Comprehensive Analysis of the Chemokine/Cytokine Profiles in Advanced Mycosis Fungoides and Atopic Dermatitis
Abstract
Mycosis fungoides (MF) is an indolent form of cutaneous T-cell lymphoma. Its early lesions are important to be distinguished from atopic dermatitis (AD) because of their similar immune microenvironment. We have previously demonstrated that several chemokines are involved in the pathogenesis of advanced MF. Therefore, we sought to comprehensively analyze the changes in the immune environment during the advanced phase of MF by focusing on serum cytokines and chemokines and to identify potential biomarkers for the early detection of the transition to the advanced phase of MF. Sera from 11 cases of advanced-stage MF, 16 cases of mild AD (median EASI score = 5.75), 16 cases of severe AD (median EASI score = 28.1), and 21 healthy volunteers were analyzed using the Bio-Plex 40 multiplex immunoassay system. The results revealed significant increases in immunosuppressive macrophage-related factors (IL-4, MIF, CCL3) in MF patients compared to those with AD and healthy controls. In contrast, IL-2, CCL1, CCL7, CCL21, CCL25, and CCL26 were significantly increased in severe AD patients compared to MF patients and healthy controls. Our findings suggest that several chemokines and cytokines contribute to an immunosuppressive environment favorable for tumor growth, distinguishing MF from AD. Moreover, T-cell proliferation and migration factors, which are mainly involved in maintaining inflammation, are elevated in severe AD compared to MF. In addition to elucidating the differences in the pathogenesis of these diseases, these factors may be important in the differential diagnosis of early MF and AD. Further studies are warranted to confirm these findings.
1. Introduction
Mycosis fungoides (MF) is an indolent form of cutaneous T-cell lymphoma (CTCL) [1], and its severe lesions are important to be distinguished from atopic dermatitis (AD) to avoid the progression of MF [2]. Indeed, several cases of misdiagnosed AD and the use of biologics for CTCL, including MF, have led to worsening of the lymphomas [3–5]. For example, a recent report suggests an increased risk of CTCL in a cohort of AD patients who used dupilumab (humanized anti-IL-4 receptor alpha (Rα) antibody that block both IL-4 and IL-13) (odds ratio 4.1003, 95% confidence interval 2.055–8.192) [3]. Notably, risk was not increased for other cutaneous or lymphoid malignancies [3]. A recent systematic review also suggests that biologics for psoriasis might induce CTCL, tough the number of cases of CTCL is limited. Most cases of CTCL were reported in the context of TNF inhibitor use with no cases reported in the setting of IL-23 inhibitor use [4]. Notably, another report suggests that 54.2% of inadequate responders were ultimately diagnosed with MF by detecting T-cell receptor clonality in patients with severe AD who were refractory to dupilumab [5]. They concluded that insufficient response to dupilumab treatment may help uncover early MF on an existing AD background [5]. Taken together, since distinguishing MF from AD is important to avoid the progression of MF due to biologics for AD [3, 5], we sought to comprehensively analyze the differences between severe AD and advanced MF by focusing on serum cytokines and chemokines to identify potential biomarkers for the early detection of the transition to the advanced phase of MF.
2. Patients and Methods
2.1. Patients, Ethics Statement for Human Experiments
This study aims to identify diagnostic factors associated with the transition of MF to the advanced stage, particularly in cases with acquired UV resistance. Sera from 11 cases of MF, 16 cases of mild AD, 16 cases of severe AD, and 21 healthy volunteers were collected (Table 1). The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (Permit No: 2021-1-1214). All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
| Median age | 59 |
| Gender | |
| Male | 7 |
| Female | 4 |
| Stage | |
| IB | 2 |
| IIB | 7 |
| IIIA | 2 |
| Median sIL-2R (IU/mL) | 571 |
| Median LDH (IU/mL) | 199 |
2.2. Serum Levels of Cytokines and Chemokines
Serum levels of 40 cytokines and chemokines described below were analyzed simultaneously using the Bio-Plex-Pro Human Chemokine Panel, 40-Plex immunoassay system, which contains the largest number of cytokines and chemokines test in a commercially available kit (Bio-RAD, Tokyo, Japan) according to the manufacturer’s protocol: CXCL1. CXCL2, CXCL5, CXCL6, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, CXCL16, CCL1, CCL2, CCL3, CCL7, CCL8, CCL11, CCL13, CCL15, CCL17, CCL19, CCL20, CCL21, CCL22, CCL23, CCL24, CCL25, CCL26, CCL27, CX3CL3, MIF, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-16, GM-CSF, IFN-γ, TNF-α.
2.3. Statistical Methods
To analyze differences in cytokine and chemokine production among the four groups, we conducted a one-way analysis of variance (ANOVA). When significant differences were observed, we identified the groups contributing to the significant p value (p < 0.05) by the Kruskal–Wallis test. Correlation coefficients were determined by using Spearman’s rank correlation test. All analyses were conducted using GraphPad Prism 10.3.1.509.
3. Results
3.1. Demographic Data
The demographic characteristics of MF patients are shown in Table 1. The median ages of MF, mild AD, severe AD patients, and healthy controls were 59 (42–71), 31 (10–55), 37 (13–65), and 50 (9–84), respectively. The clinical stages of MF patients were as follows: 2 cases of stage IB, 7 cases of stage IIB, and 2 cases of stage IIIA. Sera from all MF cases were obtained immediately before the induction of systemic therapy.
The demographic characteristics of severe and mild AD patients are shown in Table 2. The median eczema area and severity index (EASI) scores for severe and mild AD were 28.1 (19.1–49.9) and 5.75 (1.4–9.1), respectively. The median levels of thymus and activation-regulated chemokine (TARC) in severe and mild AD were 1650 pg/mL (371–30,000) and 410 pg/mL (121–1082), respectively. The median total IgE levels in severe and mild AD were 4493 IU/mL (1047–14,790) and 5232 IU/mL (197–13,188), respectively.
| Severe AD | Mild AD | |
|---|---|---|
| Median age | 37 | 31 |
| Gender | ||
| Male | 13 | 10 |
| Female | 3 | 6 |
| Median EASI score | 28.1 | 5.75 |
| Median TARC (pg/mL) | 1650 | 410 |
| Median total IgE (IU/mL) | 4493 | 5232 |
3.2. Comprehensive Analysis of Serum Chemokines and Cytokines
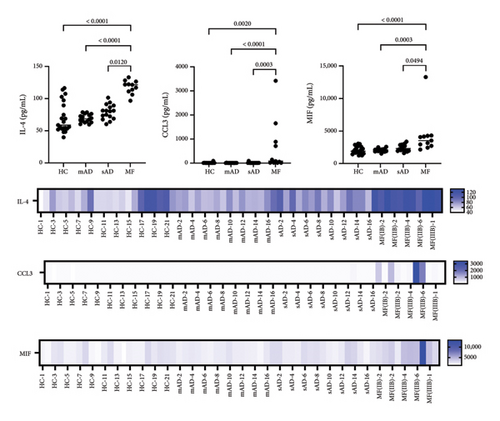
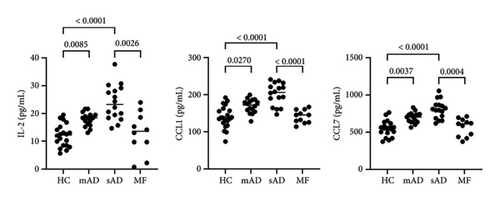
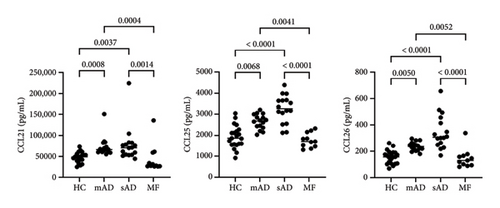
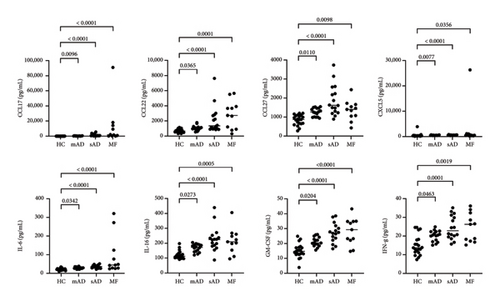
All data are provided in the Supporting Figure 1. Of the 40 chemokines and cytokines, IL-4, MIF, and CCL3 were significantly increased in MF compared to mild AD, severe AD, and healthy controls (Figure 1). In contrast, CCL21, CCL25, and CCL26 were significantly decreased in MF compared to mild AD and severe AD (Figure 2(a)). IL-2, CCL1, and CCL7 were significantly decreased in MF compared to severe AD (Figure 2(b)). CCL17, CCL22, CCL27, CXCL5, IL-6, IL-16, GM-CSF, and IFN-γ were significantly increased in MF, mild AD, and severe AD compared to healthy controls (Figure 3). The serum levels of TARC (TARC: CCL17) is strongly correlated (0.70 < r < 0.90) with CCL19 and CCL25 in mAD, and with CCL3, CCL8, CCL11, CCL17, CX3CL1, IFNg, IL-8 in sAD. The serum levels of IgE is strongly with CCL19 in mild AD. The serum levels of IL-2R is strongly with CXCL1, CXCL13, CX3CL1, GM-CSF, IL-10, CCL17, CCL19, CCL23, CCL25, CCL26 in MF.
4. Discussion
To avoid worsening lymphoma in the clinic [3–5], it is important to distinguish MF from severe AD. In this study, we found a significant increase in serum IL-4, CCL3, and MIF in MF patients compared to those with severe AD. IL-4 has previously been reported as a cytokine in MF, predominantly in advanced stages [6]. More recently, IL-4 induced 1 (IL4I1), an enzyme that converts L-amino acids to α-keto acids and is involved in immune suppression, has gained attention in oncology [7]. IL4I1-mediated catabolism of tryptophan produces indoles and kynurenic acid, which activate the aryl hydrocarbon receptor (AhR), thereby promoting cancer progression and suppressing anti-tumor immunity [7]. In hematology, IL4I1 has been reported to suppress T-cell proliferation in primary mediastinal B-cell lymphoma [8]. Since IL4I1 can reduce the activity of CD8+ T cells and promote the development of inducible regulatory (iTreg) cells [9, 10], elevated IL-4 in the sera of MF patients may indicate disease progression due to suppression of the tumor immune response. Notably, as in previous reports, serum IL-2 levels did not differ between MF and healthy controls [11]. In contrast, serum IL-2 levels were significantly elevated in atopic patients compared to healthy controls [12]. In fact, in the present study, IL-2 was also significantly decreased in MF compared to severe AD, suggesting immune suppression in MF.
In addition to IL-4, CCL3 was increased in MF patients’ sera compared to severe AD. CCL3 has been reported to exacerbate tumors in several cancer types, in association with the invasion of tumor-associated macrophages (TAMs), the infiltration of monocytes into the tumor, and their differentiation into M2 polarized TAMs [13]. In hematological cancers, CCL3 has also been reported as a possible prognostic factor that promotes angiogenesis in extranodal NK/T-cell lymphoma [14]. Since M2 macrophages in MF also promote angiogenesis and are involved in tumor progression [15], CCL3 may be a multifaceted factor in exacerbating MF. MIF has also been reported to correlate with macrophage polarity and prognosis in several cancer types [16, 17]. Moreover, single-cell RNA sequencing for CTCL revealed that MIF polarized TAMs into the M2 immunosuppressive phenotype through the CD74/MIF signaling pathway [16]. In summary, TAM-related chemokines, which are known to be poor prognostic factors in other cancers, are significantly elevated in MF compared to severe AD.
In contrast to tumor-promoting factors such as IL-4, CCL3, and MIF, Th2 inflammatory chemokines such as CCL1, CCL25, and CCL26 were significantly increased in severe AD compared to MF. Notably, CCL1 and CCL26 have been reported to be correlated with pruritus in CTCL in addition to LDH and IgE, but are much higher in severe AD [18]. In the present study, CCL1 and CCL26 were predominantly higher in severe AD. It has been suggested that pruritus is a common symptom in MF, but it is not useful in comparison with AD. CCL25 is a ligand for CCR9 and is involved in a wide range of inflammatory diseases [19].
In the present study, cytokines and chemokines that have been previously reported to correlate with AD or MF (CCL17, CCL22, etc.) were not extracted as differential markers in the results of our study [20–22]. Since these chemokines were studied independently in both AD and MF, it is possible that there were no differences in their comparison in our present study. Indeed, Serum CCL17 and CCL22 are elevated in each stage of AD, proportional to disease severity, compared to healthy controls in our present study (Supporting Table). Since the number of patients in this study is small, further multicenter, prospective studies with larger numbers of patients are needed to confirm the results of present study in future.
In summary, we found distinct cytokine/chemokine profiles in MF and severe AD. These differences in cytokines and chemokines are characteristic of the pathogenesis of tumorigenic and inflammatory skin diseases, respectively. Similar to previous reports [23, 24], IL-4 shows a statistically significant increase in both AD and MF cohorts, with a significantly higher level in MF compared to AD. However, while CCL3 and MIF levels are statistically higher in MF than in severe AD, the differences are relatively small. Therefore, further validation studies are needed to confirm the significance of these findings in future. The present study suggests potential biomarkers for differentiating MF from severe AD. Since this study has a small sample size, future multicenter, prospective studies with larger cohorts are needed to validate our findings [25].
Ethics Statement
The protocol was approved by the institutional review board of Tohoku University Hospital and the institutional review board of each participating center.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Taku Fujimura and Yoshihide Asano contributed to the writing of the manuscript. Taku Fujimura designed the research study. Manami Takahashi-Watanabe, Taku Fujimura, and Emi Yamazaki collected and analyzed the data. Manami Takahashi-Watanabe, Taku Fujimura, Emi Yamazaki, Ryo Amagai, Kenta Oka, Yumi Kambayashi, Maki Ozawa, Tomoko Chiba, Mayuko Onodera-Amagai, and Toshiya Takahashi treated the patients and collected the clinical data and samples. Yoshihide Asano and Yoshihide Asano supervised the study.
Funding
This study was supported in part by the Japan Agency for Medical Research and Development (JP23ym0126041) and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23K19525).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




