A Randomized Controlled Study of the Efficacy of a Topical Antifungal, Antimicrobial, Anti-Inflammatory, and Antiseborrheic Cream in Treating Mild to Moderate Facial Seborrheic Dermatitis
Abstract
Seborrheic dermatitis (SD) presents treatment challenges due to its complex pathogenesis. A nonsteroidal cream (NSC) with antifungal, antimicrobial, anti-inflammatory, and antiseborrheic properties containing piloctone olamine, biosaccharide gum-2, stearyl glycyrrhetinate, and zinc PCA has shown promise in previous studies with the design that lacked control groups and demographic diversity. To validate its efficacy, this randomized, assessor-blinded, controlled trial included 50 Thai subjects with mild-to-moderate facial SD. Participants were randomized 1:1 to apply either NSC or a hydrophilic cream (control) twice daily for 8 weeks. Assessments at week (W) 0, 4, 8, and 12 (4 weeks posttreatment) included severity scores (mSDASI), Investigator Global Assessment (IGA), pruritus, and skin biophysics (erythema index, hydration, TEWL, and sebum levels). The use of triamcinolone acetonide 0.02% cream as rescue medication was recorded. Both NSC (n = 25) and control (n = 25) groups showed clinical improvement from W4, peaking at W8 and continuing to W12. The NSC group’s mSDASI significantly differed from controls at W8 (p = 0.043). The proportion of subjects achieving successful IGA (> 80% improvement) was significantly higher in the NSC group at W8 (44% vs. 16%, p = 0.048). NSC also significantly reduced erythema index at all visits and sebum levels at W4, with lower trends toward pruritus and rescue medication use during treatment. No adverse effects were reported. Overall, NSC demonstrated positive therapeutic effects for mild to moderate facial SD in Southeast Asians, highlighting its potential as a treatment option.
Trail Registration: Thai Clinical Trials Registry: TCTR20230811003
1. Introduction
Seborrheic dermatitis (SD) is a common dermatologic condition that presents as chronic patches or plaques with erythema, scaling, and pruritus, commonly in seborrheic areas [1].
The pathogenesis of SD remains complex. Factors such as immune dysregulation, sebum overproduction, skin barrier dysfunction, overgrowth of Malassezia yeast, and dysbiosis of skin microbiota might contribute to its development [1, 2]. SD is prevalent in immune-compromised individuals [3] and often correlates with sebaceous gland activity. However, it is noteworthy that some SD patients might exhibit normal sebum production. Malassezia yeast feeds on lipids and produces metabolites that trigger inflammation when the skin barrier is compromised [1, 4]. Staphylococcus epidermidis also predominated in the Thai population’s skin microbiome at SD lesion [2].
Treatment modalities in mild-to-moderate cases commonly include topical corticosteroids, calcineurin inhibitors, and antifungals. Despite their effectiveness, these treatments have documented adverse effects [5, 6].
A nonsteroidal cream (NSC) with antifungal, antimicrobial, anti-inflammatory, and anti-seborrheic properties offers a novel treatment option [7]. The active substances of NSC are piloctone olamine, antifungal and antimicrobial [8]; biosaccharide gum-2, anti-inflammatory and antifungal [9]; stearyl glycyrrhetinate, anti-inflammatory [8, 10]; and zinc salt of L-pyrrolidone carboxylate (zinc PCA), anti-inflammatory and antiseborrheic [11, 12].
The previous study in a European country [13] demonstrated in vivo results of NSC, showing antifungal, anti-inflammatory, and antimicrobial properties over 2 weeks in 12 males. Additional findings indicated disease resolution in 17 subjects for up to 60 days in the same country [14] and in twenty HIV-seropositive individuals for up to 12 weeks in South America [15]. Despite these findings, the studies [13–15] had a small number of cases and used an open-label design. Furthermore, these studies did not include a control, given that SD may remit spontaneously [16], and improvement could be attributed to other unmeasured ingredients (i.e., humectants and moisturizers) that enhance skin barriers [17]. Lastly, no current study has explored its effects on Southeast Asians or its recurrence after treatment discontinuation.
To address the limitations above, this study primarily aims to compare the efficacy of NSC with control in treating mild to moderate facial SD in the Thai population and explore posttreatment effects and changes in skin properties.
2. Materials and Methods
2.1. Study Design and Ethical Consideration
This study was a randomized, assessor-blinded, controlled trial conducted at King Chulalongkorn Memorial Hospital (KCMH), Bangkok. It was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University (COA no. 0313/2023) . Written informed consent was obtained from all subjects before enrollment following the Declaration of Helsinki. The patients in this manuscript have given written informed consent to the publication of their case details.
2.2. Study Subjects
We recruited subjects aged 18–65 diagnosed with facial SD by a board-certified dermatologist (C.K.). Inclusion criteria comprised individuals with mild-to-moderate facial SD, as determined by the modified Seborrheic Dermatitis Area Severity Index (mSDASI), adapted from the SDASI [18], who could adhere to the protocol and attend all visits throughout 12 weeks. Exclusion criteria included individuals with concurrent active skin disorders on the face, recent use of topical or systemic anti-inflammatory or immunosuppressive medications within the past 4 weeks, known allergy or sensitivity to cosmeceutical ingredients, immunocompromised status, breastfeeding, or pregnancy.
2.3. Procedures and Measurement
Subjects meeting the criteria were stratified and randomized by sex, age, and disease severity using a computer-based program, with a technician not involved in the study assigning them to two groups in a 1:1 ratio. Each subject was instructed to apply a fingertip-sized amount of either NSC (Nutradeica, ISDIN, Spain) or a hydrophilic cream (control) (KCMH in-house preparation) to the entire face twice daily for 8 weeks. In cases of worsening or intolerable symptoms, triamcinolone acetonide 0.02% cream was provided twice daily as a rescue medication for application to lesions. Throughout the study, subjects were instructed to refrain from using other topical medications or products except for routine skin care, including cleansers, makeup, and sunscreen. All ingredients of the study materials are listed in the Supporting Table 1.
2.4. Assessment
All subjects underwent assessments at weeks 0, 4, 8, and 12 (4 weeks posttreatment) by blinded investigators (K.C. and P.R.). The clinical evaluation of the severity of facial SD was determined using mSDASI. mSDASI is a scoring system adapted from the SDASI [18] precisely for this study. Unlike the SDASI, mSDASI excludes pruritus as a subjective measure and does not include areas outside the facial region (scalp, auricular, intermammary, and back). Erythema and desquamation were scored as follows: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Erythema and desquamation scores were summed for four facial areas (forehead, nasolabial, eyebrow, cheek, or chin). Originally, each site was multiplied by a constant for the area. mSDASI was not constant since the original SDASI constants were equal for all face-related areas. Thus, we maintained equal weight in the calculation. mSDASI scores were derived from the sum of erythema (0–12) and desquamation (0–12) scores. The total mSDASI scores range from 0 to 24 and are graded by tertiles: 0–8 = mild; 9–16 = moderate; and 17–24 = severe.
Clinical responses were evaluated using both Investigator Global Assessment (IGA) and Patient Global Assessment (PGA), utilizing a 5-point scale: excellent response (> 80% improvement); good response (50%–80% improvement); mild response (1%–50% improvement); no response (no change); and worsening. Successful treatment was defined as an excellent response. The primary outcome was assessed based on mSDASI scores and successful IGA at week 8.
Skin biophysics (erythema index, hydration, transepidermal water loss (TEWL), and sebum levels) were measured using a Multi Probe Adapter System (Cutometerdual MPA580; Courage + Khazaka, Cologne, Germany). The erythema index was measured at the same point as the most prominent redness area. Hydration, TEWL, and sebum levels were measured at an intersection of a horizontal line from the nasal ala and a vertical line from the lateral cantus. The measurements were performed in a controlled environment with 15–20 min of acclimatization (temperature of 18°C–21°C and relative humidity of 40%–60%). Values were averaged from individual, noncontinuous, triplicate measurements.
Pruritus was measured using a verbal analog scale ranging from 0 (no itching) to 10 (most severe itching). The number of rescue medication use days was recorded during and after treatment. Subjects were asked to rate patient satisfaction scores (PSS) from 0 (not satisfied) to 10 (most satisfied) at the last visit.
2.5. Statistical Analysis
The sample size was calculated at 50 using G∗power 3.1.9.7 (Universität Düsseldorf, Düsseldorf, Germany) with effect size = 0.25, alpha error = 0.05, and power = 0.8, allowing a 10% dropout rate. Noncontinuous variables were expressed as the frequency and percentage and analyzed using Chi-square or Fisher exact tests. Continuous variables were expressed as the mean ± standard deviation (SD) or median with interquartile range (IQR) for normal and non-normal distributions and analyzed using an unpaired t-test or Wilcoxon rank-sum test to compare group outcomes. Linear mixed model regression with interaction between groups and time was used to compare skin biophysics outcomes. A p value < 0.05 was considered statistically significant. Analyses were performed in Stata 15.1 (StataCorp, College Station, TX, USA), and graphs were created using GraphPad Software.
3. Results
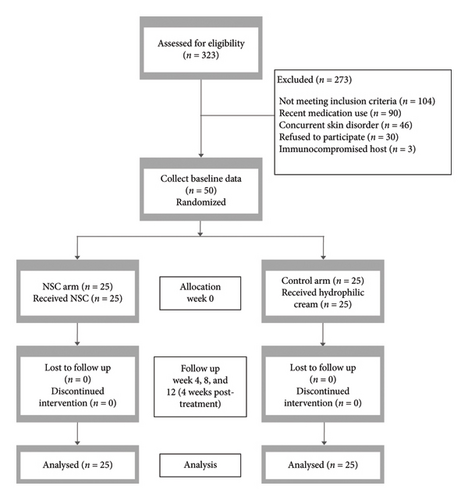
From June 2023 to March 2024, 323 patients were screened, and 50 Thai subjects who met the criteria were enrolled and randomized into the NSC group (n = 25) and the control group (n = 25) (Figure 1). Baseline characteristics were similar between the groups (Table 1). All subjects adhered to the protocol and completed all visits. The last patient completed their final visit in May 2024.
| Characteristics | NSC (N = 25) | Control (N = 25) | p value |
|---|---|---|---|
| Age, mean ± SD (years)a | 41.0 ± 12.2 | 39.1 ± 10.1 | 0.547 |
| Gender, n (%)b | |||
| Male | 15 (60) | 15 (60) | 1.000 |
| Female | 10 (40) | 10 (40) | |
| Fitzpatrick skin type, n (%)c | |||
| III | 7 (28) | 9 (36) | 0.816 |
| IV | 16 (64) | 15 (60) | |
| V | 2 (8) | 1 (4) | |
| Underlying disease, n (%)c | |||
| Dyslipidemia | 2 (8) | 4 (16) | 0.966 |
| Hypertension | 1 (4) | 2 (8) | |
| Diabetes mellitus type 2 | 1 (4) | 2 (8) | |
| Hypothyroidism | 1 (4) | 1 (4) | |
| Mood disorder | 2 (8) | 1 (4) | |
| Cardiac disease | 0 (0) | 1 (4) | |
| None | 19 (76) | 16 (64) | |
| Disease severity, n (%)b | |||
| Mild | 12 (48) | 13 (52) | 0.777 |
| Moderate | 13 (52) | 12 (48) | |
| mSDASI, median (IQR)d | 9 (7, 11) | 8 (7, 11) | 0.950 |
- Note: Statistical tests used included the unpaired t-test (a), Chi-square test (b), Fisher’s exact test (c), and Wilcoxon rank-sum test (d).
- Abbreviations: IQR = interquartile range, mSDASI = the modified seborrheic dermatitis area severity index, NSC = a nonsteroidal cream.
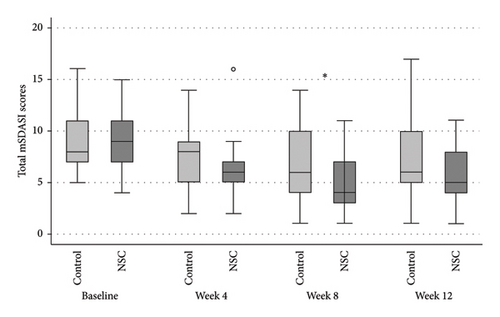
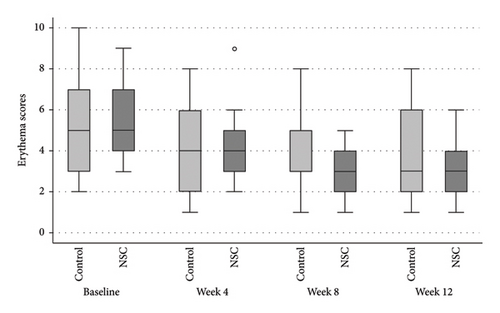
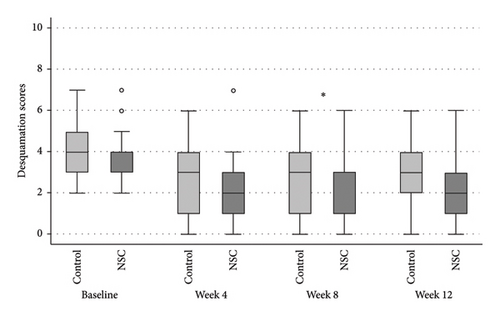
The NSC and control groups exhibited a decline in all mSDASI scores (Figure 2), starting at week 4, reaching their peak at week 8, and persisting through week 12. At week 8, the total mSDASI (median [IQR]) was significantly lower in NSC compared to the control (4 [3, 7]) versus (6 [4, 10]; p = 0.043; Figure 2(a)). Similarly, the desquamation scores in NSC were statistically lower in NSC than in the control (1 [1, 3] vs. 3 [1, 4]; p = 0.020; Figure 2(c)). At week 12, the scores slightly increased in both groups, without significant differences between groups.
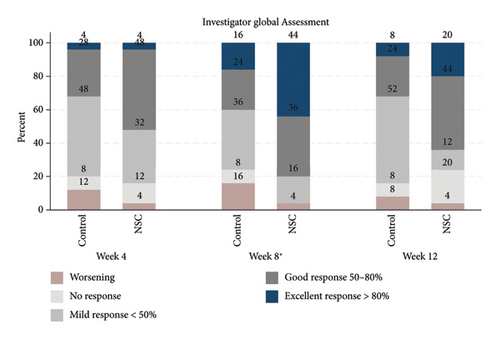
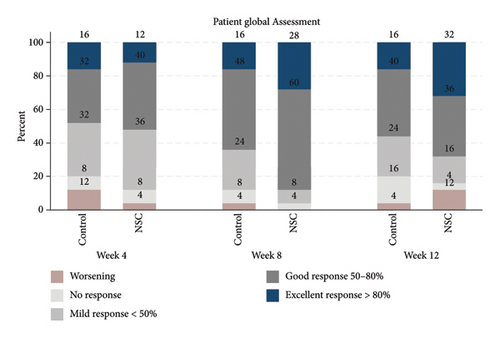
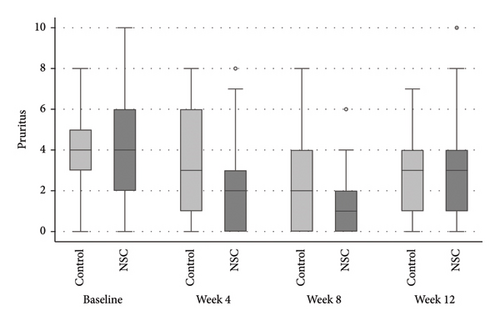
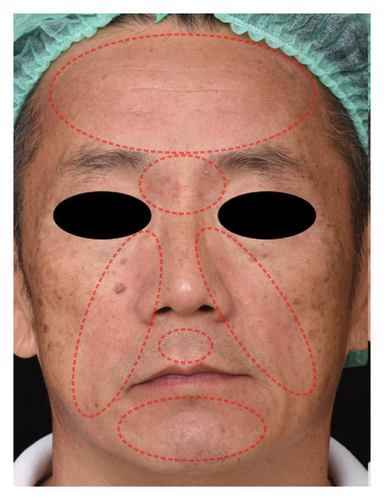
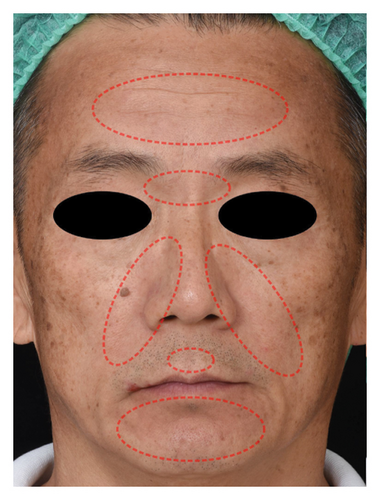
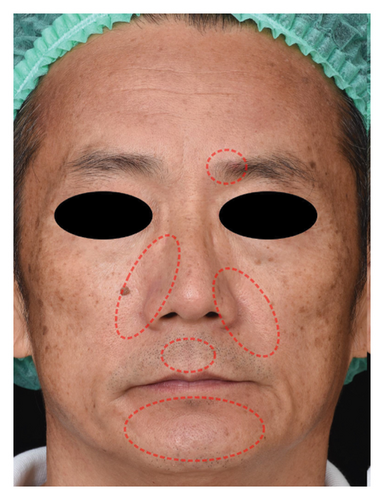
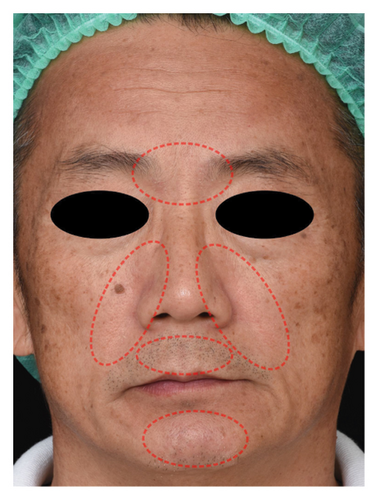
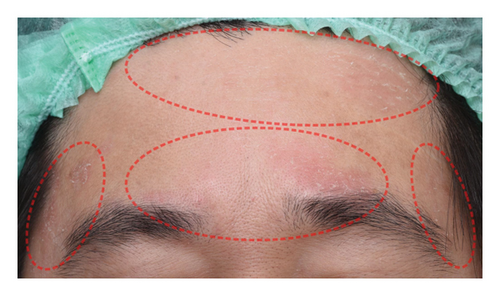
The successful IGA and PGA ratios were comparable in both groups at week 4 but appeared to favor the NSC at week 8 and 12 (Figure 3). At week 8, a significantly higher proportion of subjects achieved a successful IGA in the NSC (11, 44%) compared to the control (4, 16%) (p = 0.045) (Figure 3(a)). Similarly, the successful PGA at week 8 was higher in the NSC (7, 28%) compared with the control (4, 16%), with a rate of good response of 15 (60%) in the NSC (Figure 3(b)). Subjects in both groups reported a reduction in pruritus since week 4, with a downward trend in the NSC; however, no statistical differences between two groups were detected (Figure 4). Clinical photography of representative cases in the NSC demonstrated in Figures 5 and 6.
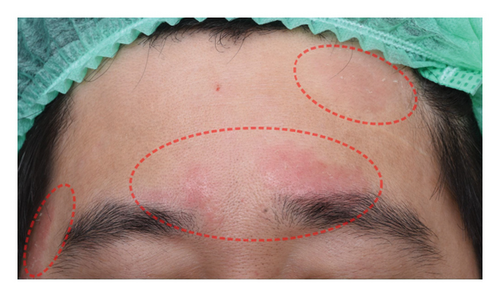
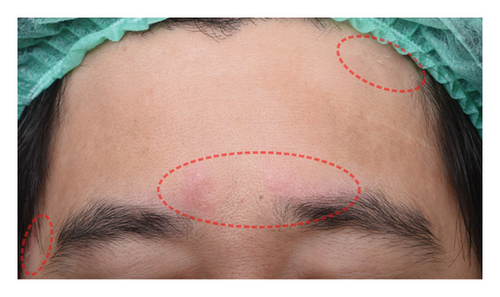
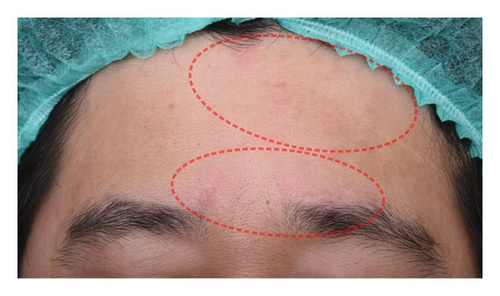
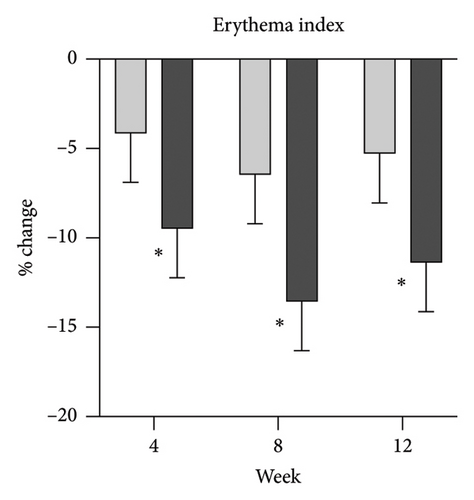
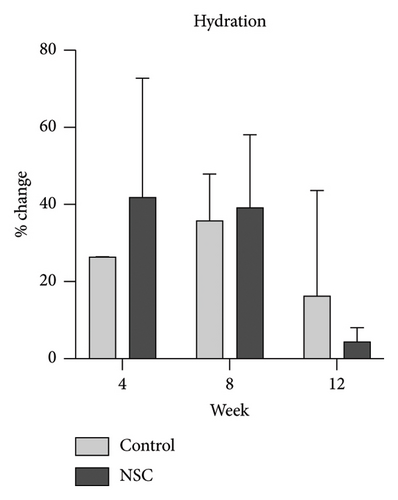
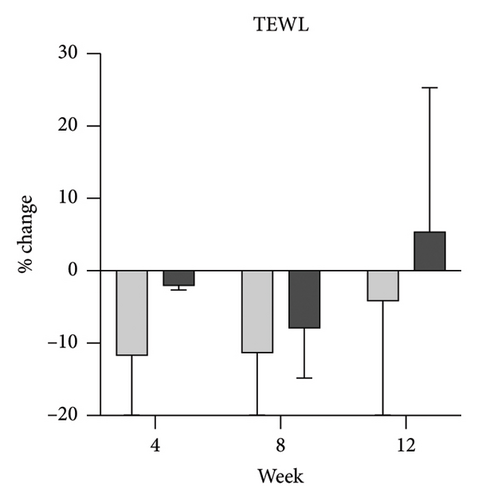
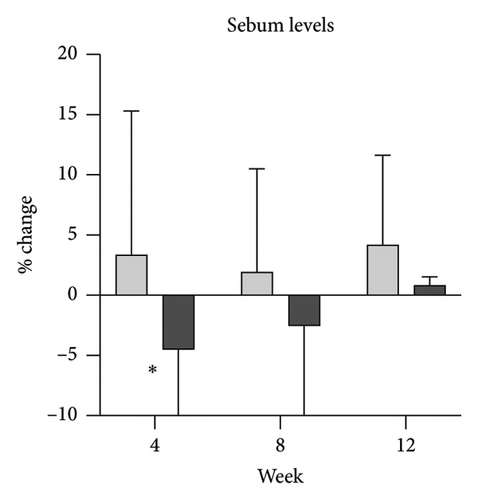
Skin biophysics values were adjusted using percentage changes from the baseline. The erythema index showed improvement throughout the follow-up period, with an estimated mean reduction of 13.5% at week 8 in the NSC (Figure 7(a)). Sebum levels were reduced during the treatment period, with estimated mean decreases of 4.5% at week 4% and 2.5% at week 8 in the NSC (Figure 7(d)). Hydration and TEWL showed improvement in both groups (Figures 7(b) and 7(c)). Significant differences between groups were detected in percentage changes in erythema index at all visits: week 4, −5.33 [95% CI: −1.80, 0.84]; week 8, −7.10 [95% CI: −11.00, −3.21]; week 12, −6.08 [−9.98, −2.19]; and in sebum levels at week 4, −7.87 [95% CI: −13.64, −2.11].
During treatment, the number of subjects using rescue medication was slightly lower in the NSC group (9, 36%) compared with the control group (12, 48%). Median days of rescue medication use were similar between groups (0 [0, 2] in NSC vs. 0 [0, 7] in control). At week 12, the median days of rescue medication use and the number of subjects requiring it were 2 [0, 3] and 15 (60%) in the NSC group and 1 [0, 4] and 13 (52%) in the control group. A total of 21 (84%) patients in the NSC group and 17 (68%) in the control group experienced worsening IGA or the need for rescue medication. Subjects in the NSC group reported a higher median PSS (9 [8, 9]) compared with the control group (8 [7, 9]). No significant differences were observed in rescue medication use, relapse, or PSS. In addition, no adverse effects were reported.
Exploratory post hoc analyses were conducted using the Friedman test within groups and subgroup analyses by severity level (Supporting Table 2). In the moderate severity, most parameters followed the pooled analysis trend. In the NSC, all mSDASI scores changed significantly across visits (p < 0.001). Total scores significantly differed between groups from week 4 (p = 0.034), with erythema scores differing at weeks 8 and 12 (p = 0.008 and 0.018, respectively), while no significant changes in total or desquamation scores were found in the control.
In the mild severity, most scores followed a similar trend, with significant changes across visits in all scores for both the NSC (p < 0.001) and control (p < 0.05). However, no significant between-group differences were detected.
4. Discussion
This study explored the efficacy of a topical antifungal, antimicrobial, anti-inflammatory, and antiseborrheic cream containing piloctone olamine, biosaccharide gum-2, stearyl glycyrrhetinate, and zinc PCA compared with a control using an assessor-blinded design in the Thai population. We evaluated the severity of SD using clinical parameters semiquantitatively and skin biophysics parameters objectively; furthermore, we extended 4 weeks posttreatment period to assess changes and recurrence to evaluate the enduring effect of the product.
The NSC group exhibited a rapid reduction in severity, including erythema, desquamation, and pruritus, which was evident from the first follow-up visit and continued to improve through week 8. As the primary outcomes, median total mSDASI, erythema, and desquamation scores in the NSC group decreased from 9 to 4 (−5), 5 to 3 (−2), and 3 to 1 (−2) at week 8, respectively. Based on a previous SDASI study defining a 50% reduction as the primary endpoint [18], we considered a reduction of ≥ 2 points in erythema and desquamation scores or ≥ 4 points in total score as a clinically meaningful improvement. IGA also indicated over 90% improvement, with 44% demonstrating an excellent response at week 8. These findings align with a previous report [14] that showed a 90% improved IGA with a 53% excellent response at Day 60. Despite a slightly lower response and most patients experiencing relapse at 4 weeks posttreatment, IGA still showed a 76% improvement, with median severity scores shifting from moderate to mild, confirming persistent benefits after discontinuation. Data from previous studies [13] support these findings, as NSC has demonstrated efficacy in antifungal, antimicrobial, and anti-inflammatory properties. Within 2 weeks, significant reductions of over 75%–98% were reported for Malassezia furfur, Staphylococcus epidermidis, and inflammatory mediators on the face and chest areas.
Skin barrier impairment is a key factor in SD, and hydration alone can help alleviate symptoms [17]. TEWL reflects barrier integrity, with increased TEWL linked to SD-related dysfunction, while hydration influences skin homeostasis and severity [19, 20].
As expected, hydration and TEWL showed no significant differences between groups, likely due to the moisturizing effects in both formulations. The improvement observed with the hydrophilic cream supports this and underscores the importance of a controlled study design. NSC’s superior effect stems from a synergy of barrier-restoring properties and additional therapeutic mechanisms. Post hoc findings suggest that NSC provides greater benefits than a basic moisturizer in moderate cases, though the difference is less pronounced in mild cases. This insight may help guide treatment selection, where simpler emollients may suffice for mild SD, while moderate cases may benefit more from NSC.
We observed a reduction in sebum levels, a parameter not previously evaluated, likely due to the antiseborrheic properties of zinc PCA [11, 12]. The percentage difference between groups was 8% at week 4, decreasing to 5% by week 8, possibly due to tachyphylaxis [21], improved hydration [22], or uncontrolled factors such as temperature and activity [23, 24]. While this modest difference has uncertain clinical significance, it could benefit patients with oily skin. In contrast, isotretinoin achieves up to a 70% sebum reduction with marked SD improvement [25], suggesting that a more substantial decrease may be required for significant clinical effects. The role of sebum levels in SD severity remains unclear, as studies have found no consistent link between sebum reduction and symptom improvement [26].
Over 60%–80% of patients achieved disease clearance with 80% severity score improvement after using topical calcineurin inhibitors for 1–2 weeks, and 80% experienced milder relapsing symptoms after 2–4 weeks of discontinuation [27]. Previous studies [28, 29] demonstrated an 80%–90% response with at least a 50% improvement using topical 2% ketoconazole at week 4, with 70% experiencing relapse. Local adverse effects such as dryness, burning, and tingling sensations were reported in 10%–37% of the cases with topical calcineurin inhibitors [27] and 5%–32% with topical ketoconazole [30]. However, most studies did not exclusively focus on facial areas, and methodological variations make direct comparisons challenging. Despite these differences, NSC demonstrated an 80% response rate with at least a 50% improvement by IGA at week 8, which is comparable to ketoconazole 2%. However, NSC required a longer duration to reach its optimal effect. The relapse ratio and pattern were consistent across all treatments, presenting with milder symptoms, suggesting the need for maintenance therapy. Notably, NSC demonstrated good tolerability with no reported adverse effects, making it a promising option for long-term daily use. Moreover, comparing the efficacy to an antifungal dermocosmetic cream containing climbazole/piroctone olamine [26], which helps reduce erythema and sebum levels but shows no significant difference in symptom scores compared to an emollient cream, NSC provides a targeted multimechanistic approach that may enhance its clinical utility beyond a single mechanism.
Considering that Asian skin is more reactive to irritants [31] and access to dermatologists is limited in many Asian countries [32], a well-formulated OTC product could offer a practical solution for SD management in this population beyond basic emollients. However, its higher price (∼USD 22 for 50 mL) compared to common OTC moisturizers may limit widespread adoption.
Environmental factors such as heat and humidity may also exacerbate SD in tropical regions by increasing sebum production [31, 33], while disrupted microbial balance due to Malassezia spp. [34] and Staphylococcus spp. [2] further contribute to inflammation and skin barrier impairment. Given these challenges, effective SD management in this unique environment may require antiseborrheic, antifungal, anti-inflammatory, and barrier-restoring properties to address these factors.
The primary limitation of our study is the inclusion of only mild-to-moderate forms of SD, which typically do not require as aggressive treatment as a severe form of SD. Another limitation is that participants were allowed to use rescue medication and their routine skincare products at their discretion, potentially obscuring the actual therapeutic effect; however, the limitation of only mild-potency steroid use could reflect reduced steroid exposure, and the use of concomitant topical medications and cosmeceutical products is expected in real-world practice.
In addition, variability in assessment timing due to recruitment challenges and inconsistencies in follow-up scheduling may have influenced disease fluctuations from seasonal temperature changes. We aimed to mitigate this by randomization, but standardized assessment intervals could improve data accuracy in future studies.
Lastly, due to the inability to provide identical packaging, subjects were not blinded to the study product, and some ingredients differed between the creams. However, assessor-blinded evaluations were conducted to minimize bias, and no significant differences in moisturizing effects were found. Future studies could reduce bias by using repackaging with identical, texture-matched formulations under a double-blind design.
5. Conclusion
NSC demonstrated a positive therapeutic effect for mild-to-moderate SD with favorable tolerability in Southeast Asians. Future studies should assess its efficacy in severe SD, compare it with other treatments, and conduct long-term follow-up to optimize SD management.
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University (March 16, 2023/COA no. 0313/2023).
Consent
Informed consent was obtained from all participants included in the study.
The authors affirm that human research participants provided informed consent to publish the images in Figures 5 and 6.
Disclosure
The sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of data, or in the preparation, review, or approval of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Kridipop Charoenchaipiyakul, Patcharapong Rujirawan, Nopadon Noppakun, Yuda Chongpison, and Chanat Kumtornrut: conceptualization; Kridipop Charoenchaipiyakul, Patcharapong Rujirawan, Yuda Chongpison, and Chanat Kumtornrut: methodology; Kridipop Charoenchaipiyakul, Patcharapong Rujirawan, Yuda Chongpison, and Chanat Kumtornrut: formal analysis and investigation; Kridipop Charoenchaipiyakul, Yuda Chongpison, and Chanat Kumtornrut: writing – original draft preparation; Kridipop Charoenchaipiyakul, Patcharapong Rujirawan, Nopadon Noppakun, Yuda Chongpison, and Chanat Kumtornrut: writing – review and editing; Chanat Kumtornrut: funding acquisition and resources; Nopadon Noppakun and Chanat Kumtornrut: supervision.
Funding
ISDIN Thailand supported the study materials but did not include financial funding.
Acknowledgments
We gratefully acknowledge ISDIN Thailand for their support in providing study materials and sincerely appreciate all participants for their contributions.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Not all data are included in the manuscript and supporting tables but can be obtained from the corresponding author upon reasonable request.




