Phospholipid Detection From Surgical Smoke Distinguishes Basal Cell Carcinoma: A Proof-of-Principle Study
Abstract
Background: Basal cell carcinoma (BCC) is a nonmelanocytic skin cancer and the most common malignancy in Caucasians. Diagnostics and treatment of BCC cause significant health-related stress for many patients and costs for public health care systems. Differential mobility spectrometry (DMS) is a sensitive method for detection of gaseous molecules. The DMS-derived automatic tissue analysis system (ATAS) utilises diathermy-generated surgical smoke to distinguish cancerous tissue from normal tissue based on lipid profiling between the tissues.
Objectives: The aim of this study was to create a surrogate porcine model to test the feasibility of the ATAS in lipid detection of skin. Another objective was to determine whether BCC of human skin could be identified from healthy skin using lipid profiling.
Methods: Porcine ear skin was used to establish a three-group porcine model for lipid profile detection. Lecithin was chosen as a marker to demonstrate elevated phospholipid levels in one of the groups. We also recruited five BCC patients to collect BCC tumour biopsies and healthy skin biopsies to test the model in human samples. In both models, all samples were processed with the ATAS to test the accuracy of lipid profiling and resolution between the groups.
Results: In the porcine model, the classification accuracy was 74.5% for three groups (unprocessed porcine skin, fine-grained porcine skin, and lecithin-marked fine-grained porcine skin) and 91.8% for two groups (unprocessed porcine skin and fine-grained porcine skin combined into one group in comparison to lecithin-marked fine-grained porcine skin). The support vector machine (SVM) classifier model trained on porcine surrogate samples was then used to analyse a small number of human BCC and healthy skin samples with 95% accuracy.
Conclusion: DMS-based differentiation of porcine skin samples based on surgical smoke is possible. This study is a step towards a method to distinguish human BCC from healthy skin from surgical smoke by the ATAS. The presented skin identification of DMS analysis of surgical smoke opens the possibility to research the method in a larger sample number of human BCC and healthy skin samples as well as develop the method and ATAS towards a clinical tool for margin assessment.
1. Background
Basal cell carcinoma (BCC) is a nonmelanocytic skin cancer (NMSC) originating from the basal layer of epidermis and counting for up to 75% of all skin cancers [1, 2]. BCC is the most common malignancy in the Caucasian adult population with lifetime risk of up to 30% [3]. Worldwide, the incidence of BCC is rising because of life-long cumulative sun exposure and ageing populations [4]. Nevertheless, the mortality rate in NMSCs has remained low since most tumours are curable and they rarely metastasize [5]. The subtypes of BCC are superficial, nodular, micronodular, sclerosing, infiltrative and morpheiform lesions [6]. Most BCCs are located on sun-exposed areas such as face and scalp. The mean invasion depth in BCC tumours is approximately 0.50–4.0 mm, and the tumour diameter can vary from a few millimetres to several centimetres [7].
Low-risk superficial BCCs and thin nodular BCCs can usually be managed with nonsurgical treatment such as topical therapies, cryotherapy, laser ablation and photodynamic therapy. Surgery is the primary treatment for all the other subtypes of BCCs although hedgehog inhibitors and radiotherapy can be used as adjuvant treatment or as an alternative to surgery for a selected group of patients [8–10].
The aim of the surgery is to remove the tumour entirely while minimizing the removal of healthy tissue to optimize functional and cosmetic outcome. The preoperative determination of the boundaries of the tumour is based on macroscopic examination by the surgeon. Nevertheless, the macroscopically clear boundaries can harbour microscopic tumour growth that can only be detected postoperatively in microscopic examination. Involved margin leads to one or more reoperations which increase the risk of complications such as delayed wound healing, infections and impaired functional or/and cosmetic outcome as well as cause additional stress for the patient. In addition, it increases costs related to the treatment process.
Mohs micrographic surgery (MMS) was developed in the 1930s to enable intraoperative margin assessment for BCC, in particular. During MMS, the surgeon removes the visible tumour, and fresh frozen section margins are assessed. The excision is extended until margins are clear. MMS has been shown to enable accurate intraoperative margin assessment but is cost- and labour-intensive [11]. Immunohistochemistry has later been combined with fresh frozen tissue to further delineate tumour margins during MMS [12].
Several methods have been proposed to ease intraoperative margin assessment. For example, optical coherence tomography (OCT) and its applications are techniques that exploit bandwidth light to produce cross-sectional images of examined tissue samples [13, 14]. Moving from morphological examination to molecular assessment is a compelling trend. Differential mobility spectrometry (DMS) or field asymmetric ion mobility spectrometry (FAIMS) is an analysation method that separates and detects molecules in atmospheric conditions [15]. Our team has studied DMS’ usage for real-time tissue analysis from surgical smoke. Previously, we have successfully categorized benign tissue types and differentiated breast tumours and brain tumours from benign tissue by DMS analysis of surgical smoke [16–18].
Lipids account for approximately 10% of the skin’s dry weight, and phospholipids (PLs) represent 50% of this total lipid mass [19–21]. Phosphatidylcholine (PC) is the most abundant PL throughout the epidermis and dermis [22]. PLs play important roles in cell signalling molecules, cell membrane structures and energy-storing molecules among others. PL levels are elevated in cancer cells and tissue due to changes in cancer cell lipid metabolism, which promotes rapid cell proliferation and division by streamlining the construction of cell membranes and cell migration, enabling more aggressive growth [23, 24]. PL levels are significantly elevated in BCC and actinic keratosis (AK) compared to normal skin [21], and there is a substantial upregulation of PC, PI and SM also in melanocytes and melanoma cells when they are radiated with UVA-radiation, which is a well-known risk factor of skin cancer [25], highlighting the importance of lipid metabolism in carcinogenesis of skin cells.
DMS analysis requires that the tumour material is transformed into gaseous form. This is inherently achieved when tumour is excised with an electrosurgical instrument. Energy instruments generate heat and cause a thermal effect on the tissue. Although conventional scalpel is predominant in skin surgery, energy instruments can be used with similar cosmetic outcomes, and they may enable faster healing [26, 27]. Thus, analysis of surgical smoke with intent of margin assessment is compatible with current clinical practise.
Skin cancer tumours have significantly higher amounts of total PLs (40 mg/g) when compared to normal skin (20 mg/g) [21] and even other cancer type tumours such as breast cancer (10–20 mg/g) [28]. The analysis set up of this study is two-phase. Firstly, we assessed DMS’ ability to differentiate elevated PL levels of samples with added PL mixture lecithin from control samples using porcine epidermis. Detection of elevated PL amounts is of interest to transition towards a more profound analysis methodology. Secondly, we evaluated DMS’ ability to differentiate human BCC punch biopsy samples from human skin control samples using a small patient-derived tissue set.
2. Materials and Methods
2.1. DMS Equipment
A DMS-based application called the automatic tissue analysis system (ATAS) was used in the study. The function of the ATAS is based on a surgical smoke analysis by a DMS sensor [29]. Surgical smoke produced by an electrosurgical instrument from an incised tissue is driven into a DMS analyser via a smoke evacuator. DMS operates in ambient air pressure, and for separation, ions are driven into an asymmetrically changing electric field where their trajectories differ based on their characteristic changes in ion mobility in high and low electric fields. The ion composition of the sample can then be presented as its distinct two-dimensional dispersion plot or “smell fingerprint” [15, 30]. The operating principle of the DMS is illustrated in Figure 1.
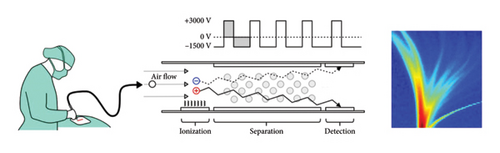
Study equipment consisted of the Ionvision DMS-analyser (Olfactomics Ltd, Finland), and a monopolar surgical blade Itkacut 350 MB diathermy device (Innokas Medical, Finland) was connected to the analysis system via a SafeAir Smoke Evacuator compact surgical smoke evacuator (Stryker Corp, USA). The system is described in detail in the study by Karjalainen et al. [30]. The diathermy power was set to 15 W to avoid excess charring of tissue during sample processing. The power setting was lower than typically used in skin surgery to compensate Colorado tip size and prevent charring in small-sized samples. We solely used the cut function of the diathermy and excluded the coagulation function, which is clinically a preferred method in skin incisions.
2.2. Samples
To test the feasibility of lipid detection from smoke with DMS, we established a surrogate porcine model as well as a BCC patient human model. Porcine skin significantly resembles human skin by histological properties as well as organic composition. The recommended experimental area is the outer side of the porcine auricle. On this area of the porcine ear, the epidermis is approximately 81 μm [19] relating to the epidermal thickness of human buttocks [31]. Previous studies show that the lipid and PL concentrations in porcine and human skin are closely related. The total amount of lipid compounds is 8% in porcine skin dry weight and 10% in human skin dry weight [20], whereas the total amount of PLs is 62.3% in porcine epidermis and approximately 50% in human epidermis [19, 21]. In human skin, the normal concentration of lipids is 50 mg/g, of which the PL concentration is 20 mg/g. In BCC tumours, the concentration of PLs is 40 mg/g which is two times greater than in normal skin [21].
2.3. Porcine Model
To respect the 3R’s principle of reduction, refinement, and replacement in animal testing [32, 33], we used porcine skin that was offal and a by-product of the food industry. We obtained porcine ears from a local abattoir and used the epidermis of the outer side of the auricle. To demonstrate the increased PL concentration, we used lecithin. Lecithin is an example of glycerophospholipids which are amphiphilic fatty substances occurring in animal tissues [34]. Altered lipid metabolism of cancer cells ultimately leads to an overall increase of multiple PLs rather than specific ones (23), (24), so it was concluded that the overall concentration of PL is more crucial than the individual concentrations of separate PLs. Lecithin suits well as a marker as it contains proportionally more PC, the most abundant PL in cells and potential cancer biomarker [35], than other PL compounds. It was observed that as an injection, lecithin did not infiltrate avascular porcine epidermis evenly and, thus, decided to prepare a mixture of fine-grained porcine epidermis and lecithin to achieve a stable concentration. The fine-grained porcine outer-ear epidermis was weighed and divided into two analogous portions. Lecithin was measured and carefully mixed to the other portion to double the PL concentration. In the porcine setting, we had three control groups: unprocessed porcine epidermis, fine-grained porcine epidermis and fine-grained lecithin marked porcine epidermis, as shown in Figures 2 and 3.
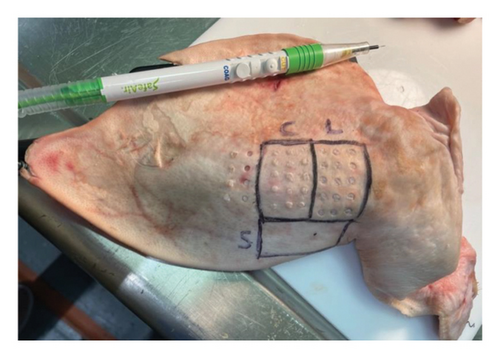
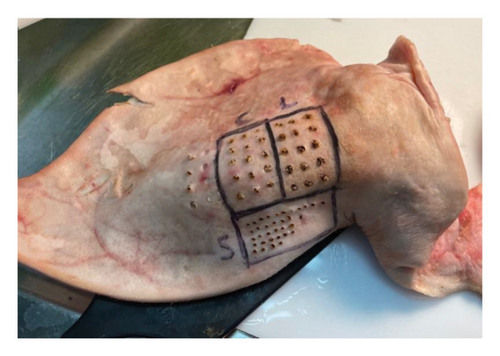
2.4. Human Model
To demonstrate the feasibility of BCC detection of PL profiling in human skin, we recruited five voluntary patients diagnosed with BCC and perioperatively collected healthy skin and tumour samples for comparison. The patients were recruited between the 9th of December 2022 and the 8th of March 2023 at the Tampere University Hospital, Finland. Ethical approval was obtained from the local Ethics Committee of Tampere University Hospital (code R20010L). The study was conducted following the World Medical Association’s Declaration of Helsinki. Informed consent was obtained from each patient in writing. Inclusion criteria included patients over 18 years of age recently diagnosed with an operative treatment requiring BCC and tumour size of 1.5 cm or larger. The age of the patients was between 49 and 93 years with a median of 75.4 years. All patients had a BCC tumour in the head, neck or chest area. Operations were carried out following the national guidelines [10]. Perioperatively, a 4-mm punch biopsy was taken from the centre of the BCC lesion from a nonulcerative area. The tumour was then surgically removed with healthy skin margins, and a same-sized 4-mm control biopsy was obtained from the healthy skin margin outside the tumour. All samples were stored in a freezer at −70°C.
Patient data were collected from electronic health records, as shown in Table 1. The resected tumour specimens were microscopically examined by a skin pathologist. Histological data were gathered from a structured histopathology report. According to the histopathological reports, there was BCC tumour growth in the immediacy of the punch biopsy in each specimen. Consequently, a presumption was made that the tumour punch biopsies included BCC tumour growth. In one of the samples, ulceration was present at the biopsy site both macroscopically and histologically.
| Sample number | Tumour location | Tumour diameter in mm (max) | Tumour diameter in mm (min) | Histology (subtypes present) | Observations |
|---|---|---|---|---|---|
| 1 | Head | 45 | 25 | Nodular, micronodular and sclerosing | Ulceration was present at the biopsy site |
| 2 | Head | 30 | 70 | Superficial, nodular and sclerosing | |
| 3 | Chest | 20 | 15 | Superficial and nodular | |
| 4 | Neck | 30 | 25 | Nodular | |
| 5 | Head | 40 | 40 | Nodular |
- Note: The table presents the location, size and histopathological findings of each tumour. In sample number 3, ulceration was present at the biopsy site.
2.5. Measurements
To demonstrate the accuracy of PL recognition by the ATAS, we made short approximately 1 s lasting puncture cuts into control tissues with a Colorado tip diathermy. The samples were processed by single operator, a board-certified plastic surgeon. Surgical loupes (Optergo VinKep) with a magnification of 4.3x were used to enhance precision. The diathermy tip was cleansed with a saline retted gauze after each cut.
In the porcine model, the puncture cuts were made in a random order between the different control groups. The diathermy smoke was conducted to the ATAS to evaluate the accuracy of lipid detection between the control groups.
In the human model, initially three puncture cuts were made in each 4-mm BCC tumour biopsy and 4-mm control biopsy. During the procedure, we noticed that after two puncture cuts the dermal effect of the diathermy to the 4-mm biopsy was notable. Consequently, we decided to evaluate first and second puncture cuts and rule out the third puncture cut to get reliable results. Diathermy smoke was then processed with the ATAS to evaluate healthy skin and tumour recognition.
In total, 98 incisions were made on porcine samples (unprocessed skin, fine-grained skin and lecithin-marked fine-grained skin) of which all were included in the analysis. In addition, 30 incisions were made on human samples (BCC samples and control samples), of which 20 incisions were included in the final analysis due to excessive charring of the tissue in final cuts.
2.6. Data Analysis
Parametrization of the SVM classifier model was performed with porcine surrogate samples. The SVC implementation in scikit learn was used with linear kernel function. The grid-searched parameters of the model were the regularization parameter C and the kernel coefficient gamma. This approach was taken to avoid overfitting to the patient data.
Statistically significant areas were selected using the Kolmogorov–Smirnov (KS) method. As the method works between two classes, the unprocessed skin and fine-grained skin were combined into one group. These areas were subsequently used to classify human samples with leave-one-patient-out cross validation. Leave-one-patient-out was chosen as the primary method due to the small number of unique patients. The cross-validation method results in an 80/20 training/testing split of the dataset. As leave-one-patient-out is prone to overfitting, other group-cross validation splits were also used. 4-fold and 3-fold cross validations were tested.
3. Results
In the porcine model, the classification accuracy was 74.5% for three groups (unprocessed porcine skin, fine-grained porcine skin as the control group and lecithin-marked fine-grained porcine skin as the sample group) and 91.8% for two groups (unprocessed porcine skin and fine-grained porcine skin combined into one group in comparison to lecithin-marked fine-grained porcine skin). Confusion matrixes for both porcine sample analyses are presented in Figures 4 and 5.
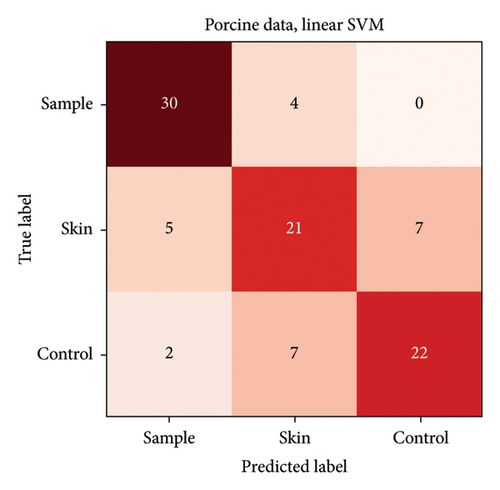
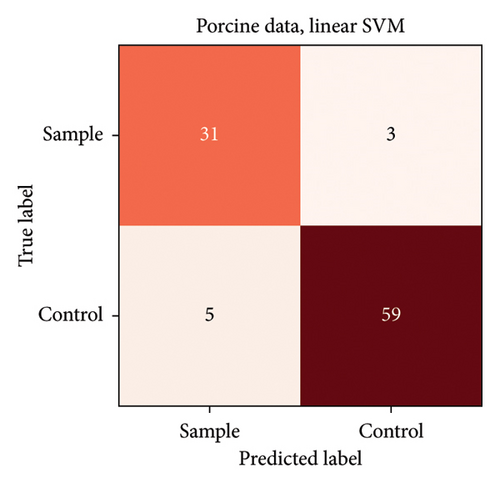
The highest misclassification rate in the porcine model was between unprocessed porcine skin and fine-grained porcine skin. This is a favourable result meaning that the graining process did not overwhelmingly alter the signal of the tissue. Therefore, the three groups could be combined into a binary classification setting, and the lecithin-marked fine-grained porcine skin was distinguished from the other two groups regardless of the texture of the nonlecithin-marked porcine skin.
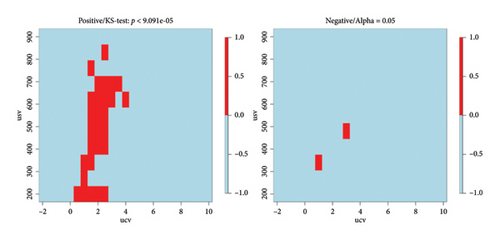
Statistically significant areas from the dispersion plots were identified using the KS test. The original significance level of 0.05 was adjusted with Bonferroni correction to limit the possibility of accepting an area as significant purely by chance. The adjusted significance level was 9.091 ∗ 10−5. Figure 6 shows that most significant areas can be found from the positive ion side and a few also from the negative ion side.
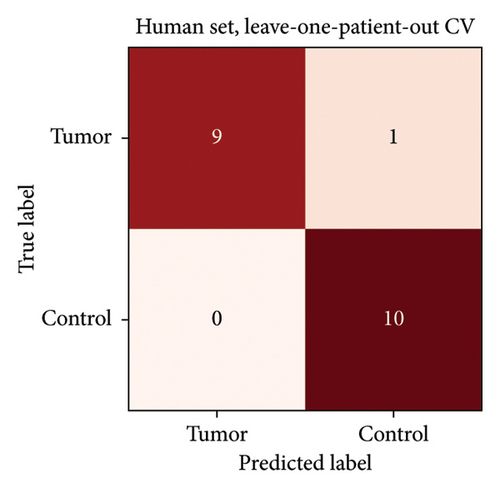
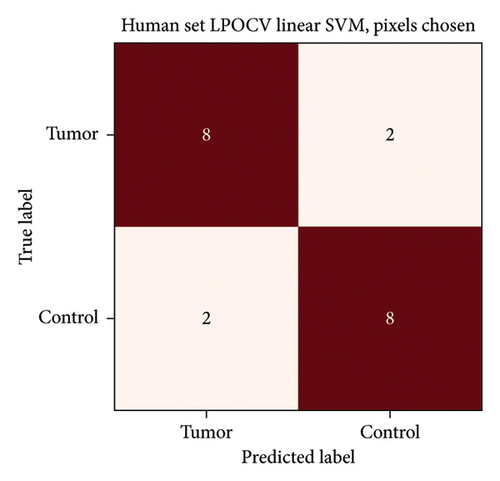
Initially, three incisions were made into a single sample which produced a SVM CA of 81%. Closer examination of the measurements revealed that primarily the third burns were misclassified presumably due to excessive charring of the sample. After exclusion of the third incisions, the proof-of-concept human set achieved a high CA (95%) only misclassifying 1 tumour sample as a control. This specific tumour sample was macroscopically and histologically ulcerated. The confusion matrix is presented in Figure 7. 4-fold cross validation also gave 95% CA, and 3-fold gave 70% CA. The lower result with 3-fold cross-validation is to be expected, as the number of samples used for training decreases.
Preparametrized SVM produced CA, sensitivity and specificity of 95%, 90% and 100%, respectively, and 80%, 80% and 80% with the parametrized model limited to areas with statistically significant differences between control and lecithin-enriched porcine samples. These are illustrated in Figure 8.
4. Conclusion
In this study, we showed that DMS analysis of surgical smoke is capable of discriminating BCC from noncancerous skin using a classification algorithm that relies solely on lipid features of lecithin-enriched porcine skin, demonstrating that the lipid profile of the tissue is the determining factor for discrimination in BCC. This contrasts with previous DMS studies where the identification of malignant and benign tissue types has relied on a ‘black box method’. Even though most BCC tumours are curable, BCC diagnosis and cancer treatment have a negative impact on the quality of life and cause stress to the patients [36]. In addition, BCC treatment causes substantial costs for the health care system. Considering NMSC’s considerable incidence globally and its economic burden on the health care system [37, 38], it is necessary to study DMS’ applicability as an intraoperative margin assessment tool in dermatological surgery.
To our knowledge, the identification of BCC from surgical smoke has been carried out in one previous study where BCC samples and benign skin and adipose tissue samples were analysed retrospectively ex vivo (CA of 90%) and prospectively during surgery (CA of < 50%) with REIMS from surgical smoke [39]. The researchers analysed BCC, skin and adipose samples from 47 patients and tested the classification performance on a pilot sample of 5 patients, making the study setup comparable to ours.
The inferior results of the intraoperative setup were multifactorial with weighty emphasis on the modality of the tissue samples, which formed a very heterogenous group, and tissue types were categorized by a pathologist only visually, used for the training data set before the in vivo set up [39]. It is noteworthy that adding adipose tissue to the setup might produce overly optimistic CA results because its composition differs significantly from benign and malignant skin samples. The larger sample sizes in the REIMS study are an evident asset which produces greater CAs and reliability. Both REIMS and DMS studies demonstrate that BCC is clearly distinguishable from healthy skin via analysis of surgical smoke. DMS achieved a high CA with a small ex vivo data set, and the limitations of the referred REIMS study are noteworthy when transitioning into an in vivo setup in the future with the Resect DMS analysis system.
Otherwise, rapid identification of malignant skin lesions has been mainly focused on imaging techniques to streamline the analysis of skin biopsies [40]. Pyun et al. studied the use of noninvasive laser-induced plasma spectroscopy (LIPS) where an ultrashort pulsed laser is used to gain biochemical information on skin lesions in real time by producing an emission spectrum of the studied sample. This method achieved high specificity (97%) and sensitivity (98%) and does not require sample preparation [41]. However, the analysis does require an additional device and work step to be performed, thus interrupting workflow if used intraoperatively. High-resolution millimetre imaging (HRMMI) has also been used to identify patient-derived benign and malignant skin lesions [42] but faces the same limitations as LIPS. This proof of the principle study shows DMS’ potential as a rapid tissue analysis method providing seamless workflow in the field of dermatological surgery.
Measurements from the porcine model set up enabled locating statistically significant data points to try to enhance separability between sample classes. Parametrized SVM produced a slightly inferior CA compared to the preparametrized model (95% vs. 80%) which is mostly likely due to the small sample size and differences in biological composition of the porcine and human samples. A bigger sample set would give reliability to see if the analysis of lecithin and porcine epidermis mixture could, in fact, enhance the classification of human epidermal tumour samples. Lecithin samples likely produce ions especially from PC. Porcine epidermis is very similar to that of human, but still human basal carcinomas have elevated levels of multiple PL subclasses and other lipid fractions such as cholesterol and triglycerides [21] which makes it less analogous compared to the porcine samples. It is noteworthy that the parametrized model produced adequate results which support PC-derived target ions as potential biomarkers for pretargeting significant areas to identify cancer tissue more accurately from surgical smoke via DMS.
The possible limitations of surgical smoke-based tissue analysis and margin recognition are, for example, tumour ulceration and high-grade growth patterns. Tumour ulceration or necrosis can cause cell destruction that could alter the concentration of molecules within the tumour. In this case, the DMS-based surgical smoke analysis might not give a reliable result with present techniques [43]. BCC tumours with high-grade growth patterns can include satellite lesions that can deteriorate accurate margin recognition. Further research on the topic might give answers and solutions to these challenges.
In addition, in this setting, the patient number was small for an encompassing analysis meaning that the results should be interpreted with caution. The utilization of a simple, lipid-based classifier and microscopic verification of all samples reduces the risk of bias to an extent. In the future, we aim to validate the method in a larger BCC patient population, which also enables us to analyse differences between BCC histological subtypes and anatomical sites.
Overall, this study illustrated the potential of DMS to distinguish BCC from healthy skin based on PL content of the tissue, making the analytical pipeline transparent. The results of this study should be repeated in a larger patient population.
Disclosure
The study sponsors did not have any involvement in the study design; collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Conflicts of Interest
Meri Mäkelä (employee), Anton Kontunen (part-time employee, shareholder), Markus Karjalainen (part-time employee, shareholder), Niku Oksala (part-time employee, shareholder) and Antti Roine (employee, shareholder) are affiliated with Olfactomics Oy, which is about to commercialize proprietary technology for the detection of diseases by ion mobility spectrometry.
The remaining authors declare no conflicts of interest.
Author Contributions
Anni Salminen: writing – original draft, visualization, writing – review and editing, data collection and formal analysis. Patrik Sioris: writing – original draft, visualization and writing – review and editing. Juha Jernman: conceptualization, data collection and writing – review and editing. Nele Veide: data collection and writing – review and editing. Anton Kontunen: formal analysis, software, visualization and writing – review and editing. Meri Mäkelä: investigation, software, formal analysis, data curation, visualization, writing – original draft and writing – review and editing. Markus Karjalainen: formal analysis, software and writing – review and editing. Minna Kelloniemi: funding acquisition, supervision, resources and writing – review and editing. Niku Oksala: funding acquisition, supervision, resources, conceptualization, methodology and writing – review and editing. Antti Roine: funding acquisition, conceptualization, methodology, supervision, writing – review and editing and project administration.
Funding
Anni Salminen declares funding from The Finnish Medical Foundation (grant number 6336), and this work was supported by the Competitive State Research Financing of the Expert Responsibility Area of the Tampere University Hospital (grant nos. T62864, 9AC044 and T69934). Patrik Sioris declares funding from the Instrumentarium Science Foundation (grant number 230034) and the Emil Aaltonen Science Foundation. Markus Karjalainen declares funding from the Finnish Cultural Foundation, the Pirkanmaa Regional Fund and the Sigfrid Juselius Foundation. Anton Kontunen declares funding from the Doctoral School of Tampere University, The Finnish Foundation for Technology Promotion (grant number 7671) and the Emil Aaltonen Foundation (grant number 210073K).
This study has received funding from the ATTRACT project funded by the European Commission from the Horizon 2020 research and innovation programme (grant agreement 777222). This study was also financially supported by Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital and Pirkanmaa Hospital District (grant numbers 9AA057, 9x040, 9v044, 9T044, 9U042, 9s045, 150618 and 151B03); Sigfrid Juselius Foundation (decision number 240148); competitive funding to strengthen university research profiles were funded by the Academy of Finland (decision number 292477) and from the Tampere Tuberculosis Foundation.
Acknowledgements
The authors would like to thank Eero Lääperi, Marika Kuuskeri, Johanna Palve, Katriina Joensuu and Elina Halme for valuable comments in preparation of the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.