Efficacy and Safety of GR1802 in Patients With Moderate-to-Severe Atopic Dermatitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial
Abstract
Background: GR1802 is a newly developed, fully humanized monoclonal antibody targeting the interleukin-4 receptor alpha (IL-4Rα) subunit, which is a component common to both the IL-4 and IL-13 receptor complexes.
Objectives: Our objective was to assess the efficacy of GR1802 in adult patients presenting with moderate-to-severe atopic dermatitis.
Methods: In this clinical trial, patients with moderate-to-severe atopic dermatitis were randomly assigned to receive either 300 mg GR1802, 150 mg GR1802, or a placebo every 2 weeks for 16 weeks. Primary endpoints were the Eczema Area and Severity Index (EASI-75) response rates at Week 16. Secondary efficacy outcomes included responders at various evaluation points from baseline to study end: IGA 0/1 with ≥ 2-point improvement; EASI-75, EASI-90, and EASI-50; and ≥ 3- or ≥ 4-point improvements in weekly average daily PP-NRS score. Safety was evaluated throughout the study.
Results: From August 2022 to February 2023, 120 patients were randomized to receive either GR1802 150 mg (n = 40), GR1802 300 mg (n = 40), or placebo (n = 40), with 107 completing the study. GR1802 demonstrated a higher proportion of patients achieving EASI-75 at Week 16 compared with the placebo group, and the observed differences in EASI-75 response rates were 39.1% (GR1802 300 mg vs. placebo, 95% CI 20.0–58.2, p = 0.0002) and 19.4% (GR1802 150 mg vs. placebo, 95% CI −0.8–39.7, p = 0.0740). The GR1802 300 mg group also showed greater efficacy on secondary endpoints compared to the GR1802 150 mg group. Serious adverse events occurred in 10% of placebo patients, 2.5% of the GR1802 150 mg group, and none in the GR1802 300 mg group. Treatment-emergent AEs occurred in 75.0% of the GR1802 150 mg group, 82.5% of the GR1802 300 mg group, and 85.0% of the placebo group.
Conclusions: GR1802 was well tolerated and effective in moderate-to-severe AD patients, showing a dose–response trend at 150–300 mg.
Trial Registration: Chinese Registry of Clinical Trials: ChiCTR2100051917
1. Introduction
Atopic dermatitis (AD) is a chronic and recurrent inflammatory skin condition marked by intense pruritus and the presence of scaly, dry, eczematous lesions. In severe instances, AD can result in significant psychological distress, severe sleep disturbances, and diminished quality of life, ultimately leading to disability and substantial socioeconomic burdens [1]. The worldwide prevalence of AD has steadily risen over the last 3 decades, especially with rates in children in developed nations reaching 10%–20% [2–5]. The rate of growth in AD prevalence in China has lagged behind that of developed Western countries, Japan, and South Korea but has shown significant acceleration over the last decade. In 2014, based on clinician diagnostic criteria, the prevalence of AD among children aged 1–7 years in 12 cities in China was reported to be 12.94% [6], with a prevalence of 30.48% among infants aged 1–12 months [7]. The present systemic treatment regimen for moderate-to-severe AD primarily consists of nonspecific anti-inflammatory agents, such as corticosteroids, aimed at preventing exacerbations and enhancing epidermal function. Nonetheless, the use of systemic corticosteroids is generally discouraged in pediatric patients due to the potential for rebound effects following short-term therapy and the risk of long-term toxicity. Targeted biological therapy represents a promising approach for localized treatment of refractory AD patients, offering a reduced incidence of adverse effects [8, 9].
Th2 inflammation is a fundamental feature of AD, and IL-4 and IL-13 are important cytokines that mediate the pathogenesis of AD, mainly produced by Th2 cells, eosinophils, and Type 2 intrinsic lymphoid cells, etc. Th2 inflammatory factors can inhibit the expression of keratinocyte barrier–associated proteins, which further disrupts the skin barrier function. Dupilumab, a fully human monoclonal antibody targeting the interleukin-4 receptor alpha (IL-4Rα), functions by inhibiting signaling of IL-4 and IL-13, thereby serving as a therapeutic agent for Type 2 immune-mediated autoimmune-related diseases and has demonstrated significant therapeutic efficacy in various Phase 3 clinical trials for patients with moderate-to-severe AD [8, 10].
GR1802 is a humanized monoclonal antibody that demonstrates specificity in its binding to IL-4Ra, consequently impeding its interaction with IL-4 and IL-13. As evidenced by cross-species reactivity studies, GR1802 targets a different epitope on IL-4Rα compared to dupilumab. GR1802 binds to IL-4Rα from both human and crab-eating monkey samples, whereas dupilumab exclusively binds to human IL-4Rα. We assume that these differences in binding affinities may lead to varied clinical outcomes in treating patients with AD. Currently, GR1802 is also being developed to treat asthma, chronic rhinosinusitis, and allergic rhinitis, all of which share the same underlying pathology of Type 2 inflammatory disease. Here, we report the results of a Phase II study that we carried out to assess the efficacy, safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of GR1802 in AD subjects.
2. Methods
2.1. Study Design
The study was a Phase II, multicenter, double-blind, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of GR1802 injection in patients with moderate-to-severe AD across 30 sites in China. This trial was registered on the Chinese Clinical Trial Registry website (https://www.chictr.org.cn) in October 2021. The research encompassed a 4-week screening phase, a 16-week double-blind intervention phase, and an 8-week safety follow-up phase. The protocol, devised by the sponsor and investigators, received approval from all hospital institutional review boards. Prior to participation, all study subjects provided written consent after receiving comprehensive information about the study. The trial was conducted in strict accordance with the principles of Good Clinical Practice, the Declaration of Helsinki, and the relevant laws and regulations of China. Approval for the trial’s execution and related documentation was secured from the ethics committee at all study sites (Supporting Table S1).
2.2. Participants
Patients were assessed for eligibility at a screening visit no more than 28 days before treatment initiation. Patient eligibility criteria were defined as follows: age 18–70 years; patients who have been diagnosed with moderate-to-severe AD for at least 12 months; patients who demonstrate a baseline Investigator’s Global Assessment (IGA) score of ≥ 3, an EASI score of ≥ 16, and ≥ 10% BSA of AD involvement; and patients who were inadequately controlled with topical corticosteroids. Exclusion criteria included use of systemic immunosuppressants within 4 weeks and topical corticosteroid or calcineurin inhibitor within 2 weeks; experienced acute conjunctivitis/keratitis within 4 weeks before randomization; and presence of skin comorbidities at screening that may interfere with study evaluation.
2.3. Randomization and Treatment
In this Phase 2 study, patients were randomized in a 1:1:1 ratio to receive GR1802 injection 300/150 mg or placebo Q2W for 16 weeks using stratification factors (baseline IGA of 3 or ≥ 4) through the IWRS system. GR1802 injection and placebo were placed in prefilled syringes that were indistinguishable in appearance.
During the trial, participants were instructed to consistently apply the moisturizer (Cetaphil Moisturizing Cream) provided by the sponsor twice daily. In cases where it was deemed necessary for managing severe AD symptoms, rescue medications such as topical corticosteroids, topical calcineurin inhibitors, or systemic glucocorticoids were administered at the investigator’s discretion. Participants who received systemic glucocorticoids were removed from the study medication.
2.4. Outcomes
The main objective of the study was to determine the percentage of Eczema Area and Severity Index (EASI) responders who showed an improvement of at least 75% in their EASI scores compared to baseline at the 16-week mark.
Secondary efficacy outcomes included responders at other evaluation points from baseline to end of the study: IGA 0/1 with ≥ 2-point improvement; EASI-75; EASI-90; EASI-50; and ≥ 3- or ≥ 4-point improvements in weekly average of daily PP-NRS score. Additional secondary efficacy outcomes include change from baseline in EASI score and change from baseline in BSA of AD involvement.
The subgroup analyses, which were predefined and stratified based on baseline IGA scores (3 and above), were conducted in a manner consistent with the primary analysis for the primary outcomes.
The safety endpoints of the study encompassed adverse events (AEs), vital signs, physical examinations, electrocardiogram (ECG) readings, and clinical laboratory tests. Additionally, the study evaluated the PD response and immunogenicity of GR1802. Serum biomarkers, such as thymus activation-regulated chemokine (TARC), IgE levels, and eosinophil count, were measured to assess the PD response. The potential immunogenicity of GR1802 was investigated by measuring the presence of antidrug antibodies (ADAs) and neutralizing antibodies (Nabs).
2.5. Statistical Analysis
Based on preclinical data showing similar efficacy to dupilumab, the sample size calculation for the Phase 2 trial of GR1802 was based on an EASI-75 response rate of 51% at 300 mg Q2W dose in the dupilumab SOLO1 trial and 15% in the placebo group at Week 16. With a two-sided test and significance level of 0.05%, and 80% power, a total of 120 subjects (40 per group) were determined for the study, accounting for potential dropouts.
The primary population used for the efficacy analysis was the full analysis set (FAS), including all patients who had undergone randomization and had used the study drug at least once, obeying the intend-to-treated (ITT) principle. The safety set (SS) comprised all patients who were administered any study drug, while the pharmacokinetics set (PKCS) and immunogenicity set (IMGS) consisted of all patients who received any study drug and yielded at least one corresponding qualified result. The SS, PKCS, and IMGS were analyzed on an as-treated basis. The CMH test was used to analyze the difference in the proportion of subjects achieving EASI-75 between each test group and the placebo group, stratified by IGA score at randomization (3, ≥ 4); 95% confidence intervals for the difference in proportions were estimated using the stratified MN approach, with corresponding p values reported. Different types of strategy for intercurrent events according to the types proposed in E9 (R1) draft addendum were considered in this study for primary and secondary outcome analysis. If rescue medication was used or participants had missing values of endpoints for any visit, the patient would be treated as a nonresponder from when the rescue was used. SAS Version 9.4 was used for statistical analysis.
2.6. Role of the Funding Source
Genrix Biopharmaceutical sponsored the trial, supplied the trial treatments, and worked in conjunction with the investigators to develop the trial’s design and oversee the collection, analysis, interpretation of data, and preparation of the manuscript. All authors have given their approval to the final version of the manuscript and have taken on the ultimate responsibility for the decision to submit it for publication.
3. Results
3.1. Participants
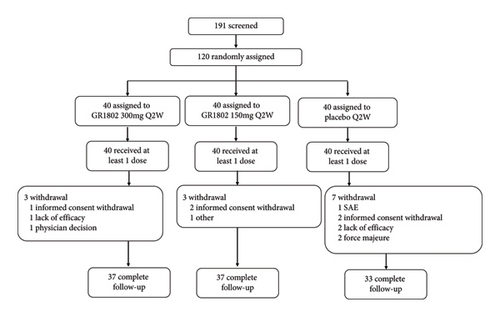
Of 191 patients screened for eligibility from August 26, 2022, to February 11, 2023, 71 failed to participate in this study. A total of 120 patients were selected to receive study treatment and were randomized to GR1802 300 mg (n = 40) and GR1802 150 mg (n = 40) or matched placebo (n = 40). Thirteen subjects (6 from the GR1802 group and 7 from placebo) dropped out before the end of the study, the most common reason being “withdrawal of consent” (Figure 1). The baseline characteristics are summarized in Table 1 and comparable between the 3 groups. The mean (SD) of baseline efficacy endpoints for all enrolled patients were as follows: EASI (22.6 [7.3]), IGA (3.5 [0.6]), BSA (33.8 [16.0]), and PP-NRS (6.9[1.7]).
| Characteristics | GR1802 150 mg (n = 40) |
GR1802 300 mg (n = 40) |
Placebo (n = 40) |
Total (n = 120) |
|---|---|---|---|---|
| Age (y) | 41.4 (14.8) | 42.3 (16.2) | 43.0 (16.3) | 42.2 (15.7) |
| Sex | ||||
| Female, n (%) | 12 (30.0) | 11 (27.5) | 16 (40.0) | 39 (32.5) |
| Male, n (%) | 28 (70.0) | 29 (72.5) | 24 (60.0) | 81 (67.5) |
| Race, Han, n (%) | 38 (95.0) | 39 (97.5) | 38 (95.0) | 115 (95.8) |
| Weight (kg) | 69.2 (12.1) | 71.9 (13.2) | 66.3 (14.5) | 69.1 (13.4) |
| Baseline EASI score | 23.0 (6.9) | 23.3 (8.2) | 21.4 (6.7) | 22.6 (7.3) |
| Baseline IGA score of 3, n (%) | 22 (55.0) | 22 (55.0) | 22 (55.0) | 66 (55.0) |
| Baseline IGA score of 4, n (%) | 16 (40.0) | 16 (40.0) | 18 (45.0) | 50 (41.7) |
| Baseline IGA score of 5, n (%) | 2 (5.0) | 2 (5.0) | 0 | 4 (3.3) |
| Baseline %BSA affected by AD | 36.2 (14.8) | 35.2 (17.1) | 30.0 (15.6) | 33.8 (16.0) |
| Baseline %BSA affected by AD, n (%) | ||||
| < 50 | 34 (85.0) | 35 (87.5) | 35 (87.5) | 104 (86.7) |
| 50–75 | 6 (15.0) | 4 (10.0) | 4 (10.0) | 14 (11.7) |
| > 75 | 0 | 1 (2.5) | 1 (2.5) | 2 (1.7) |
| Baseline PP-NRS score | 6.9 (1.7) | 6.9 (1.8) | 7.0 (1.7) | 6.9 (1.7) |
| Baseline PP-NRS score, n (%) | ||||
| < 4 | 1 (2.5) | 2 (5.0) | 1 (2.5) | 4 (3.3) |
| 4–7 | 24 (60.0) | 22 (55.0) | 25 (62.5) | 71 (59.2) |
| > 7 | 15 (37.5) | 16 (40.0) | 14 (35.0) | 45 (37.5) |
| ≥ 4 | 39 (97.5) | 38 (95.0) | 39 (97.5) | 116 (96.7) |
- Note: Data are n (%) or mean (SD), unless otherwise specified.
- Abbreviations: BMI, body mass index; BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; PP-NRS, Peak Pruritus Numerical Rating Scale.
3.2. Primary Endpoints
The EASI-75 response rates in the GR1802 300 mg, 150 mg, and placebo groups at Week 16 were 75.0% (30/40), 52.5% (21/40), and 32.5% (13/40), respectively. The observed differences in EASI-75 response rates were 39.1% (GR1802 300 mg vs. placebo, 95% CI 20.0–58.2) and 19.4% (GR1802 150 mg vs. placebo, 95% CI −0.8–39.7), with corresponding p values of 0.0002 and 0.0740, respectively (Figure 2). Sensitivity analyses were conducted employing the normal approximation (Wald) method for calculating 95% confidence intervals and the last observation carried forward (LOCF) technique for addressing missing data, respectively. These analyses yielded results consistent with those of the primary analysis.
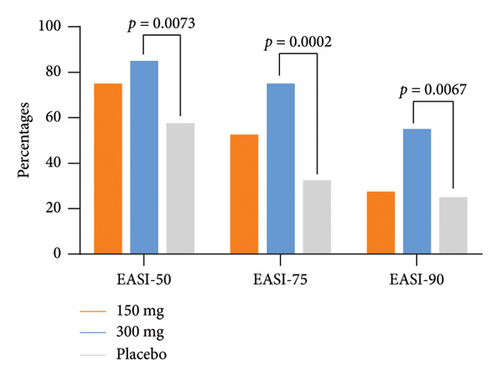
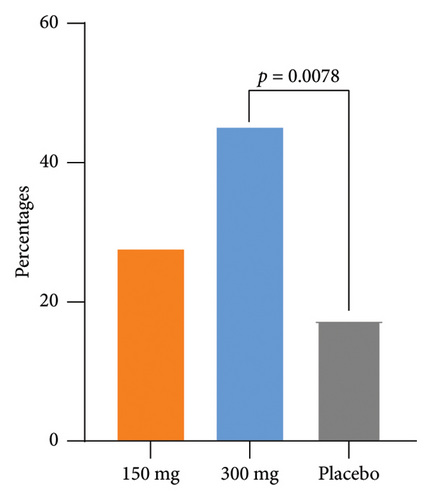
3.3. Secondary Endpoints
The response rates of EASI-50 and EASI-90 were significantly higher in the GR1802 300 mg group at Week 16 compared to the placebo group (85.0% vs. 57.5% and 55.0% vs. 25.0%, p = 0.0073 and 0.0067, respectively) (Table 2). Participants also achieved significantly higher IGA response (IGA score of 0/1 and a reduction of ≥ 2 points from baseline) rate (45.0% vs. 17.5%, p = 0.0078) (Table 2) and greater LS mean percent reductions in %BSA affected (−25.0 vs.−18.3, p = 0.0483) from baseline to Week 16. The metrics observed above in the 150 mg group indicated initial disparities in efficacy when compared to the placebo group, although these differences did not reach statistical significance.
| Items | GR1802 150 mg (n = 40) |
GR1802 300 mg (n = 40) |
Placebo (n = 40) |
|---|---|---|---|
| Patient with EASI-75 at Week 16 | |||
| Number, n (%) | 21 (52.5) | 30 (75.0) | 13 (32.5) |
| Difference (95% CI) vs. placebo, % | 19.4 (−0.8, 39.7) | 39.1 (20.0, 58.2) | |
| p value vs. placebo | 0.0740 | 0.0002 | |
| Patients with IGA 0-1 and ≥ 2-point improvement from baseline at Week 16 | |||
| Number, n (%) | 11 (27.5) | 18 (45.0) | 7 (17.5) |
| Difference (95% CI) vs. placebo, % | 10.1 (−8.5, 28.6) | 25.3 (6.4, 44.3) | |
| p value vs. placebo | 0.2892 | 0.0078 | |
| Patients with EASI-50 at Week 16 | |||
| Number, n (%) | 30 (75.0) | 34 (85.0) | 23 (57.5) |
| Difference (95% CI)vs. placebo, % | 17.6 (−2.0, 37.2) | 25.9 (7.0, 44.8) | |
| p value vs. placebo | 0.0973 | 0.0073 | |
| Patients with EASI-90 at Week 16 | |||
| Number, n (%) | 11 (27.5) | 22 (55.0) | 10 (25.0) |
| Difference (95% CI) vs. placebo, % | 2.7 (−16.5, 21.9) | 28.1 (8.3, 47.9) | |
| p value vs. placebo | 0.7999 | 0.0067 | |
| LS means change in AD-affected %BSA from baseline to EOS | |||
| Mean ± SE | −23.59 (2.203) | −26.16 (2.152) | −20.86 (2.346) |
| Difference (95% CI) vs. placebo, % | −2.73 (−9.11, 3.66) | −5.30 (−11.62, 1.02) | |
| p value vs. placebo | 0.3991 | 0.0991 | |
| LS mean percent change in EASI score from baseline to EOS | |||
| Mean ± SE | −72.48 (4.677) | −80.51 (4.550) | −73.93 (5.090) |
| Difference (95% CI) vs. placebo, % | 1.45 (−12.26, 15.16) | −6.58 (−20.12, 6.96) | |
| p value vs. placebo | 0.8343 | 0.3374 | |
| Patients with ≥ 3-point reduction in weekly average of daily PP-NRS score from baseline at Week 16 | |||
| Number, n (%) | 29 (72.5) | 26 (65.0) | 15 (37.5) |
| Difference (95% CI) vs. placebo, % | 32.0 (12.2, 51.9) | 25.2 (4.8, 45.6) | |
| p value vs. placebo | 0.0019 | 0.0151 | |
| Patients with ≥ 4-point reduction in weekly average of daily PP-NRS score from baseline at Week 16 | |||
| Number, n (%) | 18 (45.0) | 22 (55.0) | 10 (25.0) |
| Difference (95% CI) vs. placebo, % | 19.5 (−0.2, 39.2) | 27.7 (8.0, 47.5) | |
| p value vs. placebo | 0.0640 | 0.0064 | |
| LS mean percent change in weekly average of daily PP-NRS score from baseline to EOS | |||
| Mean (SE) | −49.24 (5.899) | −51.34 (5.720) | −31.33 (6.352) |
| Difference (95% CI) vs. placebo, % | −17.91 (−35.10, −0.72) | −20.01 (−36.96, −3.06) | |
| p value vs. placebo | 0.0413 | 0.0212 |
- Abbreviations: %BSA, percentage of body surface area; AD, atopic dermatitis; CI, confidence interval; EASI, Eczema Area and Severity Index; EOS, End of study; IGA, Investigator’s Global Assessment; LS, least squares; PP-NRS, Peak Pruritus Numerical Rating Scale; SE, standard error.
As for patient-reported outcomes, higher NRS reduction of ≥ 3 rate was observed in both the GR1802 groups compared to placebo (300 mg [65.0%] and 150 mg [72.5%] vs. placebo [37.5], p = 0.0151 and 0.0019, respectively). Similar results were observed in the case of the proportions of patients achieving ≥ 4-point improvements (300 mg [55.0%] and 150 mg [45.0%] vs. placebo [25.0], p = 0.0064 and 0.0640, respectively).
The group receiving a dosage of 300 mg exhibited noticeable effects by Week 6, as evidenced by a statistically significant increase in the proportion of participants achieving EASI-90 improvement compared to those in the placebo group. Furthermore, as the duration of dosing progressed, a greater number of participants in the treatment group experienced substantial enhancements in skin lesions. Specifically, 75.0%, 45.0%, and 37.5% of participants in the 300 mg, 150 mg, and placebo groups, respectively, demonstrated improvement in EASI-75 at Week 24. The EASI response rates at Week 24 in the 300 mg group, 10-week postfinal dose, continued to show significance compared to the 150 mg group. This sustained efficacy difference, along with prolonged maintenance of efficacy, highlights an initial quantitative correlation between the two dosage groups. The proportions of EASI-50, EASI-75, and EASI-90 responders from baseline at each time point are shown in Figure 3.
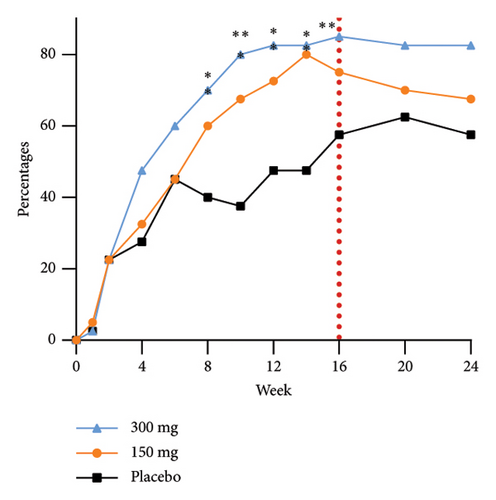
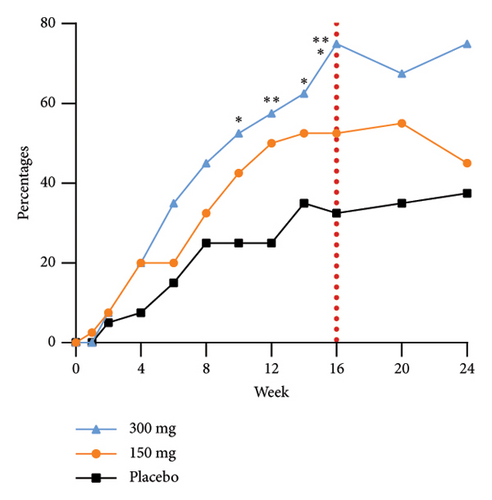
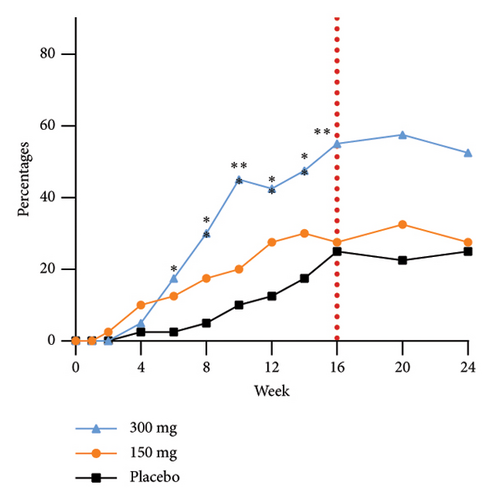
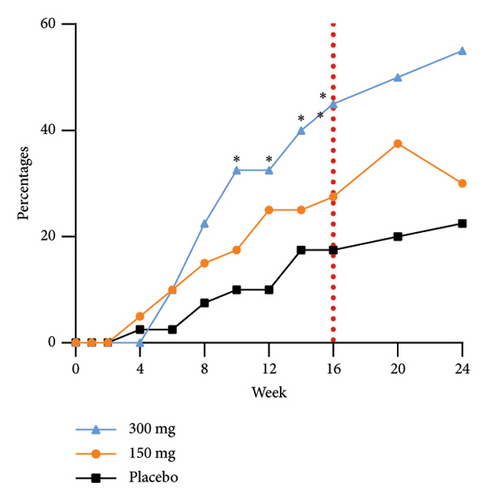
The efficacy outcomes for subjects with an IGA score of three in the subgroup analyses were found to be in line with the results of the primary analyses. Furthermore, the proportion of subjects who achieved an EASI-75 response at Week 16 of treatment was notably different between the GR1802 300 mg group and the 150 mg group compared to the placebo group for subjects with an IGA score of 4 or higher. These findings suggest that individuals with higher baseline IGA scores are more likely to experience positive effects from the treatment.
In this study, rescue therapy was administered to three subjects (7.5%) in the 150 mg group, one subject (2.5%) in the 300 mg group, and eight subjects (20.0%) in the placebo group. A total of five subjects who used rescue therapy before the W16 primary efficacy endpoint visit and completed the W16 visit, one in the GR1802 150 mg group (2.5%) and four in the placebo group (10.0%), were treated as nonresponders in the analysis of the primary efficacy.
3.4. Safety
There were no remarkable differences in the frequency of AEs among the GR1802 300 mg, 150 mg group, and placebo groups (82.5% [33/40], 75.0% [30/40] vs. 85.0% [34/40]) (Table 3). Drug-related TEAEs generally were similar among treatment groups (25.0% [10/40], 30.0% [12/40] vs. 25.0% [10/40]). No deaths or TEAEs leading to permanent discontinuation of the drug were reported. No SAEs were reported in the GR1802 300 mg group. Three SAEs (biliary cyst, pancreatitis, and intestinal obstruction) were reported in 1 participant in the GR1802 150 mg group. The biliary cyst was deemed to be associated with a preexisting condition, whereas pancreatitis was considered secondary to the biliary cyst. Surgical intervention addressing these two conditions resulted in a postoperative intestinal obstruction. Eight SAEs were reported in 4 patients in the placebo group (large intestine polyp, incarcerated inguinal hernia, gastritis erosive, AD, peripheral nerve lesion, and carpal tunnel syndrome). All SAEs were considered unrelated to the investigational medicinal products.
| GR1802 | Placebo (n = 40) n (%) | ||
|---|---|---|---|
| 150 mg (n = 40) n (%) | 300 mg (n = 40) n (%) | ||
| Any treatment-emergent adverse events (TEAE) | 30 (75.0) | 33 (82.5) | 34 (85.0) |
| Any serious adverse events | 1 (2.5) | 0 | 4 (10.0) |
| Any adverse event leading to permanent treatment discontinuation | 0 | 0 | 2 (5.0) |
| Any treatment-related adverse events | 12 (30.0) | 10 (25.0) | 10 (25.0) |
| Grade ≥ 3 TEAE | 4 (10.0) | 2 (5.0) | 5 (12.5) |
| TEAEs occurring in ≥ 10% of patients in any treatment group (MedDRA PT) | |||
| Upper respiratory tract infection, n (%) | 4 (10.0) | 5 (12.5) | 3 (7.5) |
| White blood count increased, n (%) | 4 (10.0) | 1 (2.5) | 1 (2.5) |
| Increased blood bilirubin, n (%) | 2 (5.0) | 2 (5.0) | 4 (10.0) |
| COVID-19, n (%) | 2 (5.0) | 5 (12.5) | 4 (10.0) |
| Suspected COVID-19, n (%) | 4 (10.0) | 3 (7.5) | 3 (7.5) |
| Hypercholesterolemia, n (%) | 2 (5.0) | 1 (2.5) | 4 (10.0) |
| Atopic dermatitis, n (%) | 3 (7.5) | 0 | 5 (12.5) |
- Note: Data are presented as number of patients (%).
The most prevalent AEs in the GR1802 group, defined as those occurring in ≥ 10% of participants in any treatment group, included increased white cell count, upper respiratory tract infection, suspected COVID-19, COVID-19, hyperuricemia, hypercholesterolemia, increased blood bilirubin, and AD. Patients administered with placebo exhibited a greater prevalence of AD flare (coded as AD under the preferred term of MedDRA as AEs) compared to those receiving GR1802 treatment, with incidence rates of 12.5% for placebo, 0% for GR1802 300 mg, and 7.5% for GR1802 150 mg. No adverse changes in vital signs, physical examinations, ECGs, and clinical laboratory tests were observed following the administration of GR1802. Of these, there was no imbalance in these AEs among the treatment groups.
3.5. Biomarkers
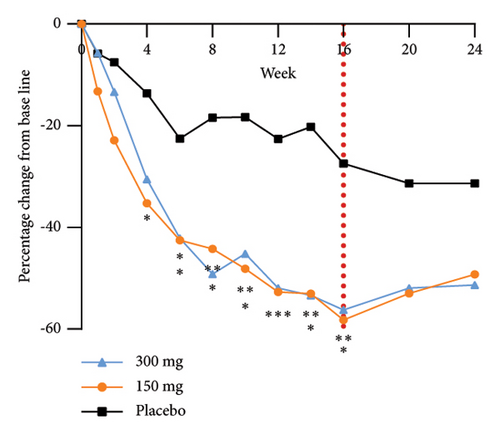
The analysis of exploratory endpoints demonstrated that administration of GR1802, at both 300 mg and 150 mg dosages, led to a significant reduction in serum concentrations of biomarkers associated with Type 2 inflammation, namely, IgE and TARC, by Week 16 (Figure 4). The eosinophil count in the GR1802 300 mg group exhibited a noticeable decrease over the course of treatment in comparison to the placebo group, with no statistically significant variance between the three groups in the posttreatment follow-up period.
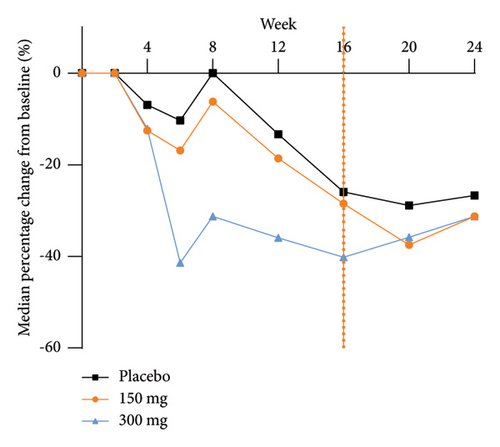
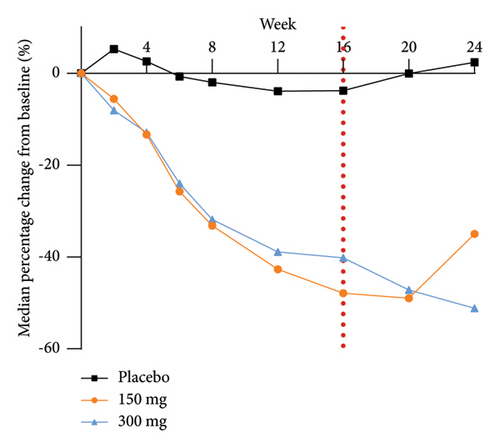
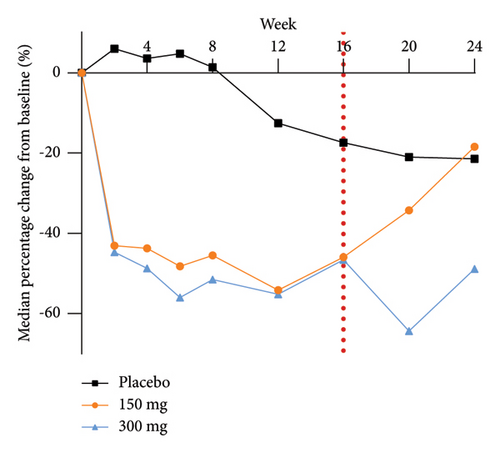
3.6. PK and Immunogenicity
The participants in this study underwent infrequent blood sampling, and the average trough concentration of the 300 mg group exhibited a slight increase with each subsequent dose, eventually achieving a relatively stable level following a doubling of the initial dose. The immunogenicity evaluation of GR1802 revealed that the rates of ADAs following treatment were 27.5% (11/40) in the 150 mg group and 15.8% (6/38) in the 300 mg group. Subjects with elevated ADA titers exhibited an impact on blood levels; however, there was no observed escalation in the incidence of AEs linked to immunogenicity in subjects with heightened ADA titers.
4. Discussion
The introduction of biologic therapies that target Type 2 inflammation has profoundly altered the treatment approach for AD. Clinical trials of dupilumab have shown that dupilumab improved AD symptoms by blocking IL-4Ra [8, 10]. In this study, the novel anti-IL-4Ra antibody, GR1802, demonstrated significant efficacy in ameliorating clinical manifestations of AD, such as pruritus (PP-NRS), skin lesions (measured objectively with EASI, IGA, and BSA), and overall quality of life. The group administered with 300 mg exhibited a dose–response relationship compared to the group receiving 150 mg.
Multiple clinical trials examining IL-4Ra receptor inhibitors in Chinese patients with AD have yielded findings on the efficacy including dupilumab, CBP-201, and CM310 [11–13]. The current study found that the GR1802 300 mg group exhibited significant efficacy, with a placebo-adjusted response rate of EASI-75 (42.5%) and IGA 0/1 (27.5%) at Week 16, which was comparable to the results of dupilumab in a Chinese Phase 3 trial (42.8% and 22.0% for EASI-75 and IGA 0/1, respectively), CM310 in a Phase 2b trial in China (50.0% and 10.0% for EASI-75 and IGA 0/1, respectively), and CBP-201 in a pivotal trial in China (39.5% and 22.8% for EASI-75 and IGA 0/1, respectively). However, these results were not from a head-to-head comparison analysis.
The validity of indirect comparisons of clinical trial outcomes in AD may be compromised by substantial variability in factors such as the characteristics of the populations enrolled and elements of the study methodology. The increased response rate in the placebo group in the current study (EASI-75 of 32.5%), in comparison to other trials, may be linked to the lower baseline average EASI scores among participants. The mean baseline EASI score in the placebo group of the current study was 21.43, compared to 31.0 in a Phase 3 trial of the approved anti–IL-4Ra therapy, dupilumab, and 26.25 in a Phase 2 trial of CM310, both of which were conducted with Chinese patients suffering from AD [11, 13]. Multiple studies have shown a correlation between the initial severity of AD and the efficacy of EASI responses. In all cases, patients with less severe AD exhibited greater EASI responses in placebo arms compared to those with more severe AD.
Referring to the Phase III randomized controlled study of dupilumab in Chinese patients with AD (not head-to-head comparison), the trends in the two trials were similar, with subjects in the dupilumab/GR1802-treated group using less rescue therapy than those in the placebo group in all cases. However, the overall incidence of rescue therapy was lower in this trial, with the incidence of rescue therapy (vs. placebo) at equivalent dose levels (i.e., dupilumab/GR1802 300 mg) being (19.5% vs. 50.6%) vs. (2.5% vs. 20.0%), respectively [11]. Due to the implementation of nonresponder imputation for these subjects, the reduced use of rescue medication in this study, compared to AD trials involving other medications, may have contributed to an elevated placebo effect.
Significantly, the group administered with 300 mg exhibited superior efficacy compared to the 150 mg group across various measures, such as EASI-50/75/90, IGA0/1, and BSA. This obvious dose–effect relationship could potentially be attributed to the observation that the administration of GR1802 injection displays a nonlinear pharmacokinetic pattern within the dosage range of 150–300 mg. Specifically, there is a notable over twofold rise in the steady-state trough concentration of GR1802 in the 300 mg group compared to the 150 mg group, along with a decreased incidence of ADAs.
However, there was no statistically significant difference in effectiveness between the 150 mg and 300 mg groups in terms of reducing pruritus, suggesting that GR1802 may have differing blood concentration thresholds for alleviating itch and lesions in patients with AD. Likewise, Chen et al. found that dupilumab effectively reduces pruritus in adult patients with AD, regardless of any observed enhancements in skin condition [14]. Moreover, the biomarker studies of this trial exhibited comparable outcomes. TARC serves as a chemotactic factor that exhibits selective attraction toward Th2 cells [15]. The levels of TARC exhibited a rapid decline across all test groups following drug administration in comparison to the placebo, persisting at low levels throughout the administration period and gradually increasing postdiscontinuation. Notably, there were no significant differences observed between the 300 and 150 mg groups during the administration phase. The trend of total IgE levels over a 16-week period exhibited similarities to those of TARC levels. These findings imply that TARC and IgE levels may serve as more sensitive biomarkers for assessing pruritic symptoms than biomarkers related to improvements in skin lesions.
The safety profile of GR1802 was similar to that of placebo. The predominant AEs observed in the GR1802 group were mostly associated with the COVID-19 pandemic (including upper respiratory tract infection, white blood count increased, COVID-19, and suspected COVID-19) with no discernible increase in incidence compared to the placebo group. The elevated incidence of AD observed in the placebo group may be linked to the impact of GR1802 on the restoration of skin-barrier integrity and reduction of scratching behavior. Conjunctivitis, a prevalent adverse effect of dupilumab in individuals with AD (occurring in approximately 10% of cases), was observed at a frequency of 6.8% in the GR1802 group during the study, compared to a rate of 2.3% in the placebo group. This occurrence, while lower than that associated with dupilumab, warrants confirmation through a more extensive Phase 3 clinical trial [11, 16].
This trial was constrained by a number of limitations, including the absence of an examination into the treatment’s long-term safety and efficacy in the context of a Phase 2 clinical trial. Furthermore, the study only included a relatively small sample size of Chinese patients with moderate-to-severe AD. A Phase 3 clinical trial of GR1802 is currently being conducted in a larger population of individuals with AD, with an extended duration of treatment.
5. Conclusions
In summary, the administration of GR1802 resulted in a significant improvement in the signs and symptoms of moderate-to-severe AD in adult patients, demonstrating a favorable safety profile. The group receiving 300 mg exhibited superior initial efficacy compared to the group receiving 150 mg, thereby providing support for the selection of dosages for subsequent Phase 3 clinical trials. This study offers additional support for the notion that IL-4Ra serves as a crucial target for addressing AD and suggests that GR1802 shows promise as a biologic therapy for adults presenting with moderate-to-severe AD.
Conflicts of Interest
Wei Wang is an employee of Chongqing Genrix Biopharmaceutical Co. Limited. The other authors declare no conflicts of interest.
Author Contributions
All authors developed the initial research concept and contributed to protocol development, study procedures, and delivery of the study. S.W. wrote the manuscript. S.W. and J.X. had full access to the data and verified the data. All authors had full access to the study data, approved the final version of the manuscript, and had final responsibility for the submission.
Funding
This study was sponsored by Chongqing Genrix Biopharmaceutical Co. Limited.
Acknowledgments
This study was sponsored by Chongqing Genrix Biopharmaceutical Co. Limited. The authors would like to thank Zhongyi Hu for statistical analysis support.
Supporting Information
The IRB approval documentation (including institutional lists and approval numbers) from all participating centers is available in the Supporting Information.
Open Research
Data Availability Statement
Data are available on request from the authors.




