Allopurinol-Induced Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review of Case Reports and Case Series
Abstract
Background: Allopurinol is a known cause of mucocutaneous adverse drug reactions, including Stevens–Johnson syndrome and toxic epidermal necrolysis. The aim of this systematic review was to characterize the clinical presentation, identify risk factors, and evaluate the best treatment strategies for allopurinol-induced severe skin reactions.
Methods: The PubMed, Embase, and Scopus databases were systematically searched to identify English case reports and case series of allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis. Animal studies, reviews, book chapters, randomized and nonrandomized human studies, observational studies, and conference abstracts were excluded. The Joanna Briggs Institute (JBI) critical appraisal checklists were used to assess the quality of the included studies.
Results: Forty-seven case reports and 21 case series were included in the analysis, which reported 91 individual patient datasets. The reaction occurred after a median of 16 days (8.5 days in those with prior reactions to allopurinol). Rapid dose escalation was observed in half of the patients (21 of 43) for whom dose-increment schedules were reported. Mucosal involvement was observed in 72 (90.0%) patients. Corticosteroids, IVIG, cyclosporine, and plasma exchange were the most common treatment modalities. Twenty-one patients (23.6%) died, and 68 (76.4%) were discharged.
Conclusion: Although gout is 2–3 times more common in men, the numbers of cases were similar in both sexes, likely due to higher reporting rates in women. Rapid dose escalation is a risk factor for the occurrence of severe skin reactions. Corticosteroids, IVIG, and plasma exchange appear to be reasonable treatment options.
1. Introduction
Allopurinol, a xanthine oxidase inhibitor, is commonly used for the treatment of gout, cancer therapy-induced hyperuricemia, and recurrent calcium oxalate calculi [1]. Moreover, it is increasingly being used in patients with asymptomatic hyperuricemia [2].
Although allopurinol is generally well tolerated, it has been associated with severe hypersensitivity reactions, including Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) [2]. These reactions occur in approximately 1 in 1000 patients receiving allopurinol and are associated with a mortality rate of 20%–25% [3]. Many patients indicated for allopurinol therapy have other underlying medical conditions, such as chronic kidney disease and cardiovascular disorders, which are considered independent risk factors for hypersensitivity reactions to allopurinol [3].
SJS and TEN are considered the same reaction, and the difference lies in the extent of skin involvement. In SJS, SJS/TEN overlap, and TEN, skin detachment occurs in less than 10%, 10%–30%, and greater than 30% of the body surface area (BSA), respectively. These reactions begin with general malaise, fever, and flu-like symptoms, followed by the appearance of pruritic macules and painful blisters. The mucosal surfaces of the eyes, oral cavity, and genitalia are frequently affected. In addition, other organs, such as the cardiovascular, pulmonary, and gastrointestinal systems, are involved [4].
Although case reports of severe allopurinol-induced skin reactions have been published, they have not been comprehensively reviewed. We conducted a systematic review of biomedical databases to identify case reports and case series of allopurinol-induced SJS/TEN. The aims of this systematic review were to characterize the clinical presentation, identify contributing factors, and evaluate the efficacy of various treatment modalities used in the management of these reactions.
2. Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [5]. After the study protocol was reviewed, the research council and the research ethics committee of the Alborz University of Medical Sciences approved the study (IR.ABZUMS.REC.1401.155).
2.1. Literature Search
A systematic literature search was conducted via a predefined search strategy in the PubMed, Embase, and Scopus databases. Both free text and Medical Subject Heading (MeSH) terms were used to perform the literature search. The search strategy is presented in the Supporting Table 1. All English case reports or case series studies that reported at least one case of SJS, TEN, or SJS/TEN overlap associated with allopurinol use were included. Animal studies, reviews, book chapters, randomized and nonrandomized human studies, observational studies, and conference abstracts were excluded. No age or time limits were applied. The last date of the search in the PubMed database was March 31, 2023; that in Scopus was April 2, 2023; and that in Embase was May 6, 2023.
2.2. Study Selection
Two independent researchers (SB and MM) explored the titles and abstracts of the records. Relevant studies were exported to EndNote (Version 21.1 for Windows; Thomson Reuters, Philadelphia, PA, USA). References of narrative reviews, systematic reviews, case reports, and case series were also searched to find additional studies.
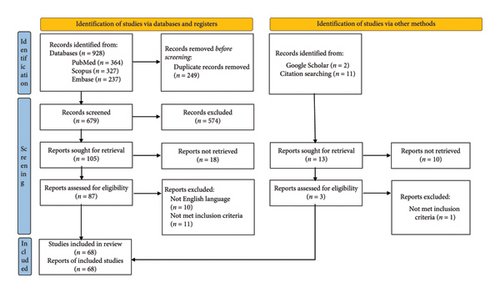
The duplicates were then removed, and the two investigators evaluated each record according to the inclusion criteria of the study. In cases of disagreement, a third investigator (SE) was consulted, and the decision to include the study was made on the basis of consensus. Figure 1 shows the PRISMA flowchart of the study.
2.3. Data Extraction
Two authors performed the data extraction (SB and MM). A comprehensive set of data, including the first author’s name, year of publication, age, sex, race, and country of the patient, indication of allopurinol, dosage administered, duration of drug use before the onset of rash, HLA-B∗5801 status, history of drug allergy, signs and symptoms of the reaction, concomitant medications, laboratory data, paraclinical findings, SCORTEN score [6], management strategies, and patient outcome, was extracted from each study.
In addition, the Naranjo adverse drug reaction probability scale was recorded for each patient [7]. If the author(s) of the case report did not mention the Naranjo score, we calculated it on the basis of the data of the report.
2.4. Quality Assessment
Two investigators (SB and MM) evaluated the quality of the studies via the Joanna Briggs Institute (JBI) critical appraisal checklists for case reports [8] and case series [9]. A third investigator (SE) was consulted in case of disagreement.
2.5. Statistical Analysis
Data analysis was conducted via IBM SPSS Statistics Software (Version 26.0. IBM Corp., Armonk, NY, USA). Frequencies and percentages were used to describe qualitative variables.
3. Results
3.1. Characteristics of the Studies
As presented in Figure 1, 928 articles were identified through the database search. After the removal of duplicates, full-text searches, and eligibility assessments, 66 articles were included in the review. Two additional records were also retrieved via Google Scholar and reference searching. Finally, 68 articles, including 47 case reports and 21 case series, were included in the study, which reported 91 individual patient datasets.
The quality assessment of the studies is presented in Supporting Table 2 (case reports) and Supporting Table 3 (case series) of the Supporting file. The overall quality of case reports was good, but most studies did not report or were unclear about treatment-related adverse effects. Except for two questions, the case series studies were of good quality. First, 12 of the 21 studies did not include consecutive patients. Second, 14 of the 21 studies were unclear regarding complete patient inclusion.
3.2. Patient Characteristics
The detailed characteristics of the included patients are presented in Table 1. Forty-eight (52.7%) patients were male, and 43 (47.3%) were female. The patients’ ages ranged from 8 to 96 years, with a median of 62.
| Case no. | Author/year | Age/sex | Dose (mg)/duration (days) | Presentation | Naranjo score | SCORTEN score | Mucosal involvement/BSA% | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kantor, 1970 [10] | 72/M | 100 BD/7 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Death |
| 2 | Stratigos et al., 1972 [11] | 58/F | 600 QD/2 | TEN | Probable | NR | −/NR | Supportive, corticosteroid | Death |
| 3 | Ellman et al., 1975 [12] | 76/F | 100 QID/24 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Death |
| 4 | 60/F | 100 TID/30 | TEN | Probable | NR | +/NR | Corticosteroid | Discharge | |
| 5 | 45/M | 100 QID/27 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 6 | Chan et al., 1977 [13] | 58/F | 100 TID/21 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 7 | 35/M | 100 TID/28 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 8 | 62/M | 100 TID/21 | TEN | Possible | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 9 | Bennett et al., 1977 [14] | 54/M | 100 TID/21 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 10 | Assaad et al., 1978 [15] | 38/M | 200 TID/37 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 11 | Lang, 1979 [16] | 45/M | NR/30 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 12 | 61/M | NR/14 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 13 | 76/F | NR/42 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Death | |
| 14 | 62/F | NR/14 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 15 | 47/F | NR/21 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge | |
| 16 | Marra et al., 1982 [17] | 47/F | NR | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 17 | Davies et al., 1983 [18] | 53/M | NR/60 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 18 | Vinciullo, 1984 [19] | 24/M | NR/29 | TEN | Probable | NR | −/NR | Supportive, corticosteroid | Discharge |
| 19 | Pennell et al., 1984 [20] | 71/M | 100 BD/38 | SJS | Possible | NR | +/NR | Corticosteroid | Death |
| 20 | Dan et al., 1984 [21] | 84/M | NR/5 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 21 | Edwards and Ridder et al., 1985 [22] | 46/M | 100 TID/28 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 22 | Renwick, 1985 [23] | 78/M | 300 QD/7 | SJS | Probable | NR | +/NR | Supportive | Death |
| 23 | Auböck et al., 1985 [24] | 52/F | 300 QD/14 | TEN | Probable | NR | NR | Supportive, corticosteroid | Discharge |
| 24 | 72/F | 300 QD/35 | TEN | Possible | NR | NR | Supportive, corticosteroid | Discharge | |
| 25 | 73/M | 300 QD/7 | TEN | Probable | NR | NR | Supportive, corticosteroid | Discharge | |
| 26 | 77/F | 300 QD/28 | TEN | Probable | NR | NR | Supportive, corticosteroid | Discharge | |
| 27 | 62/F | 300 QD/21 | TEN | Probable | NR | NR | Supportive, corticosteroid | Death | |
| 28 | 32/M | 300 QD/14 | TEN | Probable | NR | NR | Supportive, corticosteroid | Discharge | |
| 29 | Sakellariou et al., 1991 [25] | 65/M | NR/10 | TEN | Probable | NR | +/50 | Supportive, plasma exchange, corticosteroid, IVIG | Discharge |
| 30 | Heng et al., 1991 [26] | 47/M | NR/2 | TEN | Probable | NR | +/NR | Corticosteroid, cyclophosphamide | Discharge |
| 31 | Ashworth, 1992 [27] | 70/F | 300 QD/25 | SJS | Possible | NR | +/NR | Supportive | Discharge |
| 32 | Alfandari et al., 1994 [28] | 32/M | NR | TEN | Possible | NR | +/30 | NR | Discharge |
| 33 | Halevy et al., 1999 [29] | 58/M | 300 QD/13 | SJS | Possible | NR | +/10 | Supportive | Discharge |
| 34 | Bashir et al., 2000 [30] | 65/F | 300 BID/14 | SJS | Probable | NR | +/36 | Supportive, corticosteroid | Discharge |
| 35 | Zakrzewski et al., 2002 [31] | 35/F | 300 QD/4 | TEN | Possible | NR | −/50 | Supportive | Death |
| 36 | Yeung et al., 2005 [32] | 76/F | NR | TEN | Probable | 3 | NR/70 | Supportive, IVIG | Discharge |
| 37 | 29/M | NR | TEN | Probable | 1 | NR/50 | Supportive, corticosteroid, IVIG | Discharge | |
| 38 | 82/M | NR | TEN | Probable | 3 | NR/40 | Supportive, IVIG | Discharge | |
| 39 | 51/F | NR | SJS | Probable | 4 | NR/10 | Supportive, IVIG | Discharge | |
| 40 | 77/F | NR | SJS/TEN | Probable | NR | NR/20 | Supportive, corticosteroid, IVIG | Discharge | |
| 41 | Kakeda et al., 2005 [33] | 68/M | NR/23 | TEN | Probable | NR | +/NR | Supportive, corticosteroid | Death |
| 42 | Saxena et al., 2005 [34] | 72/M | NR/21 | TEN | Probable | NR | +/80 | Supportive, corticosteroid | Death |
| 43 | Stenton et al., 2005 [35] | 81/M | 300 QD/1 | TEN | Probable | NR | +/NR | Supportive, corticosteroid, IVIG | Discharge |
| 44 | Paquet et al., 2007 [36] | 51/F | 200 QD/14 | TEN | Probable | NR | +/80 | Supportive, IVIG | Discharge |
| 45 | Dainichi et al., 2007 [37] | 57/M | NR/8 | SJS | Probable | NR | +/NR | NR | NR |
| 46 | 93/M | NR/14 | TEN | Probable | NR | +/NR | NR | NR | |
| 47 | Kemen et al., 2009 [38] | 8/F | NR/14 | TEN | Probable | NR | −/95 | Supportive, IVIG | Discharge |
| 48 | Hsieh et al., 2009 [39] | 82/F | 100 QD/56 | SJS | Possible | NR | +/20 | Supportive, corticosteroid | Discharge |
| 49 | Ventura et al., 2010 [40] | 76/F | 150 QD/9 | TEN | Probable | NR | +/70 | Corticosteroid | Death |
| 50 | 77/F | 300 QD/NR | TEN | Probable | NR | +/30 | Supportive, corticosteroid | Death | |
| 51 | Hanken et al., 2010 [41] | 80/F | NR/14 | TEN | Probable | NR | −/45 | Supportive, corticosteroid | Discharge |
| 52 | Fernando et al., 2010 [42] | 35/M | NR/14 | SJS/TEN | Probable | NR | +/NR | Supportive, IVIG, cyclosporine | Discharge |
| 53 | Lee et al., 2010 [43] | 63/F | 200 QD/7 | SJS/TEN | Probable | NR | +/20 | Supportive, corticosteroid, IVIG | Discharge |
| 54 | Ranu et el., 2011 [44] | 81/F | NR/10 | TEN | Probable | NR | +/60 | Supportive, IVIG | Death |
| 55 | 67/M | NR/14 | TEN | Probable | NR | +/80 | Supportive, corticosteroid, IVIG | Death | |
| 56 | 56/F | NR/30 | TEN | Probable | NR | −/70 | Supportive, IVIG | Discharge | |
| 57 | 78/F | NR/21 | TEN | Probable | NR | +/90 | Supportive, IVIG | Death | |
| 58 | Comparin et al., 2012 [45] | 55/F | NR/15 | TEN | Probable | 4 | +/80 | Corticosteroid | Discharge |
| 59 | Lee et al., 2012 [46] | 68/F | NR/14 | SJS | Definite | NR | +/NR | Supportive | Discharge |
| 60 | Kamal et al., 2012 [47] | 62/M | NR/0.5 | TEN | Probable | 5 | +/90 | Supportive, IVIG | Discharge |
| 61 | Sawicki et al., 2013 [48] | 64/M | NR/2920 | SJS | Probable | NR | +/NR | IVIG | Discharge |
| 62 | Lee et el., 2013 [49] | 72/M | 300 QD/7 | SJS | Probable | NR | −/NR | Supportive, corticosteroid, cyclosporine | Discharge |
| 63 | Watanabe et al., 2013 [50] | 73/F | 300 QD/12 | TEN | Probable | NR | +/80 | Supportive, plasma exchange, corticosteroid, IVIG | Death |
| 64 | Caldarola et al., 2015 [51] | 82/M | 300 QD/10 | SJS | Probable | NR | +/70 | Corticosteroid | Death |
| 65 | Villano et al, 2015 [52] | 60/F | NR/1 | TEN | Probable | 3 | +/NR | Supportive | Discharge |
| 66 | Paik et al., 2015 [53] | 43/F | 100 TID/10 | SJS | Probable | NR | +/9 | Supportive, corticosteroid | Discharge |
| 67 | Rodgers et al., 2016 [54] | 60/F | NR/21 | TEN | Probable | 4 | +/80 | Supportive, IVIG | Discharge |
| 68 | Casagranda et al., 2017 [55] | 86/F | NR/30 | DRESS/SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 69 | Banerjee et al., 2017 [56] | 62/M | 300 QD/2 | TEN | Probable | NR | +/50 | Supportive, corticosteroid | Death |
| 70 | Brown et al., 2017 [57] | 71/F | NR/7 | TEN | Probable | 5 | +/30 | Supportive, plasma exchange, corticosteroid, IVIG | Discharge |
| 71 | Wallenborn et al., 2017 [58] | 44/M | NR/21 | TEN | Probable | 2 | +/80 | Supportive, corticosteroid, infliximab | Discharge |
| 72 | Irfan et al, 2017 [59] | 45/M | NR/3 | TEN | Probable | 3 | +/50 | Supportive, plasma exchange | Discharge |
| 73 | Wang F et al., 2019 [60] | 33/M | 250 QD/9 | TEN | Probable | NR | +/60 | Supportive, corticosteroid, IVIG | Discharge |
| 74 | Buenrostro-Rubio et al., 2019 [61] | 35/M | 100 QD/21 | TEN | Probable | 3 | +/95 | Supportive, corticosteroid | Discharge |
| 75 | Claytor et al., 2019 [62] | 71/F | NR/7 | SJS/TEN | Probable | 4 | +/25 | Supportive, corticosteroid, IVIG | Death |
| 76 | Chhabra M et al., 2019 [63] | 50/F | NR/2 | SJS | Probable | NR | +/25 | Supportive, corticosteroid, cyclosporine | Discharge |
| 77 | Ponzo et al., 2019 [64] | 81/F | NR/32 | TEN | Probable | 4 | +/50 | Supportive, cyclosporine | Discharge |
| 78 | Gupta et al., 2019 [65] | 85/F | 300 QD/6 | SJS | Probable | NR | +/NR | Supportive | Discharge |
| 79 | Bozca et al., 2020 [66] | 81/F | NR/17 | TEN | Probable | 2 | +/30 | Cyclosporine | Discharge |
| 80 | Xiong et al., 2020 [67] | 47/M | NR/10 | SJS | Probable | NR | +/NR | Supportive, corticosteroid, IVIG | Discharge |
| 81 | Gopan et al., 2021 [68] | 25/M | NR/26 | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 82 | Lavu A et al, 2021 [69] | 28/M | 300 QD/20 | SJS/TEN | Probable | NR | +/13.5 | Supportive | Discharge |
| 83 | Srivastava et al., 2021 [70] | 22/M | 100 BD/3 | SJS | Probable | NR | −/NR | Supportive, corticosteroid | Discharge |
| 84 | Hoyer et al., 2021 [71] | 75/F | NR/19 | TEN | Probable | 5 | +/85 | Supportive, corticosteroid, cyclosporine | Discharge |
| 85 | Ikediobi et al., 2021 [72] | 42/M | 300 QD/53 | SJS/TEN | Probable | NR | +/80 | Supportive, corticosteroid, IVIG | Death |
| 86 | Ferdiana et al., 2022 [73] | 63/M | 200 QD/60 | SJS | Probable | NR | +/30 | Supportive, corticosteroid | Discharge |
| 87 | 43/M | NR/28 | SJS | Probable | NR | +/30 | Supportive, corticosteroid | Discharge | |
| 88 | Manciuc et al., 2022 [74] | 77/M | 100 BD/14 | SJS | Probable | 3 | +/10 | Supportive, corticosteroid | Death |
| 89 | Zeller et al., 2022 [75] | 74/M | 300 QD/34 | TEN/DRESS | Probable | 4 | +/50 | Supportive, corticosteroid, IVIG, benralizumab | Discharge |
| 90 | Reyes-Ramos et al., 2023 [76] | 58/M | NR | SJS | Probable | NR | +/NR | Supportive, corticosteroid | Discharge |
| 91 | Anis et al., 2023 [77] | 96/F | 100 QD/10 | SJS | Probable | 3 | +/10 | Supportive, corticosteroid | Discharge |
- Note: M, male; F, female; BD, twice daily; TID, three times per day; QID, four times per day: QD, once daily.
- Abbreviations: DRESS, drug reaction with eosinophilia and systemic symptoms; IVIG, intravenous immune globulins; NR, not reported; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
The daily allopurinol dose at the time of reaction ranged from 100 to 600 mg, with a median of 300 mg. The time from the initiation of allopurinol to the onset of the reaction was reported for 82 patients in our study, the median of which was 14 days (min = 0.5 days, max = 2920 days). Six patients had a history of prior skin reactions to allopurinol (patient numbers 20, 43, 46, 62, 63, and 91 in Table 1), and the median time to onset was 8.5 days (min = 1, max = 14). Among the 76 patients who experienced skin reactions for the first time, the median duration was 16 days (min = 0.5 days, max = 2920 days). The time distribution of SJS/TEN based on the number of days after the initiation of allopurinol therapy is shown in Supporting Figure 1. As shown, the reaction occurred in 51 (67.1%) patients within the first 3 weeks and 65 (85.5%) patients within 1 month after the initiation of allopurinol.
The HLA-B∗5801 genotyping results were reported for 13 patients who were positive for this variant (patient numbers 45–47, 52, 62–64, 73, 77, 82, and 85–87 in Table 1), and the status of other patients was unknown in this regard. The ethnicities of eight of these patients were reported, six of whom were South Asian or Southeast Asian.
The distribution of clinical presentations was as follows: TEN in 55 (60.4%), SJS in 28 (30.8%), SJS/TEN in 6 (6.6%), and overlap with DRESS in 2 (2.2%) patients. The percentage of body surface involved during the reaction ranged from 5% to 95% (median of 50%). Mucosal involvement was present in 72 (90.0%) patients and absent in 8 (10.0%) patients (not reported for 11 patients).
According to the Naranjo probability scale, only one reaction (1.1%) could be definitely attributed to allopurinol. The reaction was “probable” in 82 (90.1%) cases and “possible” in 8 (8.8%) cases.
In addition to supportive care, other therapeutic interventions were used to manage the reaction. These included corticosteroids (65 patients), IVIG (25 patients), cyclosporine (6 patients), plasma exchange (4 patients), infliximab, benralizumab, and cyclophosphamide (each in one patient). Among the 89 patients for whom the outcome was reported, 21 (23.6%) died and 68 (76.4%) were discharged.
4. Discussion
In this systematic review, the number of reported cases of allopurinol-induced SJS/TEN was almost equal in males and females. Notably, the prevalence of gout in men is 2–3 times greater than that in women [78]; therefore, more men are treated with allopurinol. Whether this finding indicates a greater prevalence of allopurinol-induced cutaneous reactions in women should be interpreted with caution because there are at least two points that make establishing such a relationship difficult. First, our study only retrieved SJS/TEN data and not all skin reactions caused by allopurinol. Second, women are more likely to report adverse drug reactions than men are, and there appears to be a bias in the reporting of adverse drug reactions in favor of a greater probability of reporting in women [79]. In general, on the basis of the findings of this review, establishing a relationship between sex and the occurrence of allopurinol-induced SJS/TEN is not possible.
The relationship between the starting or maintenance doses of allopurinol and the risk of SJS/TEN is controversial. Higher starting doses of allopurinol are associated with an increased risk of SJS/TEN [80]. Although it has not been confirmed by clinical trials, starting allopurinol at lower doses with gradual dose escalations has been suggested as an effective strategy to prevent SJS/TEN [81]. In patients with normal renal function, the American College of Rheumatology 2020 gout guidelines recommend starting doses of ≤ 100 mg daily [82], and the prescribing information of allopurinol suggests increasing the dose by 100 mg/day every week to prevent the risk of SJS/TEN [1]. In patients with compromised renal function, a more conservative dose escalation should be considered. Among the 91 patients in this review, the drug dose and onset time of SJS/TEN were reported for 43 patients. In 21 patients (48.9%), the dose increase rates were higher than the recommended values, confirming the correlation between rapid dose escalation and SJS/TEN occurrence.
Most cases of drug-induced SJS/TEN occur in the first 4 weeks after the initiation of the culprit drug [83]. The time between drug initiation and the occurrence of SJS/TEN differs for various pharmacologic classes. For example, the median durations reported for macrolide antibiotics [84], acetaminophen [85], and anticonvulsants [86] were 3, 4, and 15–24 days, respectively. The median time to the onset of the reaction was 14 days in our review, which was somewhat shorter than the latency times reported in previous studies [86, 87]. This difference can be attributed to the fact that our data were derived from the studies included in our review and may not fully correspond to clinical practice because many cases have not been reported.
The time to SJS/TEN occurrence was considerably shorter in the six patients with prior reactions to allopurinol. This was an expected finding, and a more rapid occurrence of hypersensitivity reactions after re-exposure to an offending drug has been reported previously [88].
Another important finding was that six of the eight patients with known ethnicities who tested positive for HLA-B∗5801 were from South or Southeast Asia. As recommended by the American College of Rheumatology 2020 gout guidelines [82], this finding also provides a rationale for HLA-B∗5801 genotyping before the initiation of allopurinol in patients of these ethnicities.
The distribution of reaction subtypes is not consistent in published studies on drug-induced severe cutaneous adverse reactions. The reported proportions differ according to the pharmacologic categories and studied populations. For example, a systematic review of SJS and TEN cases revealed that, compared with SJS, a greater percentage of TEN cases were associated with antibiotic use and anticonvulsants [89]. In the present study, 55 (60.4%) patients presented with TEN, 28 (30.8%) with SJS, and 6 (6.6%) with SJS/TEN overlap. This finding may be inconsistent with the epidemiological findings, which showed that the prevalence of SJS is 3–4 times higher than that of TEN [89]. Our findings probably do not correspond to actual clinical data and may simply reflect publication bias because more clinically severe cases are more likely to be reported.
In this review, mucosal lesions were present in 90.0% of the patients for whom they were reported. This finding is consistent with other SJS/TEN studies in which mucosal lesions were present in approximately 90% of patients.
SCORTEN is a prognostic scoring system that incorporates seven clinical and laboratory variables, including age, malignancy, heart rate, extent of epidermal detachment, and serum urea, glucose, and bicarbonate levels. All these parameters are equally weighted and transformed into dichotomous variables. Higher SCORTEN scores denote higher mortality risk; for example, a score of 0–1 predicts a 3.2% mortality rate, and a score of ≥ 5 predicts a 90% mortality rate [6]. There have been criticisms regarding the accuracy of SCORTEN in estimating the mortality rate of patients with SJS/TEN. The risk of death may be underestimated for scores < 3 and overestimated for scores > 3 [90]. Efforts have been made to optimize the SCORTEN score; however, these scores are not readily available for clinical use because SJS/TEN is a rare condition, and prospective studies with adequate sample sizes are difficult to conduct. SCORTEN scores were reported for only 19 patients in our study. Therefore, this systematic review did not have sufficient power to evaluate the predictive accuracy of SCORTEN. Interestingly, the patients with the highest scores in our study were three patients with scores of 5, and all three patients were eventually discharged. This finding is consistent with the overestimation of mortality risk in patients with SCORTEN scores > 3.
The identification and discontinuation of the culprit drug are the most important therapeutic interventions for the management of SJS/TEN. Causality assessment tools, such as Naranjo and ALDEN [91], have been developed to help identify the culprit drug. In our study, only one reaction could be definitely attributed to allopurinol on the basis of the Naranjo score; 82 reactions were probable, and 8 reactions were possible. The answers to some questions in the Naranjo score remain unknown in daily clinical practice [92]. For example, in patients with allopurinol-induced SJS/TEN, the drug is not usually readministered, a placebo is not given, and there is no known toxic concentration of allopurinol in the serum. As a result, suspected reactions to allopurinol are often classified as “probable” or “possible.” This may not be helpful in deciding whether allopurinol should be discontinued, especially when other drugs that cause similar reactions are used concurrently. This is a commonly encountered situation in clinical practice.
In addition to causality assessment tools, clinical and laboratory tests, such as oral provocation and patch testing, have been used to identify the offending drug. Although the oral provocation test is considered the gold standard for other drug reactions, it cannot be used for severe reactions such as SJS/TEN. Patch testing has been successfully used to identify the cause of severe skin reactions, such as acute generalized exanthematous pustulosis (AGEP) and DRESS; however, there is no standard preparation for performing the test. Newer in vitro tests, such as lymphocyte transformation tests (LTTs) and cytokine assays, seem promising, although they can identify the causative drug in 21%–56% and 50%–78% of patients, respectively [4]. Currently, a combination of causality assessment tools and clinical tests can help practitioners judiciously identify causative drugs.
Nearly all the patients in this review received supportive care, including fluid and electrolyte replacement, nutritional support, analgesia, and antibiotic therapy. In addition to supportive modalities, systemic immunomodulating treatments, such as corticosteroids and IVIG, were frequently used.
Systemic corticosteroids are the most widely investigated drugs for the management of SJS/TEN. The results of meta-analyses on the use of corticosteroids in SJS/TEN are controversial. While some of these studies failed to show survival benefits with the use of corticosteroids [93], others suggested improved survival [94, 95]. Unfortunately, studies conducted on corticosteroids do not have a uniform methodology. The dose and schedule of drug administration, specific corticosteroid agents used, study population, and time to start steroids after the onset of symptoms are the most important confounding factors in clinical studies, which can partly explain the different results observed in the meta-analyses.
Similar discrepancies have also been observed in studies on IVIG in patients with SJS/TEN. Although the use of IVIG has been associated with the interruption of disease progression [96] and reduced time to mucocutaneous healing [97], it has failed to show survival benefits in several studies [98, 99]. Notably, trials that used high-dose IVIG, defined as a total dose > 2 g/kg body weight, have shown reduced mortality in SJS/TEN patients [100, 101]. The different doses and regimens of IVIG and the different compositions of the available products may explain these inconsistent findings.
Less studied agents, such as cyclosporine and TNF-α inhibitors, have been associated with reduced mortality in patients with SJS/TEN. Plasma exchange, which removes drugs, metabolites, and cytokines, is a promising intervention that can be performed daily or every other day. In addition, several combinations of the abovementioned interventions with various beneficial effects have been studied in patients with SJS/TEN [4].
There were 21 deaths in the study population. The distribution was as follows: 4 of 28 patients (14.3%) with SJS, 15 of 55 patients (27.3%) with TEN, and 2 of 6 patients (33.3%) with SJS/TEN. These mortality rates are higher than those reported in the US [102]. There are two possible explanations. First, the patients included in this systematic review were from various countries with different levels of care than those in the US. Second, the US study gathered information on patients admitted between 2009 and 2012, whereas the patients in our study were reported from 1970 when the care of patients was not of current quality.
Another important issue in the interpretation of mortality rates is the role of underlying disorders, which may complicate patients’ clinical condition. Except for two patients, all patients who died in our study had at least one (average of 2.15) chronic medical condition, including hypertension, diabetes mellitus, dyslipidemia, heart failure, chronic kidney disease, chronic liver disease, atrial fibrillation, stroke, and chronic obstructive pulmonary disease (data not shown).
There are at least two areas for further research in the field of allopurinol-induced, or more generally, drug-induced, SJS/TEN. In the first step, a more discriminative causality assessment tool should be developed and validated to help practitioners identify the causative agent(s) in SJS/TEN in a timely manner. Next, the relative efficacy and safety of treatment strategies, including corticosteroids, IVIG, plasma exchange, cyclosporine, and TNF-α inhibitors, as well as their various dosages and regimens, should be investigated in multicenter prospective clinical trials.
The findings of this systematic review have at least two limitations that should be addressed. First, as mentioned above, many drug-induced adverse reactions are not reported because more severe reactions are more likely to be reported. Therefore, the analysis of the study patients, particularly quantitative variables, may not completely match the clinical picture of allopurinol-induced SJS/TEN. Second, the patients were not uniformly reported, and there were unreported or missing data for some patients.
5. Conclusion
Although the prevalence of gout is higher in men, the reported cases of allopurinol-induced SJS/TEN were similar between men and women, indicating possible reporting bias. In approximately 50% of patients who experienced SJS/TEN, the dose increment rate was > 100 mg/day every week. The median time to the onset of the reaction was 16 days after the initiation of allopurinol, with a shorter time (8.5 days) in patients with prior skin reactions. Corticosteroids, IVIG, cyclosporine, and plasma exchange, either alone or in combination, have been successfully used in several patients. Finally, nearly all patients who died of allopurinol-induced SJS/TEN had underlying chronic medical conditions.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Acknowledgments
The research council of the Alborz University of Medical Sciences supported this systematic review. The authors would also like to thank Dr. Maryam Daei for her valuable contributions to this work.
Supporting Information
Supporting Table 1. Search strategy of the study; Supporting Table 2. Results of the quality assessment of case report studies; Supporting Table 3. Results of the quality assessment of case series studies; Supporting Figure 1. Distribution of the number of SJS/TEN cases based on the duration of allopurinol use.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




