Therapeutic Strategies and Decision-Making to Optimize Psoriasis Treatment: A French National Survey Based on Virtual Case Vignettes
Abstract
Despite current treatments for psoriasis, effective disease control remains challenging for physicians. Current recommendations provide information on when treatment should be optimized, but providing guidance on how to optimize treatments in real-word practice is complex. To examine the decision-making practices of dermatologists when optimizing the management of mild or moderate-to-severe psoriasis, we conducted a nationwide, virtual case vignette-based survey among French dermatologists. Participants reviewed four case vignettes. Data on management suggestions from the dermatologists were collected using questionnaires embedded in the case vignettes. Ninety dermatologists reviewed 356 virtual case vignettes. Of these, 99.4% contained a randomly generated profile describing a virtual psoriasis patient who had responded to current therapy but required treatment optimization, and 23.0% involved mild psoriasis treated with topical treatments, 77.0% involved moderate-to-severe psoriasis treated by conventional systemics or biologics, 59.9% involved psoriasis that had moderate or severe-to-very severe impact on HRQoL, 48.6% had joint involvement, and 58.7% had persistent skin lesions. For virtual patients with mild psoriasis involving topical treatments alone (n = 82), the dermatologists suggested switching or modifying the treatment in 81.7% of cases, most commonly by initiating conventional oral systemic therapy. For virtual cases with moderate-to severe psoriasis involving conventional oral systemic therapies (N = 90), the most common suggestion was to switch treatment (54.4% of cases), most notably to biologic therapy. Switching to a second-line or subsequent-line biologic was the most common suggestion for virtual cases already undergoing biologic therapy. Overall, modifying the existing treatment was suggested for 31.5% of the virtual cases, most notably by introducing new treatment combinations (biologics with conventional systemic and/or topical treatments). Impact on HRQoL, joint involvement, and persistent skin lesions were identified as factors influencing dermatologist decision-making (p < 0.001). Our findings provide valuable insights into the current decision-making practices of French dermatologists when optimizing the management of mild or moderate-to-severe psoriasis.
1. Introduction
Psoriasis is a common, chronic, inflammatory skin disorder that can have a major effect on quality of life (QoL). The aims of treatment are to reduce disease severity, ideally achieving clearance or near-clearance of the skin lesions, and to minimize the impact of disease on the daily lives of patients [1–4]. Many parameters have to be taken into account when making treatment decisions, including disease severity and location, the impact of the disease on patient QoL, and the presence of comorbidities [5]. Numerous national and international clinical practice guidelines and treatment algorithms for psoriasis management have been published and recently updated [2–4, 6–13]. These guidelines generally recommend the use of topical treatments for mild psoriasis and conventional systemic or biologic therapies for patients with moderate-to-severe disease or for those with mild disease who do not respond to topical agents. Regular evaluations of treatment responses, based on physician-reported assessments of disease severity and patient-reported assessments of health-related QoL (HRQoL), are essential for treatment optimization. Adjustments to therapy (e.g., changes in treatment dose and administration frequency, or switching therapies) are recommended when treatment goals are not being achieved.
Despite the diversity of therapeutic options and guideline updates, maintaining control of psoriasis remains challenging for physicians [3, 14, 15]. The ideal therapeutic goal of achieving skin clearance or near-clearance is based on efficacy endpoints widely used in clinical trials, whereas this objective is not always easy to achieve in the more diverse patient populations seen in real-world practice [16–21]. Parameters such as patient demographic and social characteristics, patient medical history, and joint involvement [22–24], as well as maintenance treatment regimens (intermittent versus continuous administration), have been shown to affect the efficacy of some treatments and to severely impact the risk of psoriasis relapse and/or treatment failure [25–27]. Therapeutic adherence also has a major impact on treatment outcomes, and although reported adherence rates vary between treatments, most studies have indicated that adherence to psoriasis therapy is suboptimal [28–31]. In addition, patient satisfaction with various psoriasis management strategies has been reported as modest, with patients feeling inadequately informed about the chronicity of their disease, dissatisfied with treatment results or the adverse effects of therapy, or that their treatment preferences and therapeutic objectives differ from those of their physician [32, 33]. Current guidelines provide recommendations to help physicians decide when treatments need to be optimized. However, providing guidance as to how treatment strategies should be adjusted in real-world clinical practice is more complex.
Improving the understanding of the current daily practice of physicians treating psoriasis and gaining insights into the factors that influence the therapeutic decision-making process during treatment optimization may help to improve future guidelines, better optimize psoriasis long-term management, and increase patient satisfaction. Case vignettes are a valuable tool for examining clinical practice and physician behavior during decision-making [34–36]. The aim of this study was to use this case vignette methodology to examine the practices and factors that influenced decision-making among dermatologists when adjusting the clinical management of mild or moderate-to-severe psoriasis in case of suboptimal responses to treatment.
2. Methods
2.1. Study Design and Setting
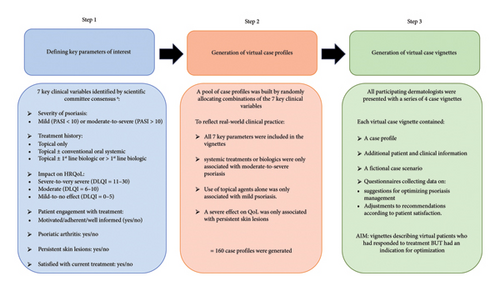
This observational, survey-based study was conducted in France between March 2022 and July 2022. The study used virtual case vignettes to collect data on therapeutic decision-making from dermatologists. The case vignettes were developed by a scientific committee comprised of experts in the management of psoriasis and decision-making processes (NQ-T, NJ, AV-L, A-BD-M, CN, CL, BR, and EM; Figure 1). All data were collected anonymously online via a dedicated platform (developed by FAST4) in accordance with the regulations of the French Data Protection Authority (CNIL). The methodology has been validated in previous studies [37].
2.2. Participants
French dermatologists registered in a large database of healthcare professionals (including 3800 dermatologists) who were working in either public hospitals and/or private practices and had experience of managing of patients with psoriasis were invited to participate in the survey and provide their suggestions for the management of virtual patients described in the case vignettes. There were no restrictions on the age or number of years of dermatology practice of potential participants.
All potential participants were contacted about the study by email or phone, and those agreeing to participate were reminded weekly to complete the survey.
2.3. Development of the Case Vignettes
Development of the virtual case vignettes involved three steps (Figure 1). First, the scientific committee identified the following key clinical parameters of interest to be included in the vignettes: (I) severity of psoriasis according to a physician-assessed score (i.e., the psoriasis area and severity index; PASI); (II) treatment (topical and/or conventional oral systemic or biologic); (III) impact of psoriasis on HRQoL based on dermatology life quality index (DLQI) scores; (IV) patient engagement with treatment; (V) joint involvement; (VI) presence of persistent lesions despite the ongoing treatment; and (VII) patient satisfaction with the ongoing treatment. Second, the parameters of interest were randomly allocated to virtual case profiles. To ensure that the profiles reflected clinical practice recommendations at the time of the study, a fixed set of disease characteristics and treatment combinations was defined as follows: treatment profiles describing the use of systemic treatments or biologics were only associated with clinical profiles indicating moderate-to-severe psoriasis; treatment profiles describing the use of topical agents alone were only associated with clinical profiles indicating mild psoriasis; and only case profiles indicating the presence of persistent skin lesions could be associated with profiles where psoriasis had a severe effect on HRQoL.
The various combinations of parameters of interest allowed for the development of 160 case profiles. Third, the case vignettes were generated by supplementing the virtual case profiles with additional variables (sociodemographic data, results of clinical and biological examinations, initial treatment regimens, etc.), adding fictional patient case scenarios to create context, and incorporating questionnaires to collect the opinions of the dermatologists. The aim was to generate case vignettes describing a virtual patient who had responded to therapy but had an indication (at least one of the seven key parameters) for optimization of treatment. An example of one of the case vignettes is provided in the Supporting information (Supporting Figure 1).
2.4. Data Collection
The dermatologists were presented with a series of four virtual case vignettes, each describing a different virtual case generated from the pool of possible case profiles.
Two questionnaires were incorporated into the virtual case vignettes to collect data concerning the diagnostic and treatment strategy suggestions of the dermatologists for optimizing the management of the virtual patients presented in the case vignettes. The first questionnaire was incorporated after the virtual patients had described the clinical features of their psoriasis (key parameters I–VI) and had provided any additional information (sociodemographic data, treatment history, and results of past clinical and biological assessments). The available options for treatment suggestions included recommending that the existing clinical management be continued or that management should be changed. Suggestions for changing management could include stopping the existing treatment entirely and switching to another treatment (biologics, conventional oral systemic therapies, topical treatment, or phototherapy) or modifying the existing treatment by changing the dose or frequency of administration, or proposing a new combination of treatments (methotrexate and biologics, biologics and topical treatments, or a combination of conventional oral systemic therapies and topical treatment). The second questionnaire was incorporated after the virtual patients had provided an indication of whether they were satisfied with their current treatment (key parameter VII) and collected data on whether the dermatologists would make any changes (switches or modifications) to their initially suggested management strategy once aware of the patient satisfaction status. The duration of each questionnaire was approximately 15 min.
Demographic data (age and gender of the participating dermatologists) were also collected. In addition, the dermatologists were asked to provide information on their type of professional activity (public hospital, private practice, or mixed) and experience managing patients with psoriasis (number of psoriasis patients seen per week).
2.5. Evaluation Criteria
The primary evaluation criterion was assessment of whether (yes/no) the dermatologists suggested changes to the treatment strategies described in the virtual case vignettes they evaluated. Other evaluation criteria included evaluation of the type of changes suggested and of the factors that influenced the decision-making of the dermatologists (including dermatologist-related factors, disease characteristics and current treatment regimens, and patient characteristics) and whether the dermatologists would suggest alternative changes to clinical management based on the satisfaction status of the virtual patients.
2.6. Study Size and Statistical Analysis
It was estimated that a minimum sample population of 70 dermatologists, evaluating a minimum of 280 case vignettes, would be needed to allow for meaningful descriptive statistical analysis of the study evaluation criteria. Quantitative data were expressed as numbers with the mean, standard deviation (SD), and 95% confidence intervals (95% CI). Qualitative data were expressed as numbers and percentages. Univariate and multivariate analyses, including the randomized parameters of interest, were conducted using a mixed-effect logistic regression model that took into account the nonindependence of evaluations provided by the same physician (physician random effect at the intercept level). After the inclusion of significant variables (p < 0.25 in the univariate analysis), a model for multiple logistic regression was developed to identify independent prognostic factors for suggesting changes to clinical management (at p < 0.05). No interactions were tested because there were no hypotheses regarding potential interactions. All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study Participants
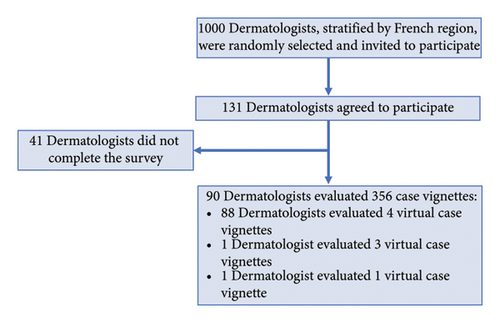
Of the 131 dermatologists who agreed to participate in the study, 90 reviewed at least one of their assigned virtual case vignettes (Figure 2). Among these dermatologists, 61.4% were aged between 25 and 40 years and 76.1% were female (Supporting Table 1). They were involved either in private practice (42.7%) or in mixed public hospital–private practice (57.3%). Two-thirds of the dermatologists (66.7%) indicated that they consulted 1–10 psoriasis patients per week.
3.2. Characteristics of the Case Vignettes
A total of 356 different virtual case vignettes were evaluated by the 90 dermatologists. Of these, 99.4% (354/356) of the case vignettes described a virtual patient who had responded to current therapy but required treatment optimization, indicated by a key clinical parameter (moderate-to-severe psoriasis, joint involvement, persistent skin lesions, and/or moderate or severe-to-very severe impact on HRQoL) in 97.5% of virtual cases (347/356) and by a lack of engagement with disease management in the remaining cases (7/356; 2%). A summary of the virtual case profiles described in the case vignettes is provided in Table 1.
Topical treatments (N = 82) |
Conventional oral systemic therapies ± topical treatments (N = 90) |
First-line biologic ± topical treatments (N = 91) |
> 1 biologic ± topical treatments (N = 93) |
Total (N = 356) |
|
|---|---|---|---|---|---|
| Severity of psoriasis | |||||
| Mild (PASI < 10) | 82 (100.0) | 0 | 0 | 0 | 82 (23.0) |
| Moderate-to-severe (PASI > 10) | 0 | 90 (100.0) | 91 (100.0) | 93 (100.0) | 274 (77.0) |
| Impact on quality of life | |||||
| Severe-to-very severe (DLQI = 11–30) | 10 (12.2) | 17 (18.9) | 20 (22.0) | 23 (24.8) | 70 (19.6) |
| Moderate (DLQI = 6–10) | 38 (46.3) | 37 (41.1) | 33 (36.2) | 35 (37.6) | 143 (40.2) |
| Mild-to-no effect (DLQI = 0–5) | 34 (41.5) | 36 (40.0) | 38 (41.8) | 35 (37.6) | 143 (40.2) |
| Patient engagement in management | |||||
| Motivated, adherent, and well informed | 41 (50.0) | 49 (54.4) | 49 (53.8) | 48 (51.6) | 187 (52.5) |
| Not very motivated, not very adherent, and not very well informed | 41 (50.0) | 41 (45.6) | 42 (46.2) | 45 (48.4) | 169 (47.5) |
| Joint involvement (yes) | 40 (48.8) | 42 (46.7) | 44 (48.4) | 47 (50.2) | 173 (48.6) |
| Persistent skin lesions (yes) | 45 (54.9) | 57 (63.3) | 52 (57.1) | 55 (59.1) | 209 (58.7) |
- Note: Data presented are the number and percentage of fictive case scenarios containing each of the key clinical parameter variables.
- Abbreviations: DLQI, dermatology life quality index; PASI, psoriasis area severity index.
In total, 23.0% of the virtual case vignettes (n = 82/356) described a patient with mild psoriasis treated with topical treatment alone. Among these mild virtual cases, psoriasis was described as having a moderate or severe-to-very severe impact on HRQoL (DLQI score > 5) in 58.5% of cases (n = 48/82), half of the virtual patients were described as engaged in their disease management, and joint involvement and persistent skin lesions were present in 48.8% and 54.9% of the virtual cases, respectively.
Of the virtual case vignettes describing a patient with moderate-to-severe psoriasis, 25.3% of the virtual patients (90/356) were being treated with conventional oral systemic therapies, with or without topical treatments. Psoriasis was described as having a moderate or severe-to very severe impact on HRQoL in 60.0% of these virtual patients (n = 54/90), 54.4% of the virtual patients were described as engaged in their disease management, and 46.7% and 63.3% had joint involvement and persistent skin lesions, respectively.
In total, 184 of the virtual case vignettes described patients with moderate-to-severe psoriasis being treated with either a first-line biologic (25.6%; n = 91/356) or a subsequent-line biologic (26.1%; n = 93/356), with or without topical treatments. Psoriasis was described as having a moderate or severe-to very severe impact on HRQoL in 58.2% of the virtual patients being managed by a first-line biologic (n = 53/91) and in 62.4% of the virtual patients being managed by a subsequent-line biologic (n = 58/93). The virtual patients managed by first-line or subsequent-line biologics were described as being engaged in their disease management in 53.8% and 51.6% of cases, having joint involvement in 48.4% and 50.2% of cases, and having persistent skin lesions in 57.1% and 59.1% of cases, respectively.
3.3. Changes to Treatment Strategies Suggested by the Dermatologists
For 77.8% of the virtual case vignettes, the dermatologists suggested that the treatment described should be changed (Table 2). In almost half of cases (45.8%), dermatologists suggested switching treatments, and for 73.6% of cases, the recommended switch involved the initiation of biologic therapy. In 31.5% of cases, the dermatologists suggested that the existing treatment should be modified, including by dosage adjustments (25.0%), the initiation of combination therapies including methotrexate with biologics (22.3%), and the use of topical treatments with biologics (19.6%) or with oral or conventional systemics (17.9%).
| Treatment profiles described in the vignettes | Total N = 356 | ||||
|---|---|---|---|---|---|
| Topical treatments (N = 82) | Conventional oral systemic therapies ± topical treatments (N = 90) | First-line biologic 1 ± topical treatments (N = 91) | ≥ 1 biologic ± topical treatments (N = 93) | ||
| Change in clinical management strategy, n (%) | 67 (81.7) | 73 (81.1) | 70 (77.0) | 67 (72.0) | 277 (77.8) |
| Switch treatment | 37 (45.1) | 49 (54.4) | 41 (45.1) | 36 (38.7) | 163 (45.8) |
| Biologics | 2 (5.4) | 43 (87.8) | 41 (100.0) | 34 (94.4) | 120 (73.6) |
| Conventional oral systemic therapies | 31 (83.8) | 3 (6.1) | 0 | 2 (5.6) | 36 (22.1) |
| Topical treatments | 1 (2.7) | 0 | 0 | 0 | 1 (0.6) |
| Phototherapy | 3 (8.1) | 3 (6.1) | 0 | 0 | 6 (3.7) |
| Modify the treatment | 29 (35.4) | 24 (26.7) | 29 (31.9) | 30 (32.3) | 122 (31.5) |
| Dose spacing | 1 (3.4) | 0 | 0 | 1 (3.3) | 2 (1.8) |
| Dose increase | 3 (10.3) | 2 (8.3) | 7 (24.1) | 3 (10.0) | 15 (13.4) |
| Dosage adjustments | 7 (24.1) | 10 (41.7) | 5 (17.2) | 6 (20.0) | 28 (25.0) |
| Methotrexate + biologics | 1 (3.4) | 3 (12.5) | 7 (24.1) | 14 (46.7) | 25 (22.3) |
| Biologics + topical treatments | 1 (3.4) | 5 (20.8) | 10 (34.5) | 6 (20.0) | 22 (19.6) |
| Combination of conventional oral systemic therapies + topical treatment | 16 (55.2) | 4 (16.7) | 0 | 0 | 20 (17.9) |
| Stop treatment entirely | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 2 (0.6) |
| Maintain existing clinical management strategy, n (%) | 15 (18.3) | 17 (18.9) | 21 (23.0) | 26 (28.0) | 79 (22.2) |
- Note: The purpose of bold is to highlight the 3 types of change in management strategy (switch treatment/modify the treatment/stop treatment entirely), so the sum of % for switch treatment, modify the treatment, stop treatment entirely = % change in clinical management strategy.
For the virtual case profiles involving mild psoriasis with treatment by topical treatments alone, the dermatologists suggested changing the treatment for 81.7% of cases, either by switching treatments (45.1% of cases) or by modifying the existing treatment regimen (35.4% of cases). The suggested switch involved the initiation of conventional oral systemic therapies for 83.8% of cases, and the suggested treatment modification involved the initiation of combination therapy with conventional oral systemic therapies and topical agents for 55.2% of cases.
For case profiles involving moderate-to-severe psoriasis with treatment by conventional oral systemic therapies with or without topical agents, the dermatologists suggested changing the treatment for 81.1% of cases by switching treatments for around half of the cases (54.4%) and by modifying the existing treatment regimen for 26.7% of cases. The suggested treatment switch involved the initiation of biologic therapy for 87.8% of cases, and the suggested treatment modification involved dosage adjustments for 41.7% of cases.
For patient profiles involving moderate-to-severe psoriasis and treatment with biologics with or without topical agents, treatment switches involving the initiation of an alternative biologic therapy were proposed for 100% of cases involving a first-line biologic therapy and for 94.4% of cases involving more than one line of biologic therapy. Suggested modifications to existing treatment varied depending on whether the case profile involved a first-line or subsequent-line biologic treatment. For case profiles involving a first-line biologic, a combination therapy with the biologic and a topical agent was the suggested modification for 34.5% of cases, whereas this modification was suggested for 20% of cases involving a subsequent-line biologic. For case profiles involving subsequent-line biologics, a combination therapy with a biologic and methotrexate was the suggested modification for 46.7% of cases, compared to 24.1% of cases involving a first-line biologic.
3.4. Suggested Changes or Maintenance of the Treatment Strategies Described in the Case Vignettes According to Dermatologist Practice Type
Mixed public hospital–private practice dermatologists reviewed 203 virtual case vignettes, and private practice dermatologists reviewed 152 vignettes (Table 3). The percentage of virtual case vignettes for which the dermatologists suggested changes to the described treatment regimen was 79.8% for the mixed hospital–private practice dermatologists and 75.0% for the private practice dermatologists. The mixed hospital–private practice dermatologists suggested switching treatments in 52.7% of cases and modifying the existing treatment in 26.6% of cases, whereas private practice-based dermatologists suggested switching treatments or modifying the existing treatment in 36.8% and 37.5% of cases, respectively. When the dermatologists suggested modifying the existing treatment, 35.2% of the mixed hospital–private practice dermatologists suggested dosage adjustments compared to 15.8% of private practice-based dermatologists. Mixed hospital–private practice dermatologists and private practice-based dermatologists suggested initiating combination therapies in 55.6% and 63.2% of cases respectively, including methotrexate and biologics (22.2% and 22.8%, respectively), biologics and topical treatments (16.7% and 21.1%, respectively), or topical treatments and conventional oral systemics (16.7% and 19.3%, respectively).
| Mixed public hospital–private N = 203 | Private practice N = 152 | |
|---|---|---|
| Change in clinical management strategy, n (%) | 162 (79.8) | 114 (75.0) |
| Switch treatment | 107 (52.7) | 56 (36.8) |
| Biologics | 78 (72.9) | 42 (75.0) |
| Conventional oral systemic therapies | 25 (23.4) | 11 (19.6) |
| Topical treatments | 1 (0.9) | 0 |
| Phototherapy | 3 (2.8) | 3 (5.4) |
| Modify the treatment | 54 (26.6) | 57 (37.5) |
| Dose spacing | 1 (1.9) | 1 (1.8) |
| Dose increase | 4 (7.4) | 11 (19.3) |
| Dosage adjustments | 19 (35.2) | 9 (15.8) |
| Methotrexate + biologics | 12 (22.2) | 13 (22.8) |
| Combination of biologics + topical treatments | 9 (16.7) | 12 (21.1) |
| Combination of conventional oral systemic therapies + topical treatment | 9 (16.7) | 11 (19.3) |
| Stop treatment entirely | 1 (0.5) | 1 (0.7) |
| Maintain existing clinical management strategy, n (%) | 41 (20.2) | 38 (25.0) |
- Note: Data presented are the number and percentage of virtual case vignettes for which the dermatologists suggested maintaining the existing treatment or changing the treatment according to the type of suggested change. N, number of virtual case vignettes reviewed by dermatologists according to practice type. Data on practice type were missing for one of the dermatologists. The purpose of bold is to highlight the 3 types of change in management strategy (switch treatment/modify the treatment/stop treatment entirely), so the sum of % for switch treatment, modify the treatment, stop treatment entirely = % change in clinical management strategy.
3.5. Psoriasis-Related Factors Influencing the Dermatologist Suggestions to Maintain or Change the Treatment Regimens Described in the Case Vignettes
The results of the multivariate analysis to assess the factors that influenced the decision-making of the dermatologists when they made suggestions to maintain, switch, or modify the treatment regiments described in the case vignettes are presented in Table 4. The impact of psoriasis on HRQoL, joint damage, and the presence of persistent skin lesions were identified as factors that led to suggested changes in the existing clinical management (p < 0.001). Among these factors, psoriasis having a severe-to-very severe impact on HRQoL (versus a mild-to-no effect) was found to have had the strongest influence on the decisions of the dermatologists (OR:8.30; [95%CI: 2.31–29.78]). In contrast, psoriasis severity, treatment history, and the level of patient engagement with treatment were not found to have influenced decision-making.
| Factors influencing dermatologist decision-making | Maintain existing clinical management, n (%) N = 79 |
Stop treatment entirely N = 2 |
Switch treatments N = 163 |
Modify treatment N = 112 |
Change existing clinical management N = 277 |
Univariate analysis p value OR [95% CI] | Multivariate analysis p value OR [95%CI] |
|---|---|---|---|---|---|---|---|
| Severity of psoriasis | 0.33 | — | |||||
| Mild (PASI < 10) | 15 (19.0) | 1 (50.0) | 37 (22.7) | 29 (25.9) | 67 (24.2) | Reference | — |
| Moderate-to-severe (PASI > 10) | 64 (81.0) | 1 (50.0) | 126 (77.3) | 83 (74.1) | 210 (75.8) | 0.74 [0.39 to 1.38] | — |
| Quality of life impact | < 0.001 | < 0.001 | |||||
| Severe-to-very severe (DLQI = 11–30) | 3 (3.8) | 0 (0.0) | 47 (28.8) | 20 (17.9) | 67 (24.2) | 11.28 [3.35 to 37.96] | 8.30 [2.31 to 29.78] |
| Moderate (DLQI = 6–10) | 28 (35.4) | 0 (0.0) | 70 (42.9) | 45 (40.2) | 115 (41.5) | 2.08 [1.21 to 3.56] | 2.19 [1.23 to 3.90] |
| Mild-to-no effect (DLQI = 0.5) | 48 (60.8) | 2 (100.0) | 46 (28.2) | 47 (42.0) | 95 (34.3) | Reference | Reference |
| Patient engagement | 0.52 | — | |||||
| Motivated, adherent, and well informed | 39 (49.4) | 0 | 98 (60.1) | 50 (44.6) | 148 (53.4) | Reference | — |
| Not very motivated, not very adherent, and not very well informed | 40 (50.6) | 2 (100.0) | 65 (39.9) | 62 (55.4) | 129 (46.6) | 0.85 [0.51 to1.41] | — |
| Joint involvement | < 0.001 | < 0.001 | |||||
| Yes | 20 (25.3) | 0 (0.0) | 100 (61.3) | 53 (47.3) | 153 (55.2) | 3.64 [2.07 to 6.39] | 4.13 [2.28 to 7.46] |
| No | 59 (74.7) | 2 (100.0) | 63 (38.7) | 59 (52.7) | 124 (44.8) | Reference | Reference |
| Persistent skin lesions | < 0.001 | < 0.001 | |||||
| Yes | 29 (36.7) | 1 (50.0) | 115 (70.6) | 64 (57.1) | 180 (65.0) | 3.20 [1.90 to 5.39] | 2.33 [1.30 to 4.16] |
| No | 50 (63.3) | 1 (50.0) | 48 (29.4) | 48 (42.9) | 97 (35.0) | Reference | Reference |
| Previous treatment described in the vignette | 0.38 | — | |||||
| 1st-line biologic ± topical treatments | 21 (26.6) | 0 (0.0) | 41 (25.2) | 29 (25.9) | 70 (25.3) | Reference | — |
| > 1 biologic ± topical treatments | 26 (32.9) | 1 (50.0 | 36 (22.1) | 30 (26.8) | 67 (24.2) | 0.77 [0.40 to 1.51] | — |
| Conventional systemic treatments including oral ± topical treatments | 17 (21.5) | 0 (0.0) | 49 (30.1) | 24 (21.4) | 73 (26.4) | 1.29 [0.63 to 2.65] | — |
| Topical treatments | 15 (19.0) | 1 (50.0) | 27 (22.7) | 29 (25.9) | 67 (24.2) | 1.34 [0.54 to 2.83] | — |
- Abbreviations: CI, confidence interval; DLQI, dermatology life quality index; OR, odds ratio; PASI, psoriasis area severity index.
3.6. Impact of Patient Satisfaction Status on Dermatologist Suggestions to Maintain or Change the Treatments Described in the Case Vignettes
For the 356 virtual case vignettes reviewed by dermatologists, 50% described a virtual patient who was satisfied with their clinical management, whereas the remaining 50% described a virtual patient who was dissatisfied (Table 5). When participating dermatologists were presented with the satisfaction status of the virtual patients, they suggested that the management they had initially suggested should be changed for almost half of the virtual case vignettes (49.2%), including for 40.4% of dissatisfied virtual cases and 57.9% of satisfied virtual cases. For the virtual case vignettes describing a patient that was dissatisfied with their treatment, the proposed change to the initially suggested treatment was to switch therapies for 77.0% of dissatisfied cases and 27.5% of satisfied cases. For the virtual case vignettes describing a patient that was satisfied with their treatment, the proposed change to the initially suggested treatment was to modify the existing therapy for 48.8% of satisfied cases and 18.0% of dissatisfied cases. Finally, once aware of the satisfaction status of the virtual patients, dermatologists indicated that they would maintain the initial treatment they suggested for 13.8% of the virtual cases, including for 5.1% of dissatisfied cases and 22.5% of satisfied cases.
| Clinical management strategy after the dermatologists were made aware of the treatment satisfaction status of the virtual patients | Satisfaction status of the virtual patient, n (%) | Total N = 356 | |
|---|---|---|---|
| Satisfied N = 178 | Not satisfied N = 178 | ||
| Change the initially proposed strategy, yes | 103 (57.9) | 72 (40.4) | 175 (49.2) |
| Switch initially proposed treatment | 49 (27.5) | 137 (77.0) | 186 (52.2) |
| Alternative treatment suggested | n = 16 | n = 83 | n = 99 |
| Biologics | 13 (81.3) | 56 (67.5) | 69 (69.7) |
| Conventional oral systemic therapies | 1 (6.3) | 19 (22.9) | 20 (20.2) |
| Topical treatments | 1 (6.3) | 3 (3.6) | 4 (4.0) |
| Phototherapy | 1 (6.3) | 5 (6.0) | 6 (6.1) |
| Modify the initially proposed treatment | 87 (48.9) | 32 (18.0) | 119 (33.4) |
| Type of modification suggested | n = 58 | n = 23 | n = 81 |
| Dose spacing | 17 (29.3) | 4 (17.4) | 21 (25.9) |
| Dose adjustments | 18 (31.0) | 5 (21.7) | 23 (28.4) |
| Methotrexate + biologics | 7 (12.1) | 4 (17.4) | 11 (13.6) |
| Biologics + topical treatments | 11 (19.0) | 3 (13.0) | 14 (17.3) |
| Conventional oral systemic therapies + topical treatment | 5 (8.6) | 7 (30.4) | 12 (14.8) |
| Stop treatment entirely | 2 (1.1) | 0 (0.0) | 2 (0.6) |
| Maintain the therapy described in the vignette | 40 (22.5) | 9 (5.1) | 49 (13.8) |
- Note: The purpose of bold is to highlight the 3 types of change in management strategy (switch treatment/modify the treatment/stop treatment entirely), so the sum of % for switch treatment, modify the treatment, stop treatment entirely = % change in clinical management strategy.
4. Discussion
This nationwide survey evaluated the decision-making processes of dermatologists during the optimization of management of mild or moderate-to-severe psoriasis. Using vignette methodology, our findings provided valuable insights into how dermatologists in France adjust or switch clinical management strategies to optimize psoriasis control. Our results showed that most dermatologists (77.9% overall) were proactive in changing therapeutic strategy when responses to treatment were suboptimal, regardless of whether they practiced in a private or mixed hospital–private setting. However, the large variation in the suggestions from the dermatologists concerning how the treatments should be adjusted highlights the lack of standardization of psoriasis management during treatment optimization in France. The impact of psoriasis on patient HRQoL and the presence of joint involvement and persistent skin lesions were identified as factors that influenced the decision-making process. Around half of the dermatologists (49.2%) indicated that they would change their proposed management strategy based on the satisfaction status of the virtual patients.
The case vignette methodology used in this survey study provided an ideal approach for assessing the decision-making practices of both hospital and private practice dermatologists for the optimization of psoriasis management in a diverse range of clinical scenarios. Case vignettes are an efficient, flexible, and reliable tool that have been widely used to assess the quality of care and variations in clinical practice [34–38]. Studies comparing data obtained from clinical vignettes to those obtained from standardized patients and medical records have shown that they allow the collection of accurate data on physician’s perceptions and responses while providing a valid reflection of real-world practice [39–42] in a sensitive and ethical manner [35, 36].
Current guidelines, including the most recent guidelines published by the French Society of Dermatology [3], identify key factors such as psoriasis severity, impact of the disease on patient HRQoL, the presence of psoriatic arthritis (PsA), and the presence and location of persistent skin lesions as indications for the need for treatment optimization. However, since the publication of the results of the Transitioning Therapies program in 2014, which provided evidence-based guidelines on appropriate treatment optimization and transitioning between therapies in the management of moderate-to-severe plaque psoriasis [43], there have been only limited attempts to address the need for more detailed practical recommendations as to how psoriasis management should be optimized. In order to clarify future guidelines for treatment optimization, it is important to improve our knowledge of the clinical practice of dermatologists in this context. Indeed, in France, little is known about therapeutic strategies being used by dermatologists to optimize psoriasis control.
In the current study, we analyzed suggestions from French dermatologists on how clinical management strategies could be adjusted to optimize psoriasis treatment. We observed considerable variation in the proposed adjustments to therapy, reinforcing the need for standardized guidelines for optimizing management when patient responses to current psoriasis treatments are suboptimal. For patients with mild psoriasis being treated with topical agents alone, suggestions to optimize therapeutic management often involved switching to a conventional oral systemic treatment or combining the topical treatment with a conventional oral systemic therapy. For those already being managed by a conventional oral systemic treatment, the changes suggested often involved switching treatments and initiating biologic therapy or adjusting the dosage of the current treatment. For patients already receiving a first-line or subsequent-line biologic, switching to an alternative biologic therapy or combining the current biologic with methotrexate or topical products was often proposed. These suggestions broadly followed the treatment algorithm described in the 2019 French guidelines. However, since these guidelines were published, accumulating data from clinical trials has provided evidence supporting the superior efficacy of biologics, used either alone or in combination with topical treatments or conventional systemic therapies such as methotrexate [19, 44–47], and real-world studies have been conducted to confirm their effectiveness and safety [48–51]. The use of biologics earlier in the treatment pathway will therefore likely have to be considered in future guidelines, particularly for patients with a rapidly relapsing disease course [8] and those with comorbid conditions [52–54].
This was also one of the first studies to assess clinical decision-making based on the type of professional practice on a nationwide scale. In our study, 79.8% of the mixed hospital–private practice and 75.0% of the private practice dermatologists suggested changes to clinical management to optimize psoriasis treatment; however, the percentage of mixed hospital–private practice dermatologists who suggested switching treatments was 52.7%, compared to 36.8% for those in private practice. This finding may be in part related to differences in prescribing practices resulting from regulations in place in France at the time of the study, which limit the right to initiate biologics and some conventional systemic therapies (e.g., cyclosporine) to dermatologists practicing in hospital settings but allow private practice dermatologists to renew these prescriptions [55, 56]. In addition, a recent study investigating the prescribing behavior of private practice dermatologists found that these healthcare professionals often consulted hospital-based dermatologists before initiating systemic therapies [56]. These restrictions and real-world practice behaviors may therefore have influenced the suggestions of the private practice dermatologists participating in our study.
Among the disease-related factors identified as influencing dermatologist suggestions for treatment optimization in our study, a severe effect on HRQoL was identified as having the largest impact on therapeutic decision-making. The detrimental impact of poorly controlled or undertreated psoriasis on HRQoL is well established [57–59]. Moreover, due to the relapsing course of the disease, psoriasis may lead to irreversible cumulative life course impairment [60–63]. As identified in our study, optimizing management to maintain remission and prolong the interval between psoriasis flares is therefore a key therapeutic target for dermatologists to reduce both the immediate and life-long burden of the disease.
Joint involvement was also identified in our study as a statistically significant factor driving suggestions for optimizing therapy. This finding is consistent with the results from the physician Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which found that both rheumatologists and dermatologists considered reducing joint pain and stiffness to be the top goal when treating patients with PsA, and that dermatologists were more aware of the impact of joint involvement on patient HRQoL [64]. However, the complexity of optimizing the management of patients with both skin and joint involvement is highlighted in the latest updates of the European League Against Rheumatism (EULAR) and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines [7, 65]. These guidelines recognize the potential importance of early diagnosis and multidisciplinary interventions to prevent permanent joint damage and take into account important advances in therapeutic research [66], leading to recommendations for the earlier escalation of treatment with biologics (TNF, IL-17, and IL-23 inhibitors) or JAK inhibitors in the case of ineffectiveness of disease-modifying antirheumatic drugs (DMARDs) to address all active disease domains.
The physician MAPP survey also reported that lesion size and location were the most important factors considered by dermatologists as contributing to disease severity [64]. In our analysis, the presence of persistent skin lesions was identified as a statistically significant factor influencing decision-making although to a lesser extent than HRQoL. This finding perhaps reflects recognition by the dermatologists participating in our study that the correlation between the extent of the lesions and patient perceptions of disease severity is not straightforward. Indeed, patient surveys have clearly shown that patients often consider their psoriasis to be more severe than suggested by assessments of PASI or affected body surface area, with patients fulfilling the clinical definition of having mild disease reporting that symptoms (e.g., itching, scales, and flaking), the location of the lesions (i.e., on sensitive or visible areas such as the nails, face, scalp, palms and soles, and genitals), and the impact of psoriasis on their HRQoL led them to define their disease as more severe than indicated by physician assessments [67–69].
Taking into account patient perceptions of their disease, as well as their treatment preferences and levels of satisfaction, should therefore be key components of the decision-making process. [33, 68]. In our study, only around half of the dermatologists (49.2%) indicated that they would change their initially proposed management strategy based on the satisfaction status of the virtual patients, although patient satisfaction did seem to have an influence on the types of changes proposed, with the subsequent changes often involving modifications for satisfied virtual patients and switching treatments for dissatisfied virtual patients. Overall, our findings indicate that there is a need to strengthen recommendations concerning the importance of patient satisfaction during the optimization of psoriasis management in France.
As noted above, this study had several key strengths, including the use of the vignette methodology to generate a diverse range of realistic clinical scenarios and the inclusion of both hospital and private practice dermatologists. A potential limitation of the study was that real-life clinical practice limitations (prescription regulations, referrals to specialists, etc.) were not considered in the study design. In addition, although gender was consistent with the national demographic reality, this study mainly included a higher proportion of younger dermatologists compared to the national mean age of dermatologists in France [70]. The fact that only one-third of our study dermatologists reported seeing more than 10 psoriasis patients per week may have also influenced the study results. Future studies using alternative participant inclusion and exclusion criteria could be performed to gain insights into the daily clinical practice of specific groups of dermatologists (e.g., according to the number of years of practice or experience treating psoriasis). In addition, the case vignette methodology could be used in future studies to evaluate decision-making practices for patients with special indications (e.g. those with comorbidities or during pregnancy) and could incorporate the use of alternative patient-reported outcome measures. Finally, given the health costs associated with long-term treatment, particularly for new biologic therapies, decision-making practices for patients with rapid and pronounced responses to therapy (i.e. super-responders) could also be evaluated using this method.
5. Conclusion
Our findings provide valuable insights into the current clinical management strategies used by dermatologists in France when optimizing the treatment of mild or moderate-to-severe psoriasis. We found that both private and mixed hospital–private practice dermatologists were proactive in suggesting changes to therapy to optimize clinical management. A high impact on HRQoL, joint involvement, and the presence of persistent skin lesions were identified as key factors influencing decision-making. This study therefore provides important data for future French clinical practice recommendations, which ideally should include guidance on how as well as when to optimize therapy for patients with psoriasis.
Conflicts of Interest
Nathalie Quiles-Tsimaratos has received grants from AbbVie, Almirall, BMS, Celgène, Janssen Cilag, LEO pharma, Lilly, Medac, Novartis, Sanofi, and UCB. Nicole Jouan has received payment or honoraria as speaker from Novartis, Amgen, UCB, and LEO Pharma. Annie Vermersch-Langlin has received grants from major dermatology laboratories, as well as payment or honoraria as a speaker at a board event organized by LEO pharma. Anne-Bénédicte Duval-Modeste has received payment or honoraria from Abbvie, LEO Pharma, Janssen, Almirall, and UCB. Cristèle Nicolas has received payment or honoraria from AMGEN, UCB, Jansen, and LEO Pharma. Caroline Lislaud and Baptiste Roux declare no conflicts of interest. Emmanuel Mahé has received grants and consulting fees from AbbVie, Novartis, Amgen, Janssen Cilag, Sanofi, UCB, Biolane, and Almirall; payment or honoraria as a speaker from Janssen Cilag, Biolane, and Almirall; support for attending meetings from AbbVie, UCB, and Sanofi; and has participated in advisory board activities for Janssen Cilag.
Funding
This study and medical writing services were funded by LEO Pharma.
Acknowledgments
The authors would like to thank Fast4 for technical support for the generation of the virtual case vignettes and data collection, Acacia Tools and TLM360 for collating the results for the first draft of the manuscript, and Drs Emma Pilling and Marielle Romet from Santé Active Edition–Synergy Pharm for medical writing and language editing, funded by LEO Pharma.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




