBV–DNA load experienced viral reactivation, both demonstrating poor compliance with antiviral drugs: one without treatment and another with only 3 months of treatment. The necessity of prophylaxis antiviral for HbsAg–negative and HBcAb–positive patients remains unclear. A retrospective study found no HBVr in such patients after various biologics therapy [22]. However, prophylaxis may be compulsory for patients with positive HbsAg or abnormal baseline HBV–DNA load, as previous research suggested these factors might be risks for reactivation [21, 23].
Currently, there is limited research analyzing the safety of IL-17 MA for psoriasis patients with concurrent HBV infection, resulting in few specific and detailed recommendations. The optimal strategy for managing psoriasis patients with HBV infection under IL-17 MA therapy remains a complex decision-making process. Factors such as HBV infection type, screening procedures, antiviral regimens, duration and dosage of biologic therapy, and the frequency of viral load monitoring can influence the incidence of HBV reactivation, making it challenging to obtain high-quality evidence. Nevertheless, based on our management experiences (Figure 3), we propose an administration strategy that emphasizes accurate screening, appropriate prophylactic antiviral therapy, and periodic monitoring [24, 25]. We stress the importance of mandatory prophylactic antiviral treatment for IBC patients and highlight the significance of monitoring HBV–DNA load. Furthermore, robust evidence may require large-scale, well-designed studies in the future to address these complex issues comprehensively.
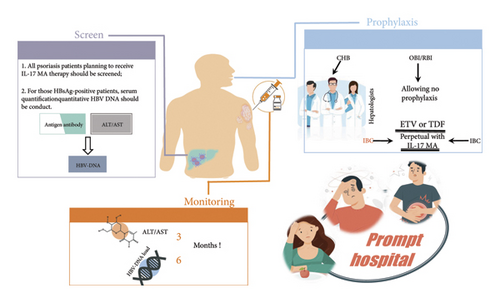
A notable strength of our study is the inclusion of a population often excluded from or underrepresented in conventional clinical trials, providing data from a retrospective analysis of these patients in real-world clinical practice, which offers a more representative perspective. However, our study has several limitations: There are fewer rescreening laboratory data compared to prescreening, likely due to cost constraints and patient compliance issues. In addition, the research method is of lower grade and lacks a comparison group for prophylactic antiviral treatment versus no prophylaxis, as well as a comparison group for reactivation rates among different infection types. Furthermore, the follow-up time was insufficient. When analyzing changes in HBsAb serum quantification, potential bias may exist due to the duration of L-17 MA therapy and sample size limitations. Nevertheless, this comparison is pioneering among homogeneous studies.
Ethics Statement
Ethics approval was obtained from the Ethics Committee of Southwest Hospital ((B)KY2023152). This study has been registered with the Medical Research Registration and Filing Information System of China (https://www.medicalresearch.org.cn/login); the registration number is MR-50-23-051172.
Consent
Patients were not required to give informed consent to the study, as each patient consented to follow-up during treatment of the IL-17 MA, as well as anonymized data obtained from clinical records were collected by the institution. The manuscript has been approved for submission by all the authors, and that all the conditions as previously stated by the ICMJE have been met.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China under grant no. 82273532.
Acknowledgments
We would like to thank TopEdit (https://www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Open Research
Data Availability Statement
The (electronic medical record) data used to support the findings of this study may be released upon application to the Ethics Committee of Southwest Hospital, which can be contacted at the following address: Gaotanyan Main Street 30, Shapingba District, Chongqing 400,038, China; Tel: 023-68754035; Fax: 023-68754035. We will provide the processed data in XLSX form once authorized by the institution.




