Electrochemotherapy for Multiple Nonmelanoma Skin Tumors in Immunosuppressed Patients: A Prospective Cohort Analysis
Abstract
Introduction: Immunosuppressed patients face a significantly higher risk of developing malignant skin tumors compared to the general population, often presenting with numerous, rapidly growing, and aggressive lesions. It can be particularly challenging to treat these tumors, especially in the head and neck region. Electrochemotherapy (ECT) emerges as a viable option for treating multiple tumors simultaneously. This study aims to compare the efficacy, toxicity, and impacts on the quality of life of ECT of nonmelanoma skin cancers (NMSCs) in immunosuppressed patients and nonimmunosuppressed patients.
Materials and Methods: A total of 156 tumors (82 target lesions) in 14 immunosuppressed patients and 183 tumors (157 target lesions) in 30 nonimmunosuppressed patients were treated with ECT using intravenous bleomycin according to the European Standard Operating Procedures of ECT (ESOPE) guidelines. Patients were monitored for at least 6 months. A prospective cohort analysis was carried out to compare tumor response, side effects, and quality of life in the two groups.
Results: 3 months after ECT, nonimmunosuppressed patients showed a significantly higher tumor response rate (p = 0.001). After 6 months, a statistically significant difference was not observed between the two groups regarding tumor response. After 3 and 6 months, there was no difference in toxicity, pain, and EQ-VAS values.
Conclusions: Our results suggest that the effectiveness and safety of ECT for treating NMSCs in immunosuppressed patients seems to be comparable to the nonimmunosuppressed patients. The emphasis on prevention, adopting a multidisciplinary approach, and optimizing immunosuppression are crucial to improving the care and management of this patient cohort.
1. Introduction
Immunosuppressed patients represent a group of a heterogeneous population, including patients with primary and secondary or acquired immunodeficiency. The latter group includes patients who have undergone splenectomy, chemotherapy, and/or radiation therapy; patients taking immunosuppressive drugs including organ transplant recipients; patients with hematological disorders; and patients infected with human immunodeficiency virus (HIV). The immunosuppressed state may be further aggravated by diabetes mellitus, chronic kidney or liver disease, advanced age, malnutrition, etc. With advances in medical sciences, the life expectancy of these patients is increasing, so the side effects of chronic immunosuppression are more common.
Immune deficiency impairs the recognition and elimination of tumor cells and the defense against oncogenic viruses, thus increasing the risk of developing cancer [1]. The most common malignancies associated with impaired immune status are nonmelanoma skin cancers (NMSCs) [2], which can be numerous, fast growing, and aggressive. After surgical resection, local recurrence and/or distant dissemination occurs often, in about 40% of cases [3].
Surgical excision is the standard treatment for NMSC, but other local treatment modalities such as topical 5-fluorouracil, photodynamic therapy (PDT), cryotherapy, topical imiquimod, topical nonsteroidal anti-inflammatory drugs such as diclofenac and piroxicam, radiotherapy, and ECT can be chosen [4–6]. Systemic treatments include immunotherapy, molecularly targeted therapy, or chemotherapy.
The management of these tumors is essentially not different from that of nonimmunosuppressed patients and is dependent on the histological type, size, localization (high-risk sites), number of tumors, symptoms (pain, bleeding, and ulceration), previous treatments, patient history of NMSCs, general condition, comorbidities, and preference of the patient [7]. However, the treatment of multiple tumors is challenging for several reasons. The large number of tumors means that surgical treatment can only be performed in several sessions, imposing a great burden on patients. With regards to systemic immunotherapies, the response is inferior to that of the immunocompetent population and adverse events may represent major risks. In organ transplant recipients, immune-checkpoint inhibitors are associated with an increased risk of rejection [8]. Sonic hedgehog inhibitors, used in the treatment of multiple or advanced basal cell carcinoma (BCC), may cause dysgeusia and weight loss, which may be difficult to bear for this vulnerable patient population.
ECT is an effective and widely used treatment for skin and subcutaneous tumors. The procedure is based on a combination of electroporation and a chemotherapeutic agent. During electroporation, an electrical pulse is applied, which causes a temporary increase in the permeability of the cell membrane, allowing substances (e.g., chemotherapeutic agents) to pass through the cell membrane that previously could not or only slightly penetrate the cell. The molecules entering the cells become “trapped” intracellularly, since electroporation is reversible, so that once the electrical impulse is removed, the cell membrane is restored. The molecules exert their cytotoxic effects and cell death occurs [9].
Preclinical studies have demonstrated that ECT induces immunogenic cell death and that the levels of certain proinflammatory cytokines (IL-1β, IL-10, IL-6, TNF-α, and IFNγ) and markers specific for macrophages and natural killer (NK) cells are elevated after treatment [10]. Studies to date have shown that the treatment in immunocompetent mice is more effective and the tumor response is longer lasting compared to immunodeficient animals [11].
ECT has the advantage of being able to treat several tumors at the same time and offers a high objective tumor response, a favorable side effect profile, repeatability, and a favorable esthetic outcome.
The aim of our study was to evaluate the efficacy, toxicity, and impacts on the quality of life of ECT for NMSCs in immunosuppressed patients compared to nonimmunosuppressed patients.
2. Materials and Methods
Fourty-four patients were included in our analysis, who underwent ECT at the Department of Dermatology and Allergology in Szeged, Hungary, between 2016 and 2024. All patients gave written informed consent (ethical approval: ECT-REPRO-002, 9/2016-SZTE). The patients had a life expectancy of more than three months, had not received any other anticancer treatment for skin tumors for at least two weeks prior to ECT, and their Eastern Cooperative Oncology Group (ECOG) status was at least 1. Exclusion criteria were documented allergic reaction to bleomycin or if the patient had previously received bleomycin treatment at a dose greater than 400,000 IU/m2. Furthermore, a history of moderate to severe neuropathy, abnormal coagulation parameters, clinically manifested cardiac arrhythmia, epilepsy, and pregnancy or lactation were among the exclusion criteria.
All patients had a histopathologically verified diagnosis of keratinocyte skin cancer. The study population consisted of two distinct groups: one comprising immunosuppressed patients and the other consisting of nonimmunosuppressed individuals.
ECT treatment was performed according to an international standardized protocol (European Standard Operating Procedures of ECT [ESOPE]) [12] using a CE-marked Cliniporator (IGEA Ltd, Modena/Carpi Italy) electric pulse generator. Due to the localization and/or the high number of malignancies, the procedure was performed under general anesthesia. Bleomycin was administered intravenously at a dose of 15,000 IU/m2. Either row or hexagonal needle electrodes were used, with repositioning of the electrodes as necessary to deliver multiple pulses for larger lesions. Eight electric pulses were delivered over 100 ms at a frequency of 5000 Hz and an amplitude of 1000 V/cm (according to the electrode distance) between 8 and 28 min after bleomycin administration (at the pharmacokinetic peak of the drug concentration).
After treatment, patients were followed regularly (every 3 months) and prospective data collection was carried out. During visits, tumor response, side effects, and quality of life questionnaires were recorded, as well as photographic documentation and the appearance of novel tumors were also documented.
2.1. Response Evaluation, Side Effects, and Quality of Life
Tumor response was evaluated according to the World Health Organization Response Evaluation Criteria for Solid Tumors (WHO RECISTs 1.0) as follows: complete regression (CR) if no visible or palpable tumor and partial regression (PR) at least a 30% reduction in the sum of lesion diameters from baseline. The objective response (OR) rate is defined as the sum of CR and PR. Progressive disease (PD) is defined as an increase of at least 20% in the sum of lesion diameters compared to the smallest value ever measured. Stable disease (SD) is defined as a change that does not fall into one of the above categories. Where assessment was not possible due to local side effects, the response was not classified (NA). In each patient, a maximum of 7 target lesions were selected. Treatment was repeated if PR was observed.
Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAEs 4.0).
Quality of life was assessed using the Euro Quality of Life—5 dimensions (EQ-5D) questionnaire, which was completed by the patients themselves. The 5D refers to five dimensions: mobility, self-care, performance of usual activities, pain/discomfort, and anxiety/depression, which were rated on a three-point scale. Patients had to rate their general health state on a scale of 0–100 (EQ-VAS) and pain on a scale of 0–10 (pain-VAS).
R 4.2.3. software was used for the statistical analysis.
3. Results
Comparative data analysis was performed between immunosuppressed (n = 14) and nonimmunosuppressed (n = 30) patients.
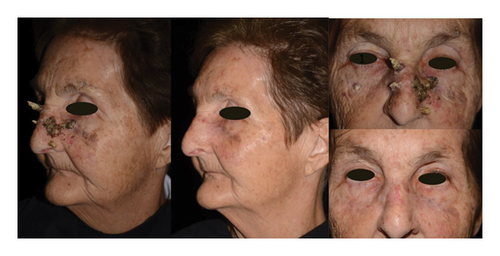
The mean age of the immunosuppressed patients was 76 years (67–90 years), and 4 women and 10 men were included. Nine patients were diagnosed with a hematological disorder: 4 patients were treated with ruxolitinib (n = 3) and hydroxycarbamide (n = 1) for polycythemia vera, 1 patient received hydroxycarbamide for essential thrombocytosis (Figure 1), while 4 patients were immunosuppressed as a result of their underlying hematological conditions such as chronic lymphoid leukemia (CLL) (n = 3) or primary myelofibrosis (n = 1), without receiving specific immunosuppressive therapy. Two patients underwent renal transplantation; they were initially treated with calcineurin inhibitor (tacrolimus), which was switched to mTOR inhibitor (everolimus) due to the appearance of multiple NMSCs. One patient with Crohn’s disease was treated with azathioprine and one with rheumatoid arthritis was treated with low-dose methylprednisolone in addition to leflunomide, and the latter was also stopped due to the appearance of skin cancers. The duration of the immunosuppressive condition was not documented in 3 cases, and for the remaining patients, the mean time from diagnosis to ECT treatment was 11 years (0.5–27 years). In one patient, the duration of the immunosuppressive condition could not be precisely determined. In this case, primary myelofibrosis was diagnosed in our clinic 6 months prior to ECT treatment. The patient had a rapidly evolving, large verrucous carcinoma in the perineal region, which was previously surgically excised, but local recurrence and progression were observed, so we decided to perform ECT. Due to the absence of any preexisting conditions to account for the rapid growth, coupled with the presence of anemia, we sought a hematological consultation. This consultation prompted a bone marrow biopsy resulting in the diagnosis of primary myelofibrosis. The clinical data of the immunosuppressed patients are presented in Table 1.
| Age | Gender | Underlying disease | Duration of immunosuppression (years) | Immunosuppressive treatment | Treated tumors (n) | Largest diameter of the largest tumor (mm) | Localization | Histology | Local recurrence | Follow-up (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. 1. | 73 | Male | B-CLL | NA | — | 5 | 27 | Head/neck | SCC | Yes | 23 |
| Pt. 2. | 70 | Male | Renal transplantation | 11 | Tacrolimus, then everolimus | 15 | 25 | Head/neck, trunk, upper limbs | SCC, BCC | Yes | 45 |
| Pt. 3. | 78 | Male | Polycythemia vera | 14 | Hydroxycarbamide | 25 | 30 | Head/neck, upper limbs | SCC | Yes | 8 |
| Pt. 4. | 79 | Male | CML, polycythemia vera | NA | Ruxolitinib | 27 | 35 | Head/neck, trunk, upper limbs | SCC | Yes | 17 |
| Pt. 5. | 76 | Male | Renal transplantation | 5 | Tacrolimus, then everolimus | 11 | 30 | Head/neck, upper limbs | SCC, BCC | No | 13 |
| Pt. 6. | 83 | Female | CLL | 14 | Tacrolimus, then everolimus | 9 | 30 | Head/neck, trunk, upper limbs | BCC | No | 11 |
| Pt. 7. | 69 | Female | Crohn’s disease | 27 | Azathioprine | 10 | 30 | Head/neck | SCC, BCC | Yes | 16 |
| Pt. 8. | 90 | Male | Polycythemia vera | NA | Ruxolitinib | 9 | 40 | Head/neck, upper limbs | BCC | No | 13 |
| Pt. 9. | 78 | Male | Rheumatoid arthritis | 20 | Methylprednisolone, leflunomide | 7 | 12 | Head/neck, upper limbs | SCC, BCC | No | 10 |
| Pt. 10. | 76 | Male | CLL | 5 | — | 11 | 15 | Head/neck, trunk, upper limbs | BCC | No | 7 |
| Pt. 11. | 72 | Female | Polycythemia vera | 1 | Ruxolitinib | 1 | 12 | Head/neck | BCC | No | 6 |
| Pt. 12. | 84 | Male | Rheumatoid arthritis | 7 | Methotrexate, then leflunomide | 17 | 35 | Head/neck | SCC, BCC | No | 6 |
| Pt. 13. | 67 | Male | Primary myelofibrosis | 0.5 | — | 1 | 160 | Perineal region | SCC | Yes | 7 |
| Pt. 14. | 75 | Female | Essential thrombocytosis | 15 | Hydroxycarbamide | 8 | 20 | Head/neck, upper limbs | SCC | No | 13 |
The mean age of the control group was 72 years (31–89), and 24 male and 6 female patients were included. The patients were followed for a minimum of 6 months.
NMSCs located in the head and neck region and trunk and upper limbs, predominantly on skin surfaces exposed to light were treated. Treated lesions included actinic keratoses (AKs), BCCs, and squamous cell carcinomas (SCCs). None of the patients had known lymph node or internal organ metastasis at the time of treatment.
In the immunosuppressed group, a total of 156 tumors and 82 target lesions were treated. In the control group, we treated 183 tumors, with 157 target lesions. The average number of lesions treated per patient was 11 in the first group and 6 in the second group. The mean of the largest diameter of the tumors was 35.8 mm (12–160 mm) in the first group and 27.6 mm (9–105 mm) in the second group. Demographics, tumor characteristics, tumor response, toxicity, and quality of life results of the two groups are presented in Table 2.
| Immunosuppressed patients | Nonimmunosuppressed patients | |
|---|---|---|
| Number of patients | 14 | 30 |
| Gender | ||
| Female | 4 | 6 |
| Male | 10 | 24 |
| Age (years) | ||
| Mean (range) | 76 (67–90) | 72 (31–89) |
| Treated tumors | 156 | 183 |
| Average number of tumors per patient | 11 | 6 |
| Target lesions | 82 | 157 |
| Largest diameter of the largest tumor (mm) | ||
| Mean (range) | 35.8 (12–160) | 27.6 (9–105) |
| Tumor response, 3 months after ECT | n = 82 | n = 157 |
| CR | 54 (66%) | 124 (79%) |
| PR | 4 (5%) | 15 (10%) |
| SD | 2 (2%) | 5 (3%) |
| PD | 0 | 2 (1%) |
| NA | 22 (27%) | 11 (7%) |
| OR | 58 (71%) | 139 (89%) |
| Tumor response, 6 months after ECT | n = 82 | n = 157 |
| CR | 73 (89%) | 125 (80%) |
| PR | 7 (9%) | 17 (11%) |
| SD | 0 | 3 (2%) |
| PD | 1 (1%) | 3 (2%) |
| NA | 1 (1%) | 9 (6%) |
| OR | 80 (98%) | 142 (90%) |
| Toxicity, 3 months after ECT | n = 14 | n = 30 |
| Odor | 0 | 0 |
| Suppuration | 1 (7%) | 2 (7%) |
| Hyperpigmentation | 6 (43%) | 4 (13%) |
| Ulceration | 5 (36%) | 7 (23%) |
| Toxicity, 6 months after ECT | n = 14 | n = 30 |
| Odor | 1 (7%) | 1 (3%) |
| Suppuration | 1 (7%) | 3 (10%) |
| Hyperpigmentation | 5 (36%) | 7 (23%) |
| Ulceration | 3 (21%) | 5 (17%) |
| Quality of life, baseline | ||
| Pain-VAS (mean) | 0 | 0.37 |
| EQ-VAS (mean) | 65.7 | 71.8 |
| Quality of life, 3 months after ECT | ||
| Pain-VAS (mean) | 0 | 1.27 |
| EQ-VAS (mean) | 68.7 | 73.6 |
| Quality of life, 6 months after ECT | ||
| Pain-VAS (mean) | 0.14 | 0.43 |
| EQ-VAS (mean) | 69.4 | 72.7 |
3.1. Tumor Response
Fisher’s exact test was used to compare tumor response rates in the two groups. Three months after treatment, tumor lesions in the nonimmunosuppressed group showed a significantly higher chance of responding to ECT treatment (p = 0.001, odds ratio = 3.18). Six months after ECT, a statistically significant difference was not observed in tumor response between the two groups (p > 0.05).
ECT treatment was repeated in 6 out of the 14 immunosuppressed patients. Among them, 3 patients showed PR in the treated area and/or experienced local recurrence; therefore, ECT was repeated 5, 11, and 12 months after the first session. In addition, surgical excision, PDT treatment, and cryotherapy were used to treat the novel tumors, which appeared in some cases outside the treated area. One patient developed histologically confirmed lymph node metastasis of SCC, and we opted for radiotherapy for the primary tumor region and metastasis. The patient with the perineal verrucous carcinoma received PD-1 inhibitor immunotherapy after ECT, due to PR after ECT treatment.
3.2. Side Effects
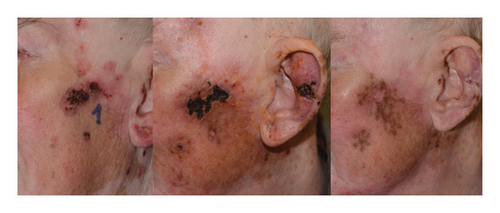
We did not experience any systemic toxicity in our patients. Local side effects included odor, suppuration, hyperpigmentation, ulceration, and transient pain well controlled with analgesics. At 3 and 6 months after ECT, there was no significant difference in toxicity between the two groups (Fisher’s exact test, p > 0.05). Temporary crusts and hyperpigmentation of the treated area are shown in Figure 2.
3.3. Quality of Life
The patients had an ECOG performance status of 0 or 1; therefore, patients did not report problems with mobility, self-care, or performing usual activities. Regarding the EQ-VAS and pain-VAS scores, there was no significant difference between the two groups (Fisher’s exact test, p > 0.05).
4. Discussion
The risk of developing skin tumors is higher in immunosuppressed patients. Most data on this matter are available in patients who have undergone solid organ transplantation. These patients have the highest incidence of developing NMSCs. BCC is the most common NMSC in the general population. However, in organ transplant recipients, the most common one is SCC. In addition, precursor lesions of SCC are also common. AKs and field cancerization appear at a significantly younger age in transplant patients compared to the nonorgan transplant patient population [13]. In these patients, invasive skin tumors develop earlier at the site of an AK. In a cohort study conducted in 2021, 2852 transplanted patients were compared with the nontransplanted population, and the incidence of skin tumors in the first group was 9-fold higher [14]. In a study from 1999, they studied 2561 Norwegian patients who had undergone kidney and heart transplantation and found that the risk of SCC in this patient population was 65 times higher than in the general population [15]. A publication in 2020 compared disease-specific survival in patients with cutaneous SCC in the head and neck region in immunosuppressed and nonimmunosuppressed patients. The results of this study found a 2.32-fold higher risk of disease-specific mortality in the first group [16].
Based on data from the literature and our study, prevention, close collaboration of the involved medical professionals, and optimizing immunosuppressive treatment are of paramount importance in the care of immunosuppressed patients. ECT treatment may be an appropriate therapeutic modality for multiple cutaneous tumors in immunosuppressed patients.
4.1. Prevention
It is clear that primary prevention and early detection of skin tumors are of paramount importance in the high-risk immunosuppressed patient population. This requires detailed patient education on regular skin self-examination and strict sun protection. The literature and the results of our own study suggest that skin cancer screening every 3–6 months would be optimal for these patients.
4.2. Multidisciplinary Cooperation
Since the treatment and care of these patients requires a multidisciplinary approach, it is recommended that all the relevant specialties (e.g., hematologists, oncologists, surgeons, radiologists, pathologists, transplant specialists, and dermatologists) should pay close attention and work closely together, possibly setting up specialized outpatient clinics where patients are regularly screened for skin tumors.
One of our patients had a rapidly growing, massive perineal tumor, which led to the diagnosis of his underlying hematological disease, which was made only 6 months before ECT treatment. The precise duration of the patient’s state of immunosuppression remains unknown. It is crucial to keep in mind that when a patient manifests multiple, aggressive, rapidly growing skin tumors unresponsive to standard treatment, an underlying immunosuppressive condition should be considered.
4.3. Optimizing Immunosuppression
Calcineurin inhibitors (cyclosporine and tacrolimus) inhibit the production of certain cytokines (e.g., IL-2) and T-cell mediated immune responses and also enhance the production of cytokines (TGF-beta and VEGF) that are associated with tumor development [17]. However, the risk of tumor development is reduced with mTOR inhibitors (sirolimus and everolimus), because they directly inhibit tumor cell formation and the production of cytokines such as VEGF. Nowadays, a significant body of evidence clearly demonstrates that switching from calcineurin inhibitors to mTOR inhibitors reduces the incidence of NMSCs [18]. It is therefore important to optimize immunosuppressive therapy in patients who are diagnosed with such tumors by their treating physicians, after weighing the benefit-risk ratio. Our two kidney transplant recipients developed 27 and 3 skin tumors while on calcineurin inhibitor treatment, and therefore we switched to mTOR inhibitors.
4.4. ECT
The advantage of ECT is that several tumors can be treated at the same time, and it can be repeated and combined with other therapeutic modalities. It is a tissue-sparing procedure with good esthetic results that is particularly important for tumors in the head and neck region. A prospective, randomized controlled trial published in 2019 compared ECT with surgical excision, which is the standard of care for BCC. There were no statistically significant differences in the efficacy or recurrence rates [19].
In a prospective study of 15 patients treated in a day surgery setting for NMSCs in the head and neck region, ECT with bleomycin achieved a 100% complete response at 6 weeks, demonstrating its feasibility and high efficacy [20].
The OR and CR after ECT in the largest study ever using WHO RECIST 1.0 criteria were 82% and 64% for BCC (n = 567) and 80% and 63% for SCC (n = 284), respectively [21]. Bertino et al. reported 95% OR and 83.1% CR for BCCs per tumor (n = 587) [22]. In another publication, Bertino et al. found 83% OR and 62% CR for SCCs per patient (n = 162) [23].
Published data suggest that ECT in combination with immunotherapy could induce a better tumor response. In the treatment of metastatic melanoma, a multicentre comparative study has already been conducted in 3 groups matched by sex, age, ECOG status, and tumor size to investigate local and systemic tumor response, toxicity, and survival. The first group received a combination of ECT and PD-1 inhibitor immunotherapy (n = 45), the second group received immunotherapy alone (n = 40), and the third group consisted of patients treated with ECT alone (n = 41). Patients treated with ECT had a significantly better local response compared to patients treated with pembrolizumab alone, and the time to local progression was the longest with the combination treatment. The systemic OR was similar in the two groups, but systemic progression occurred significantly later in patients who received combined treatment [24].
Similar studies have not been published yet with SCC, but it is expected that ECT could be used as a similar “in situ vaccination” alongside PD-1 inhibitor treatment in patients with SCC. This may be even more important in the immunosuppressed patient group due to the insufficient T-cell activation in the immunosuppressed state. We expect to see this synergistic effect in our patient with verrucous carcinoma in the perineal region, who is currently being treated with an immune-checkpoint inhibitor after ECT.
Until now, there is only one publication in the literature on the treatment of an immunosuppressed patient with ECT for skin cancer. Milicevic et al. report ECT treatment of cutaneous metastases of malignant melanoma in a liver transplant patient [25]. Targeted therapy was initiated for Stage III melanoma, which had to be discontinued due to side effects, and immunotherapy was not initiated due to the absence of disseminated disease and a major risk for organ rejection. Four ECT treatments were performed over a 6-month period. Nine months after the last treatment, after surgical removal of a lesion, the patient had no skin metastasis, and complete remission was achieved. The patient subsequently died due to systemic progression of the disease.
Our prospective cohort analysis shows that ECT seems to be as effective and safe in immunosuppressed patients as in nonimmunosuppressed patients for NMSCs. Treated tumors per patient and the largest tumor diameter were higher in the immunosuppressed group, which correlates with the findings of the abovementioned studies from the literature.
Three months after ECT, immunocompetent patients showed a significantly better tumor response. However, by the 6-month follow-up, there was no statistically significant difference in response between the two groups. The antitumor effect of ECT is based on three major mechanisms: it induces mitotic cell death by causing double-strand DNA breaks, leads to transient hypoperfusion (the vascular effect), and triggers immunogenic cell death [9]. In immunosuppressed patients, the number and/or function of both innate and adaptive immune cells are diminished [26], which might explain the delayed effect of ECT observed at the later 6-month follow-up.
5. Conclusions
In immunosuppressed patients, the incidence of NMSCs is higher and these tumors behave more aggressively compared to the tumors presenting in the general population. The prevention and proper treatment of these tumors are of paramount importance. ECT is a good choice for the treatment of skin tumors in immunosuppressed patients, as it can be used to treat many tumors effectively at the same time with few side effects. The treatment is well tolerated by this vulnerable patient population and can be repeated as needed, with favorable esthetic results. Further clinical studies are needed with a higher number of patients and also longer follow-up periods in order to evaluate the long-term efficacy of ECT in this special subgroup of patients.
Ethics Statement
This research was conducted in accordance with the Human Investigation Review Board, University of Szeged. All procedures involving human participants were approved by the Human Investigation Review Board, University of Szeged (ECT-REPRO-002, 29/02/2016). Informed consent was obtained from all individual participants included in the study. In addition, consent was obtained for the use of photographs depicting human subjects.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conducting the clinical trial and practical application of electrochemotherapy: P.R., G.V., and E.K. Writing of the manuscript: P.R. Critical reading of the manuscript: E.K. and L.K. Supervision of questionnaire completion: P.R. Dermatological examination for control: P.R., C.H., and I.C. Oncological examination for control: H.Ó., E.B., J.O., R.G. Data analysis: P.R., E.S., B.T.P. All authors have read and approved the final version of the article.
Funding
This work was supported by the Univeristy of Szeged under the grant “Open Access Fund, Grant ID: 7370” for open access publication.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




