The Effectiveness and Safety of Three Treatment Regimens of Topical Minoxidil 5.0%, Betamethasone 0.064% w/w, and Castor and Jojoba Oils for Alopecia Areata: A Multicenter Cohort Study
Abstract
Introduction: Alopecia areata (AA) is a chronic, remitting–relapsing dermatological disease that is associated with a substantial psychological impact. Despite the availability of a wide range of therapeutic options, none provides a cure for AA. This study aimed to compare the effectiveness of topical betamethasone as a monotherapy with combinations of topical betamethasone with either topical minoxidil 5% or a herbal preparation of castor and jojoba oils.
Methods: This was a multicenter, cohort study in which patients diagnosed with AA were taking one of three treatment regimens: a reference monotherapy of topical betamethasone 0.064% w/w; combined topical minoxidil and betamethasone 0.064% w/w; or combined topical betamethasone 0.064% w/w and a herbal preparation of castor and jojoba oils. The data were collected at the beginning of the study using a questionnaire. Patients were assessed at three follow-up visits for hair regrowth using trichoscopy as the primary outcome. Patient satisfaction and compliance were assessed using 10-point scales.
Results: The final sample consisted of 278 patients. Combined topical minoxidil–betamethasone therapy was significantly associated with higher rates of hair regrowth (p = 0.006), patient satisfaction (p < 0.001), and shorter median time to first improvement (p < 0.001). Combined minoxidil/betamethasone was more likely to achieve hair regrowth than the other two treatments at the multivariate level (aRR = 2.239, CI = 1.153–4.347). Moreover, hair regrowth was significantly different between the treatment groups after each phase, with hair regrowth at the final phase observed in 83.2% of patients using combined topical minoxidil and betamethasone.
Conclusions: The use of topical minoxidil–betamethasone combination for AA was superior to betamethasone monotherapy or combined with herbal preparations. Randomized clinical trials are needed to strengthen the evidence.
1. Introduction
Alopecia areata (AA) is a chronic, inflammatory, autoimmune disease that affects anagen hair follicles, presenting on a spectrum that ranges from limited, patchy, round hair loss to more severe forms where hair loss extends to the entire scalp (alopecia totalis) or entire body (alopecia universalis) [1]. In most cases, the scalp hair is mainly affected, but other locations, such as the brows, eyelashes, and beard, may also be affected [1]. Moreover, AA is associated with depression, anxiety, and low health-related quality of life (HRQoL) [2, 3].
Several studies have investigated the demographic and clinical factors influencing the severity of AA with varying findings. In most studies, early age of onset; family history of AA; and personal history of autoimmune diseases, such as thyroiditis and atopic dermatitis, were associated with increased severity of AA [4–6]. Other disease-related factors were also found to increase the severity of AA, including disease duration and nail changes [4, 6]. In addition, genetic susceptibility may play a role in the prognosis of AA, as patients with specific human leukocyte antigen (HLA) class II alleles tend to have a more severe course [7].
Multiple options have been proposed for the treatment of AA. However, AA is characterized by a chronic, relapsing nature because none of the multiple available options and approaches offers a long-term cure [8]. As a result, the US Food and Drug Administration (FDA) has not approved any treatment yet and the proposed choices are thus prescribed off-label [8]. These include systemic, topical, and intralesional corticosteroids; topical immunosuppressive agents; contact sensitizers; minoxidil; and tacrolimus [9]. Among these, topical corticosteroids are one of the most commonly used treatments for AA despite their varying efficacy [10, 11]. Minoxidil is another promising treatment option due to its safety, tolerability, and efficacy in the treatment of nonsevere AA [9, 12]. Additionally, several herbal treatments have been suggested for use in AA. While different herbal options are used in different places, essential oils, aromatherapy, and topical garlic have demonstrated the best evidence [13].
The lack of consensus on the best therapeutic options highlights the need for exploring potential monotherapies and combinations, which may contribute to improving AA treatment outcomes. To date, clinical trials and observational studies have investigated a wide range of possible combinations, with only a few exploring those of topical betamethasone, minoxidil 5%, and castor and jojoba oils [14]. This study aimed to investigate the effectiveness and safety of topical betamethasone 0.064% w/w, as a monotherapy, and in combination with either topical minoxidil 5% or a herbal preparation of castor and jojoba oil in a real-world setting. These topical options are among the most widely used in global and local dermatology practices, including castor and jojoba oils [15, 16].
2. Methods
2.1. Study Design and Settings
This three-arm, clinical cohort study was carried out from June 2023 to January 2024, corresponding to the period starting with recruitment and ending with the last follow-up. The study targeted patients who sought treatment for AA in seven dermatology centers in the major cities of the Palestinian West Bank. The study consisted of three patient groups: the reference group used a monotherapy of topical betamethasone; the second treatment group used a combination therapy of topical minoxidil 5% and topical betamethasone 0.064% w/w; and the third group used a combination therapy of topical castor and jojoba oils and topical betamethasone. Participants were instructed to wash the affected area; dry it completely before application; allow for medication absorption before covering or sleeping; and avoid washing the area with water for at least 4 h. For the three groups, the treatments were applied twice daily. The participants were provided with detailed verbal instructions to ensure proper application, with the following amount for each treatment: a pea-sized amount (0.5 cm) of betamethasone per 2 cm2 of affected area; 1 mL of sprayed minoxidil; and three drops of castor and jojoba oils per 2 cm2 of the affected area. For those who used a combination therapy, minoxidil or oils were applied first, and betamethasone was applied after waiting 30 to 60 min to allow for absorption of the first topical treatment. The study comprised three phases, with the treatment groups assessed at three follow-up visits made over 3 months. The primary outcome was dermatologist-assessed positive hair growth measured by trichoscopy. Topical castor and jojoba treatment is the most commonly used herbal preparation locally. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement was used to report the research [17].
2.2. Population and Inclusion and Exclusion Criteria
- 1.
Pregnant and lactating women
- 2.
Patients who received any treatment for AA in the 3 months preceding the study
- 3.
Patients who received a diagnosis of a dermatological condition that may be severe enough to impact the study outcome, including active psoriasis, atopic dermatitis, vitiligo, trichotillomania, fungal infection, and cutaneous sarcoidosis.
- 4.
Patients diagnosed with autoimmune conditions, including rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, Addison’s disease, multiple sclerosis, and celiac disease [18, 19]. Patients with diabetes mellitus and thyroiditis were not excluded; rather, having a personal history of these diseases was considered in the analysis.
- 5.
Patients with other confounding conditions, including chronic liver and kidney diseases, iron deficiency, amyloidosis, and infections with Treponema pallidum or human immunodeficiency virus (HIV) [19–21].
- 6.
Patients diagnosed with androgenic alopecia or extensive types of AA (alopecia totalis, alopecia universalis, and those with surface area involvement > 50%).
- 7.
Patients receiving chemotherapy or radiotherapy, or taking retinoids, cytotoxic agents, including methotrexate, acitretin, and 5-fluorouracil, for more than 3 months during the 6 months preceding the study. Despite the potential associations between multiple other drugs and hair loss, patients taking these drugs were not excluded because the pertinent evidence is mixed and largely based on case reports [22].
2.3. Sampling and Selection of Cohorts
The expected values were chosen based on a review of the literature related to minoxidil 5% and AA. One meta-analysis reported a pooled RR of 8.37 and a pooled proportion of 5.6% (π1) based on events of hair regrowth as an outcome among nonexposed groups in three clinical trials [24]. Based on these values, the sample size for each group was 17. Given the limited number of studies and study participants in each study, the sample size was recalculated, assuming a more conservative RR of 4.0, resulting in a sample size of 66 for each group. Due to expectations of dropouts, as many participants as possible were recruited.
2.4. Data Collection and Measurement
The demographic and clinical data were collected at the start of the study. The demographic section included questions about age (8–17, 18–29, 30–49, and ≥ 50 years), gender (male and female), residency (city, village, refugee camp), governorate, educational level (did not receive primary education, primary, secondary, and higher education), marital status (married and single), employment status (employed, unemployed, and student), and monthly household income level (low: < 2705 NIS; mid: 2705–10,000 NIS; and high: > 10,000 NIS). The clinical section included questions about receiving a previous diagnosis of AA; receiving a previous treatment for AA; personal histories of diabetes or autoimmune thyroiditis; and family histories of AA, diabetes type I, or autoimmune thyroiditis.
The diagnostic approach to AA was discussed and revised with each dermatologist to ensure measurement validity and reliability in different centers. AA is mainly diagnosed based on clinical examination and trichoscopy [25]. AA is characterized by patches of hair loss, mostly pigmented hair, located on normal skin. The main trichoscopic manifestation is the observation of exclamation marks described as short hair shafts that exhibit dark and thickened ends. The follicular openings filled with sebum and keratin, termed yellow dots, and secondary vellus hairs are other common trichoscopic observations. Other features include triangular hair, tapered hairs, broken hairs, and black dots [26, 27].
The primary and secondary outcomes and the side effects were measured at each of the three follow-up visits. The primary outcome of the study was dermatologist-assessed hair regrowth using trichoscopy, conducted at each of the two follow-up visits and at the end of the study.
The secondary outcomes were dermatologist-assessed hair regrowth after the first and second phases, in addition to median time to first drug response, patient satisfaction, and compliance scores. Both were measured on a 10-item graded scale as reported by the participants in an interview, conducted by the co-authors. The inter-rater reliability was pretested by interviewing 15 patients with different interviewers before the start of the study. The validity of the scales was enhanced by receiving patients’ feedback on the clarity, accuracy, and appropriateness of the questions.
2.5. Data Analysis
The Statistical Package for the Social Sciences (SPSS) software version 27.0 (IBM Corporation, USA) was used to analyze the data. The normality of the data was tested using the Shapiro–Wilk test. Both descriptive and inferential statistics were employed to analyze the data. Frequencies and percentages were used to describe the demographic and clinical variables and the side effects. The mean and standard deviation (SD) were reported for normally distributed data, and the mean rank, median, and interquartile range (IQR) were reported for non-normally distributed data. The median time to first drug response (defined by the time from the study start to the phase where hair regrowth was first observed) was calculated by including the cases that had confirmed hair regrowth at the end of the study. Differences between the three treatment groups were tested using the Chi-squared test, except for variables with a cell of less than five counts for which the Fisher’s exact test was used. The Mann–Whitney U and Kruskal–Wallis tests were used to analyze differences in patient satisfaction and compliance scores. At the multivariate level, binary logistic regression was employed to estimate adjusted risk ratio (aRR) by designing a model that is based on a clinical selection of potential confounders and includes any additional variables that demonstrated significant associations with the exposure and outcome at the bivariate level. The clinical selection of variables included a family history of AA and a personal history of thyroiditis [5, 28]. The Hosmer and Lemeshow test was used to test for goodness of fit (p value > 0.05 indicates a good fit). The precision was determined with a significance level of p value < 0.05 and a 95% confidence interval (CI). Complete-case analysis was employed by omitting participants with missing data from the final analysis.
2.6. Ethical Considerations
The approval for conducting the study was obtained from the Institutional Review Board (IRB) at An-Najah National University (ANNU) (reference number: stu.MRC-118-56-4-23). All patients enrolled in the study provided voluntary and informed consent. Patients younger than 18 years were enrolled after a parent or legal guardian gave consent for them. The privacy of the patients was ensured. The relevant data were only used for research purposes and discarded safely thereafter.
3. Results
3.1. Participants Characteristics
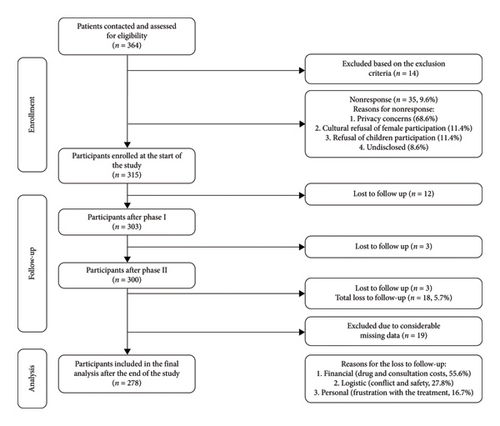
Of the 364 patients who were contacted and checked for eligibility, 14 were excluded and 35 refused to participate (response rate = 90.4%). Of the 315 who enrolled at the start of the study, 278 were included in the final analysis, with a drop-out rate of 5.7% (see further details on nonparticipation, loss to follow-up, missing data, and the reasons thereof in Figure 1). Of the final sample, 157 (56.5%) were males and 121 (43.5%) were females, with a mean age of 27.9 (SD = 12.2). Approximately half of the participants received only primary or secondary education (48.9%), and over one-third received higher education (38.1%). Almost half of the participants were employed (35.3%), and only 20.1% were unemployed. The rest were either school or university students (35.3%) (Table 1).
| Characteristics | Reference group (monotherapy of betamethasone) n (%) | Second group (combined topical betamethasone and minoxidil) n (%) | Third group (combined topical betamethasone and herbal treatment) n (%) | Total | p value | |
|---|---|---|---|---|---|---|
| Age (years) | 8–17 | 8 (8) | 34 (28.6) | 5 (8.5) | 47 (16.9) | <0.001∗ |
| 18–29 | 54 (54) | 35 (29.4) | 25 (42.4) | 114 (41) | ||
| 30–49 | 31 (31) | 40 (33.6) | 27 (45.8) | 98 (35.3) | ||
| ≥ 50 | 7 (7) | 10 (8.4) | 2 (3.4) | 19 (6.8) | ||
| Alternative age classification (adolescents and adults) | Adolescents | 8 (8) | 34 (28.6) | 5 (8.5) | 47 (16.9) | <0.001∗ |
| Adults | 92 (92) | 85 (71.4) | 54 (91.5) | 231 (83.1) | ||
| Gender | Male | 58 (58) | 68 (57.1) | 31 (52.5) | 157 (56.5) | 0.784 |
| Female | 42 (42) | 51 (42.9) | 28 (47.5) | 121 (43.5) | ||
| Residency | City | 74 (74) | 72 (60.5) | 40 (67.8) | 186 (66.9) | 0.051 |
| Village | 25 (25) | 36 (30.3) | 16 (27.1) | 77 (27.7) | ||
| Camp | 1 (1) | 11 (9.2) | 3 (5.1) | 15 (5.4) | ||
| Governorate | Northern | 65 (65) | 70 (58.5) | 39 (66.1) | 174 (62.6) | 0.443 |
| Middle | 32 (32) | 48 (40.3) | 20 (33.9) | 100 (36) | ||
| Southern | 3 (3) | 1 (0.8) | 0 (0) | 4 (1.4) | ||
| Level of education | Did not receive formal education | 14 (14) | 16 (13.4) | 6 (10.2) | 36 (12.9) | 0.021∗ |
| Primary | 12 (12) | 36 (30.3) | 9 (15.3) | 57 (20.5) | ||
| Secondary | 30 (30) | 27 (22.7) | 22 (37.3) | 79 (28.4) | ||
| Higher education | 44 (44) | 40 (33.6) | 22 (37.3) | 106 (38.1) | ||
| Marital status | Single | 53 (53) | 72 (60.5) | 28 (47.5) | 153 (55) | 0.226 |
| Married | 47 (47) | 47 (39.5) | 31 (52.5) | 125 (45) | ||
| Employment status | Employed | 54 (54) | 45 (37.8) | 25 (42.4) | 124 (44.6) | 0.013∗ |
| Student | 35 (35) | 47 (39.5) | 16 (27.1) | 98 (35.3) | ||
| Unemployed | 11 (11) | 27 (22.7) | 18 (30.5) | 56 (20.1) | ||
| Household income level | Low | 15 (15) | 21 (17.6) | 9 (15.3) | 45 (16.2) | 0.696 |
| Middle | 75 (75) | 85 (71.4) | 40 (67.8) | 200 (71.9) | ||
| High | 10 (10) | 13 (10.9) | 10 (16.9) | 33 (11.9) | ||
| Received a previous diagnosis of AA | Yes | 86 (86) | 86 (72.3) | 48 (81.4) | 220 (79.1) | 0.040∗ |
| No | 14 (14) | 33 (27.7) | 11 (18.6) | 58 (20.9) | ||
| Received a previous treatment for AA | Yes | 23 (24) | 28 (27.2) | 21 (38.9) | 72 (28.5) | 0.141 |
| No | 73 (76) | 75 (72.8) | 33 (61.1) | 181 (71.5) | ||
| Personal history of DM1 | Yes | 8 (8) | 4 (3.4) | 2 (3.4) | 14 (5) | 0.268 |
| No | 92 (92) | 115 (96.6) | 57 (96.6) | 264 (95) | ||
| Personal history of thyroiditis | Yes | 12 (12) | 16 (13.4) | 8 (13.6) | 36 (12.9) | 0.939 |
| No | 88 (88) | 103 (86.6) | 51 (86.4) | 242 (87.1) | ||
| Family history of AA | Yes | 59 (59.0) | 64 (53.8) | 27 (45.8) | 150 (54.0) | 0.270 |
| No | 41 (41.0) | 55 (46.2) | 32 (54.2) | 128 (46.0) | ||
| Family history of DM1 | Yes | 13 (13) | 11 (9.2) | 5 (8.5) | 29 (10.4) | 0.569 |
| No | 87 (87) | 108 (90.8) | 54 (91.5) | 249 (89.6) | ||
| Family history of thyroiditis | Yes | 15 (15) | 6 (5) | 7 (11.9) | 28 (10.1) | 0.045∗ |
| No | 85 (85) | 113 (95) | 52 (88.1) | 250 (89.9) | ||
- Note: The Chi-squared test was used to test for statistical significance.
- Abbreviations: AA, alopecia areata; DM, diabetes mellitus.
- ∗p value is below the threshold value for significance (0.05).
The majority reported receiving a previous diagnosis of AA (79.1%), but less than one-third used a previous treatment for AA (26.3%). Of the patients reporting a positive family history of AA (54.0%), most had a first-degree family history (81.3%), followed by those who had a second (16.0%) and third-degree (2.7%) family histories of AA. The study participants received either a monotherapy of topical betamethasone (n = 100, 36.0%), combined minoxidil and betamethasone (n = 119, 42.8%), or combined castor and jojoba oils and betamethasone (n = 59, 21.2%). The three treatment groups were significantly different in age (p < 0.001), educational level (p = 0.021), employment status (p = 0.013), receiving a previous diagnosis of AA (p = 0.040), having a family history of thyroiditis (p = 0.045), hair regrowth (p = 0.006), and the patient satisfaction score (p < 0.001) (Table 1).
3.2. Drug Efficacy
3.2.1. Primary Outcome: Dermatologist-Assessed Hair Regrowth at the End of the Study
Hair regrowth was significantly different between the three groups after the first (p = 0.006), second (p < 0.001), and third phases (p < 0.001). At the conclusion of the study, 83.2% of patients using combined topical minoxidil–betamethasone showed hair regrowth, while lower proportions of patients using betamethasone monotherapy (67.0%) and combined herbal–betamethasone therapy (64.4%) showed hair regrowth (Table 2). Moreover, hair regrowth was significantly associated with the level of education (p = 0.018) and family history of AA (p = 0.015) at the bivariate level. The other variables did not demonstrate statistical significance (Table 3).
| Outcome | Reference group (monotherapy of betamethasone) N (%) |
Second group (combined topical betamethasone and minoxidil) N (%) |
Third group (combined topical betamethasone and herbal preparation) N (%) |
Total | p value |
|---|---|---|---|---|---|
| Hair regrowth after Phase 1 | |||||
| Yes | 64 (64) | 97 (81.5) | 30 (50.8) | 191 (68.7) | <0.001∗ |
| No | 36 (36) | 22 (18.5) | 29 (49.2) | 87 (31.3) | |
| Hair regrowth after Phase 2 | |||||
| Yes | 62 (62) | 109 (91.6) | 44 (74.6) | 215 (77.3) | <0.001∗ |
| No | 38 (38) | 10 (8.4) | 15 (25.4) | 63 (22.7) | |
| Hair regrowth after Phase 3 | |||||
| Yes | 67 (67) | 99 (83.2) | 38 (64.4) | 204 (73.4) | 0.006∗ |
| No | 33 (33) | 20 (16.8) | 21 (35.6) | 74 (26.6) | |
- Note: The Chi-squared test was used to test for statistical significance.
- ∗p value is below the threshold value for significance (0.05).
| Characteristic | Positive hair regrowth N (%) |
Negative hair regrowth N (%) |
Total | p value | |
|---|---|---|---|---|---|
| Age (years) | 8–17 | 39 (19.1) | 8 (10.8) | 47 (16.9) | 0.351 |
| 18–29 | 81 (39.7) | 33 (44.6) | 114 (41) | ||
| 30–49 | 69 (33.8) | 29 (39.2) | 98 (35.3) | ||
| ≥ 50 | 15 (7.4) | 4 (5.4) | 19 (6.8) | ||
| Alternative age classification (adolescents and adults) | Adolescents | 39 (19.1) | 8 (10.8) | 47 (16.9) | 0.102 |
| Adults | 165 (80.9) | 66 (89.2) | 231 (83.1) | ||
| Gender | Male | 117 (57.4) | 40 (54.1) | 157 (56.5) | 0.624 |
| Female | 87 (42.6) | 34 (45.9) | 121 (43.5) | ||
| Level of education | Did not receive formal education | 26 (12.7) | 10 (13.5) | 36 (12.9) | 0.018∗ |
| Primary education | 51 (25) | 6 (8.1) | 57 (20.5) | ||
| Secondary education | 56 (27.5) | 23 (31.1) | 79 (28.4) | ||
| Higher education | 71 (34.8) | 35 (47.3) | 106 (38.1) | ||
| Marital status | Single | 110 (53.9) | 43 (58.1) | 153 (55) | 0.535 |
| Married | 94 (46.1) | 31 (41.9) | 125 (45) | ||
| Employment status | Employed | 85 (41.7) | 39 (52.7) | 124 (44.6) | 0.253 |
| Student | 75 (36.8) | 23 (31.1) | 98 (35.3) | ||
| Unemployed | 44 (21.6) | 12 (16.2) | 56 (20.1) | ||
| Household income level | Low | 28 (13.7) | 17 (23) | 45 (16.2) | 0.163 |
| Middle | 150 (73.5) | 50 (67.6) | 200 (71.9) | ||
| High | 26 (12.7) | 7 (9.5) | 33 (11.9) | ||
| Received a previous diagnosis of AA | Yes | 159 (77.9) | 61 (82.4) | 220 (79.1) | |
| No | 45 (22.1) | 13 (17.6) | 58 (20.9) | ||
| Received a previous treatment for AA | Yes | 55 (29.9) | 17 (24.6) | 72 (28.5) | 0.409 |
| No | 129 (70.1) | 52 (75.4) | 181 (71.5) | ||
| Personal history of DM1 | Yes | 12 (5.9) | 2 (2.7) | 14 (5) | 0.367 |
| No | 192 (94.1) | 72 (97.3) | 264 (95) | ||
| Personal history of thyroiditis | Yes | 22 (10.8) | 14 (18.9) | 36 (12.9) | 0.074 |
| No | 182 (89.2) | 60 (81.1) | 242 (87.1) | ||
| Family history of AA | Yes | 119 (58.3) | 31 (41.9) | 150 (54) | 0.015∗ |
| No | 85 (41.7) | 43 (58.1) | 128 (46) | ||
| Family history of DM1 | Yes | 18 (8.8) | 11 (14.9) | 29 (10.4) | 0.145 |
| No | 186 (91.2) | 63 (85.1) | 249 (89.6) | ||
| Family history of thyroiditis | Yes | 21 (10.3) | 7 (9.5) | 28 (10.1) | 0.838 |
| No | 183 (89.7) | 67 (90.5) | 250 (89.9) | ||
- Note: The Chi-squared test was used to test for statistical significance.
- Abbreviations: AA, alopecia areata; DM, diabetes mellitus.
- ∗p value is below the threshold value for significance (0.05).
The adjusted binary logistic regression model included all the variables that were selected clinically, including family history of AA and personal history of thyroiditis, in addition to the level of education, which demonstrated significant associations with both the exposure and outcome at the bivariate level. The model showed that only using combined topical minoxidil–betamethasone therapy (aRR = 2.239, CI = 1.153–4.347) and family history of AA (aRR = 0.502, CI = 0.283–0.890) were significantly associated with hair regrowth. Using a combined minoxidil–betamethasone therapy was 123.9% more likely to induce hair regrowth than using a monotherapy of betamethasone (Table 4).
| Adjusted risk ratio (CI 95%) | Adjusted p value | |
|---|---|---|
| Level of education | ||
| Did not receive formal education | Reference | — |
| Primary education | 2.975 (0.936–9.456) | 0.065 |
| Secondary education | 1.026 (0.413–2.551) | 0.956 |
| High education | 0.722 (0.303–1.719) | 0.462 |
| Family history of alopecia areata | ||
| Negative | Reference | — |
| Positive | 0.502 (0.283–0.890) | 0.018∗ |
| Personal history of thyroiditis | ||
| Negative | Reference | — |
| Positive | 2.072 (0.942–4.559) | 0.070 |
| Treatment group | ||
| Monotherapy of betamethasone | Reference | — |
| Combined betamethasone and minoxidil | 2.239 (1.153–4.347) | 0.017∗ |
| Combined betamethasone and herbal preparation | 0.935 (0.461–1.899) | 0.853 |
- Note: Binary logistic regression was employed for the analysis. The model included potential confounders by clinical selection of variables based on the literature, in addition to the variables that demonstrated significant at the bivariate level (family history of alopecia areata); personal history of thyroiditis; educational level. Hosmer and Lemeshow test indicated a goodness of fit (p value > 0.05) for the model.
- ∗p value is below the threshold value for significance (0.05).
3.2.2. Patient Satisfaction
The patient satisfaction median score was significantly higher for the group using a combined minoxidil and betamethasone treatment (7.0/10.0, p < 0.001), followed by the group using a combined herbal–betamethasone treatment (6.0/10.0) and the referent group using betamethasone monotherapy (5.0/10.0). Only hair regrowth was significantly associated with the patient satisfaction score (p < 0.001) (Table 5).
| Variable | Mean rank | Median (Q1–Q3) | p value | |
|---|---|---|---|---|
| Age category (years) | 8–17 | 150.26 | 7 (5–9) | 0.541 |
| 18–29 | 131.79 | 6 (4–8) | ||
| 30–49 | 141.95 | 6 (4–8) | ||
| ≥ 50 | 146.53 | 7 (4–9) | ||
| Alternative age classification (adolescents and adults) | Adolescents (≤ 19) | 150.26 | 7 (5–9) | 0.311 |
| Adults (≥ 19) | 137.31 | 6 (4–8) | ||
| Gender | Male | 138.06 | 6 (4–8) | 0.733 |
| Female | 141.36 | 7 (4–8.25) | ||
| Marital status | Single | 139.01 | 7 (4–8) | 0.910 |
| Married | 140.10 | 6 (4–8) | ||
| Household income level | Low-level | 132.79 | 6 (4–8) | 0.726 |
| Mid-level | 139.71 | 6 (4–8) | ||
| High-level | 147.38 | 7 (4.75–9) | ||
| Residency | City | 145.63 | 7 (4.25–8) | 0.185 |
| Village | 126.21 | 6 (4–8) | ||
| Camp | 131.73 | 6 (4–8.25) | ||
| City | Northern | 137.47 | 7 (4.25–8) | 0.244 |
| Middle | 145.39 | 6 (4–9) | ||
| Southern | 80.75 | 3 (1–5.5) | ||
| Level of education | Did not receive formal education | 142.19 | 6.5 (5–9) | 0.086 |
| Primary | 162.75 | 8 (6–9) | ||
| Secondary | 131.47 | 6 (4–7) | ||
| High education | 132.07 | 6 (4–8) | ||
| Employment status | Employed | 131.20 | 6 (4–8) | 0.260 |
| Student | 143.63 | 7 (5–9) | ||
| Unemployed | 150.65 | 7 (5–9) | ||
| Received a previous diagnosis of AA | Yes | 138.77 | 7 (4–8) | 0.765 |
| No | 142.28 | 7 (4.75–8) | ||
| Received previous treatment for AA | Yes | 137.72 | 7 (5–9) | 0.139 |
| No | 122.73 | 6 (4–8) | ||
| Personal history of DM1 | Yes | 136.68 | 7 (4.75–8) | 0.892 |
| No | 139.65 | 6 (4–8) | ||
| Personal history for thyroiditis | Yes | 121.88 | 6 (3–8) | 0.156 |
| No | 142.12 | 6 (4.5–8) | ||
| Family history of AA | Yes | 142.64 | 7 (5–8) | 0.478 |
| No | 135.82 | 6 (4–8) | ||
| Family history of DM1 | Yes | 135.71 | 7 (4–8) | 0.787 |
| No | 139.94 | 6 (4–8) | ||
| Family history of thyroiditis | Yes | 130.38 | 6 (3.5–8) | 0.524 |
| No | 140.52 | 7 (4–8) | ||
| Treatment group | Monotherapy of betamethasone | 111.70 | 5 (3–8) | <0.001∗ |
| Combined betamethasone and minoxidil | 172.40 | 7 (6–9) | ||
| Combined betamethasone and herbal treatment | 120.25 | 6 (4–7) | ||
- Note: The Mann–Whitney U and Kruskal–Wallis tests were used to test for statistical significance as appropriate.
- Abbreviations: AA, alopecia areata; DM, diabetes mellitus.
- ∗p value is below the threshold value for significance (0.05).
3.2.3. Median Time to First Clinical Improvement
The group using combined topical minoxidil–betamethasone treatment significantly had the shortest median time to first clinical improvement with 32 days (p < 0.001), followed by the betamethasone group (34 days) and the combined herbal–betamethasone group (38 days).
3.2.4. Patient Compliance
The group using the combined herbal–betamethasone treatment had a median score of 7.0/10.0, and the other two treatment groups had equal median scores of 6.0/10.0, without reaching statistical significance (p = 0.589) (Table 6). Only having a personal history of diabetes mellitus type I was significantly associated with the compliance score (p = 0.048).
| Variable | Mean rank | Median (Q1–Q3) | p value | |
|---|---|---|---|---|
| Age category (years) | 8–17 | 141.82 | 6 (4–9) | 0.69 |
| 18–29 | 136.32 | 6 (4–8) | ||
| 30–49 | 138.17 | 6 (4–8) | ||
| ≥ 50 | 159.68 | 8 (6–9) | ||
| Alternative age classification (adolescents and adults) | Adolescents | 141.82 | 6 (4–9) | 0.31 |
| Adults | 139.03 | 6 (4–8) | ||
| Gender | Male | 143.78 | 7 (4–9) | 0.31 |
| Female | 133.94 | 6 (4–8) | ||
| Marital status | Single | 133.75 | 6 (3–8) | 0.18 |
| Married | 146.54 | 7 (5–8) | ||
| Household income level | Low-level | 134.56 | 6 (5–9) | 0.1 |
| Mid-level | 135.99 | 6 (4–8) | ||
| High-level | 167.50 | 8 (5–10) | ||
| Residency | City | 144.73 | 7 (4.25–8) | 0.12 |
| Village | 133.99 | 6 (3–8) | ||
| Camp | 102.97 | 5 (2.75–7.25) | ||
| City | Northern | 141.16 | 6 (4–8) | 0.26 |
| Middle | 139.21 | 7 (3.75–9) | ||
| Southern | 74.50 | 3 (2–4.5) | ||
| Level of education | Did not receive formal education | 128.94 | 6 (4.25–8) | 0.14 |
| Primary | 160.66 | 7 (5–9) | ||
| Secondary | 138.32 | 6 (4–8) | ||
| High education | 132.59 | 6 (3–9) | ||
| Employment status | Employed | 140.37 | 6 (4–8.75) | 0.91 |
| Student | 136.83 | 6 (4–8) | ||
| Unemployed | 142.25 | 7 (4–8) | ||
| Received a previous diagnosis of AA | Yes | 136.08 | 6 (4–8) | 0.16 |
| No | 152.49 | 7 (4.75–9) | ||
| Received previous treatment for AA | Yes | 139.74 | 7 (5–9) | 0.08 |
| No | 121.93 | 6 (4–8) | ||
| Personal history of DM1 | Yes | 180.68 | 8 (6–9.25) | 0.048∗ |
| No | 137.32 | 6 (4–8) | ||
| Personal history for thyroiditis | Yes | 130.92 | 7 (3–8) | 0.49 |
| No | 140.78 | 6 (4–8) | ||
| Family history of AA | Yes | 142.46 | 7 (5–8) | 0.504 |
| No | 136.04 | 6 (3–9) | ||
| Family history of DM1 | Yes | 123.84 | 5 (3–8) | 0.265 |
| No | 141.32 | 6 (4–8) | ||
| Family history of thyroiditis | Yes | 147.54 | 7 (4.5–9) | 0.575 |
| No | 138.60 | 6 (4–8) | ||
| Treatment group | Monotherapy of betamethasone | 136.93 | 6 (4–8) | 0.589 |
| Combined betamethasone and minoxidil | 136.95 | 6 (4–9) | ||
| Combined betamethasone and herbal treatment | 149.0 | 7 (5–8) | ||
- Note: The Mann–Whitney U and Kruskal–Wallis tests were used to assess statistical significance.
- Abbreviations: AA, alopecia areata; DM, diabetes mellitus.
- ∗p value is below the threshold value for significance (0.05).
3.3. Drug Safety
The side effects most commonly reported by patients using betamethasone monotherapy were skin atrophy (95.1%), followed by facial hair growth (15.9%) and acne (13.4%). Patients using the minoxidil–betamethasone combination reported facial hair growth (50.0%), allergy (26.3%), acne (19.7%), and headache (19.7%) as the most encountered side effects. The group using herbal–betamethasone combination reported more allergy (83.3%) than facial hair growth (33.3%) and acne (31.0%) (Table 7).
| Side effects | Monotherapy of topical betamethasone n (%) | Combined topical betamethasone and minoxidil n (%) | Combined topical betamethasone and a herbal preparation n (%) |
|---|---|---|---|
| Skin atrophy | 78 (95.1) | 7 (9.2) | 3 (7.1) |
| Facial hair growth (hypertrichosis) | 13 (15.9) | 38 (50.0) | 14 (33.3) |
| Acne | 11 (13.4) | 15 (19.7) | 13 (31.0) |
| Allergy | 9 (11.0) | 20 (26.3) | 35 (83.3) |
| Weight gain | 8 (9.8) | 12 (15.8) | 5 (11.9) |
| Dizziness | 4 (4.9) | 11 (14.5) | 3 (7.1) |
| Blurred vision | 2 (2.4) | 3 (3.9) | 0 (0.0) |
| Arrhythmia | 2 (2.4) | 5 (6.6) | 2 (4.8) |
| Telangiectasia | 2 (2.4) | 0 (0.0) | 4 (9.5) |
| Headache | 2 (2.4) | 15 (19.7) | 1 (2.4) |
| Chest pain | 1 (1.2) | 8 (10.5) | 0 (0.0) |
| Fainting | 1 (1.2) | 3 (3.9) | 1 (2.4) |
| Stretch marks | 1 (1.2) | 1 (1.3) | 1 (2.4) |
| Easy bruising | 0 (0.0) | 9 (11.8) | 1 (2.4) |
| Folliculitis | 0 (0.0) | 3 (3.9) | 3 (7.1) |
4. Discussion
While several therapeutic options have been suggested for the treatment of AA, none has been approved by the FDA [8]. This highlights the need for exploring possible effective treatments, especially since the currently available therapies require a long duration of treatment and are associated with safety and efficacy concerns [9]. The present cohort study used a trichoscopy-based assessment to compare the efficacy and safety of topical betamethasone; topical minoxidil with betamethasone; and topical herbals with betamethasone. The study revealed that the clinical outcomes and patient satisfaction were significantly higher in those who used topical minoxidil combined with topical betamethasone across all three phases compared with the other two groups, with an adequate safety profile. Moreover, the median time to initial response was significantly shorter for patients using topical minoxidil combined with betamethasone than the other groups.
The patients who used a topical minoxidil–betamethasone combination had a higher hair growth rate, patient satisfaction score, and median time to initial response. Only one study investigated the combined use of topical minoxidil and betamethasone for the treatment of AA using a clinical trial design that compared the outcomes of several other treatments. The study reported a decrease in the severity of AA after 12 weeks of commencing a topical betamethasone-minoxidil combination, but it was not superior to other treatments [14]. Alternatively, other studies focused on evaluating the effectiveness of using minoxidil for maintaining hair regrowth following a 6-week regimen of oral, rather than topical, prednisolone [29, 30].
Moreover, several other studies have examined topical minoxidil efficacy in AA, but the evidence supporting its use as a monotherapy has been inconclusive. This evidence has been based on case studies and clinical trials employing various concentrations, application frequencies, and treatment durations. A meta-analysis of minoxidil use in AA reported a significant effect when studies using 1%, 3%, and 5% topical minoxidil were included, while no evidence was found when 1% or 3% minoxidil studies were analyzed separately [24]. Of note, the studies reporting no efficacy included patients with severe AA [31–33]. Moreover, the majority of research investigating the use of topical minoxidil has been restricted to short durations, mostly less than 6 months [24]. This is particularly relevant to minoxidil and AA, as potential outcomes may require constant use for a longer duration [34, 35]. These variations in treatment protocol, combination, target population, methodology, and study design limit direct comparison between the current and other studies but suggest a promising role of topical 5% minoxidil in the treatment of AA. Similarly, the evidence for using topical betamethasone for the treatment of AA is mixed [11, 36]. In the current study, although 67.0% of patients using topical corticosteroids had positive hair regrowth, this finding was inferior to the outcome of using a combination of topical minoxidil and betamethasone (83.2%).
Furthermore, this study reported a significant improvement in patient satisfaction among patients using topical minoxidil and betamethasone. Measuring patient satisfaction is a source of subjective evidence in efficacy studies that complements the objective assessment made by physicians. This is especially important for patients with AA where misalignment between dermatologist-assessed outcomes and patient satisfaction is common [37]. Moreover, research has shown that patients with AA demonstrate low satisfaction rates. This is often due to the lack of long-term, effective, relapse-free treatment and the impact of the disease on the quality of life (QoL) [37, 38]. This is part of the broader context of low patient satisfaction in dermatology due to the need for adherence to long-term treatment for skin diseases, high patient expectations, and poor dermatologist-patient communication [39]. Due to the special nature of AA, incorporating measures of patient satisfaction and QoL in AA is recommended in future research. Several QoL scales tailored for AA have been developed and validated in the literature, such as the Hairdex [40] and the Alopecia Areata Quality of Life Questionnaire (AA-QLI) [41], which can be used for research and clinical purposes. Moreover, dermatologists should improve patient satisfaction by enhancing their communication about the nature and treatment of AA, engaging patients in treatment decisions, and addressing related patient concerns. As a result, patient satisfaction may enhance adherence to treatment and improve clinical outcomes [42].
In this study, skin atrophy was less reported by the combination therapy groups compared to the group that used betamethasone monotherapy, which may be explained by several factors. First, co-application of betamethasone with the other topical agents might have modulated betamethasone absorption by creating a barrier effect or modifying vehicle composition. In addition, the use of multiple topical agents might have influenced patients’ behaviors and led to less frequent or less concentrated application of betamethasone. Previous studies have reported that topical combination therapies can reduce corticosteroid-induced skin atrophy. Co-administration of topical retinoids, for example, reduced corticosteroid-induced skin atrophy in multiple studies. This is possibly because retinoids stimulate collagen deposition and counteract the effects of corticosteroids on proteoglycans and glycosaminoglycans [43–46]. Similarly, topical vitamin D agonists and mineralocorticoid antagonists were associated with reduced corticosteroid-induced skin atrophy, possibly due to similar effects on the extracellular matrix [47, 48]. Further studies are recommended to identify possible similar effects made by the combinations used in this study.
Acne and hypertrichosis were common side effects encountered by the three treatment groups. Skin atrophy and acne are commonly reported side effects of topical corticosteroids, while hypertrichosis is a less common side effect [49]. The probability of adverse effects of topical corticosteroids depends on several factors, including drug potency, duration of use, and age of the patient [50]. Moreover, consistent with the findings of the present study, allergic dermatitis, hypertrichosis, and headache are common side effects of 5% minoxidil reported in the literature [51, 52]. However, minoxidil 5% might be considered safe as these side effects are local and well tolerated [53]. Especially considering the chronicity of AA and the need for long-term treatment, the safety and tolerability of topical minoxidil make it a more preferred choice for patients when compared to other treatment options, such as systemic steroids.
While this study suggests that the combination of minoxidil with corticosteroids for AA treatment may be effective and safe, further research is needed to provide a substantiated evidence base for this regimen over other monotherapies. The paucity of research investigating minoxidil, especially as a combined therapy with topical corticosteroids, limits the translation of this option into clinical practice. Although observational studies provide complementary evidence in comparative effectiveness research if a rigorous methodology is used, clinical practice must be guided by a randomized trial design whose accounting for bias and confounding is unmatched by other study designs.
This study had several limitations. First, this study did not use a validated scale, such as the Severity of Alopecia Tool (SALT), which would have allowed for graded assessment of severity, increasing outcome precision, and allowing for pre-post intervention statistical analysis. However, the SALT was not routinely used by local dermatologists participating in this multi-center, real-world study. Reliable and uniform implementation of the SALT across centers would have required considerable training and resources. To reduce inter-rater variability, clinical assessment by all dermatologists was harmonized through a calibration process to align on both the diagnostic criteria and trichoscopic assessment for hair regrowth, which is a valid and practical approach that allows for detailed observation and early detection [54, 55]. Additionally, the study measured patient-reported outcomes to capture the psychological aspect of the treatment, which the SALT does not address. Second, the study included fewer participants from southern governorates whose sociocultural characteristics may differ, limiting generalizability at the national level. Moreover, the nature of an observational cohort study inherently introduces selection and information biases into the study. Additionally, the study did not measure some clinical variables that might confound the relationship between exposure and outcome, including age at disease onset, duration of disease, and presence of nail changes. Nevertheless, this was the first study that compared the three treatment regimens of topical minoxidil, corticosteroids, and herbal treatment in patients diagnosed with AA. In addition, it was the first to explore treatment options for AA in the region, contributing to the scarce research in clinical dermatology.
5. Conclusions
Dermatologists use an arsenal of treatment options in the management of AA because no single option has been universally approved. This cohort study aimed to compare the combinations of topical minoxidil, betamethasone, and herbal preparations of castor and jojoba oils with a monotherapy of topical betamethasone to evaluate hair regrowth using trichoscopic examination performed by a dermatologist. The patient group that used a topical minoxidil–betamethasone combination significantly had a higher rate of hair regrowth and patient satisfaction score, and a shorter median time to first response than the other patient groups. This study indicates that topical minoxidil–betamethasone combinations may be effective and safe in the treatment of AA. However, more studies using randomized clinical trial design are needed to improve the evidence base.
Nomenclature
-
- AA
-
- Alopecia areata
-
- HRQoL
-
- Health-related quality of life
-
- HLA
-
- Human leukocyte antigen
-
- FDA
-
- Food and Drug Administration
-
- STROBE
-
- Strengthening the Reporting of Observational Studies in Epidemiology
-
- SLE
-
- Systemic lupus erythematosus
-
- HIV
-
- Human immunodeficiency virus
-
- SPSS
-
- The statistical package for the social sciences
-
- aRR
-
- Adjusted risk ratio
-
- IRB
-
- Institutional Review Board
-
- ANNU
-
- An-Najah National University
-
- QoL
-
- Quality of life
-
- AA-QLI
-
- Alopecia areata quality of life questionnaire
-
- SALT
-
- Severity of Alopecia Tool
Ethics Statement
The Institutional Review Board (IRB) of An-Najah National University (ANNU) approved this study, with a reference number: stu.MRC-118–56-4–23. The participants who enrolled in the study provided voluntary and informed consent, and minors were enrolled after a legal guardian provided informed consent on their behalf. The research conduct followed the guidelines and regulations of the 1964 Declaration of Helsinki.
Consent
Written informed consent was obtained from all participants prior to their participation and after they were provided with adequate information about the purpose and conduct of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed to the study design. Material preparation and data collection were performed by Amir Abadi, Wala Abdeljawad, Emad Khatib, and Shorok Jaber. Data analysis was performed by Basma Damiri and Sari Taha. The first draft of the manuscript was written by Sari Taha, and all authors read, commented, and approved the final manuscript. Amir Abadi was responsible for the integrity of the data and coordination between research team members. Basma Damiri, Munther Ardah, Sari Taha, and Manal Ardah supervised the survey team, provided valuable logistical support, and enhanced the intellectual content.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors thank An-Najah National University (ANNU) for all the administrative and logistic assistance offered during the conduct of the research. The authors thank Dr. Salah Safi and Dr. Hosam Khreim for their valuable logistical support of data collection.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.