Intradermal Botulinum Toxin A for Melasma: A Randomized Split-Face Study Trial and In Vitro Study of Its Antimelanogenic Effect
Abstract
Background: Melasma is a challenging hyperpigmentation disorder without absolute treatment.
Aims: This study aimed to evaluate the effects of intradermal botulinum toxin A (BoNT-A) on melasma and the protective effects of BoNT-A on UVA-induced melanogenesis in B16F10 melanoma cells.
Patients/Methods: This study is a split-face randomized, double-blind, placebo-controlled trial in 12 melasma patients who received intradermal abobotulinumtoxinA injection into melasma lesions. An in vitro study was also conducted in B16F10 melanoma cells treated with different concentrations of BoNT-A prior to exposure to UVA. Cell viability and cellular melanogenesis were determined.
Results: The adjusted MASI scores on the BoNT-A side were significantly lower than the control at 3 months after injection, 2.8 versus 4.5 (p < 0.001), respectively. BoNT-A injection significantly reduced the MASI score at 2 and 3 months compared with the baseline of 4.1–3.2 (22%) (p < 0.001) and 2.8 (31.7%) (p < 0.001), respectively. Melanin content and tyrosinase activity in B16F10 cells with or without UVA irradiation were significantly reduced by treatment with BoNT-A in a dose-dependent manner without causing cytotoxicity.
Conclusions: BoNT-A has a potentially beneficial effect in the treatment of melasma due to its antimelanogenic effect.
Trial Registration: Clinical Trial Registry identifier: TCTR20250118001
1. Introduction
Melasma is an acquired hyperpigmentation disorder presenting with bilateral light to dark brown hyperpigmentation [1, 2]. There are a multitude of factors triggering its development: sun exposure, family history, pregnancy, changes in hormones, thyroid dysfunction, cosmetics, and medications can all contribute to the pathogenesis of melasma [1–3]. The most important factor is sun exposure, including UVA, UVB, and visible light (VL) [4]. Due to the unclear pathophysiology of melasma, existing treatment methods tend to give inconsistent results.
Recent years have seen significant advancements in the development of new therapies for melasma, driven by a better understanding of its complex pathophysiology. Traditional treatments, such as hydroquinone, corticosteroids, and chemical peels, often provide limited and inconsistent results, leading to a search for more effective and sustainable therapies. Among the newer options, laser treatments have gained prominence, particularly fractional laser, intense pulsed light (IPL), and picosecond lasers, which target melanin in the skin while minimizing damage to surrounding tissues. Tranexamic acid, both topically and orally, has also emerged as a promising treatment, with recent studies suggesting its efficacy in reducing pigmentation by inhibiting the production of melanogenic mediators and decreasing vascular component activity in the dermis. However, despite these advancements, the development of therapies that offer long-lasting, consistent, and safe results remains a challenge [5].
Recent studies demonstrated the protective effects of botulinum toxin A (BoNT-A) on skin hyperpigmentation [6, 7]. Melanocytes, like neurons, originate from ectodermal neural crest cells during embryonic development, and since BoNT-A inhibits acetylcholine release at the neuromuscular junction, it is possible that BoNT-A might influence the melanin production pathway, which could be beneficial in melasma treatment [6]. BoNT-A significantly decreased human melanocyte dendriticity under UVB irradiation. With BoNT-A injection, UVB-irradiated mouse skin was lighter with less accumulation of melanin [6]. Human studies showed that BoNT-A significantly reduced UVB-induced pigmentation on human skin [7, 8]. Moreover, a recent study showed that BoNT-A injection decreased the melanin index in healthy skin [9]. However, there is no study that evaluates the effects of BoNT-A on melasma.
In our study, we aimed to investigate the effects of intradermal BoNT-A injection on melasma in a clinical setting. In addition, we performed an in vitro study to explore the effects of BoNT-A on UVA-induced cellular melanogenesis in melanoma skin cells. The in vitro data provided valuable confirmation that BoNT-A exerts an antimelanogenic effect at the cellular level, without causing cytotoxic damage. This finding is particularly important, as it supports the safety and efficacy of BoNT-A as a treatment for melasma and confirms that the observed clinical benefits are not due to harmful effects on skin cells, which could pose risks for patients.
2. Materials and Methods
2.1. Clinical Study
2.1.1. Study Design
This study is a double-blind, split-faced, randomized-controlled pilot study in melasma patients aged 20–55 years at Samitivej Sukhumvit Hospital, Bangkok, Thailand. The study population included female patients with Fitzpatrick skin types III and IV, which represent the most common skin types in individuals with melasma in the real world. The patients had not received any melasma treatments (such as topical agents or light-based treatments) for at least 1 month prior to participation, and any botulinum toxin (BoNT) injections were withheld for at least 6 months before the study. Note that certain treatments, like laser or microneedling, may require a longer washout period. Exclusion criteria include pregnancy, lactation, use of exogenous hormones, thyroid disease, allergy or resistance to BoNT, bleeding disorders, neuromuscular disorders, active infections or skin diseases.
Since there was no previous study using BoNT-A for melasma; thus, this is the pilot study and we enrolled 12 participants in this study. Twelve subjects were randomized with simple randomization to receive BoNT-A on the randomized cheek and 0.9% normal saline solution (NSS) on the contralateral side. Intradermal injections of abobotulinumtoxinA (Dysport) with a 7.5 mL dilution in a 500 Unit vial (66.67 Units/mL) were performed by a highly experienced dermatologist with 30G needles. The injection wheal size was 2 mm in diameter with 2 mm apart between the wheals in the melasma area.
Simple randomization was performed using computer-generated random numbers. Randomization was revealed to the dermatologist just before the start of each procedure. Both dermatologists and participants were blinded. The syringes containing BoNT-A and saline were prelabeled with codes known only to a research coordinator who was not involved in the injections. The dermatologist performing the injections was blinded to these codes and received only coded syringes.
This study was approved by the Human Research Ethics Committee, Bangkok Health Research Center, Thailand (BHQ-IRB 2021-11-35, COA No. 2022-18). Informed consent was obtained from all participants in the study, and the consent process covered all necessary details of the study, including its purpose, procedures, risks, and potential benefits. The CONSORT flowchart is shown in Figure 1.

In this study, all raw data from the clinical and in vitro experiments, including participant data and experimental results, are provided as supporting files for full transparency and reproducibility. These raw data files are available in Supporting Informations 1 and 2.
2.1.2. Clinical Assessment
The primary outcome was melasma area and severity index (MASI) score. The secondary outcome was physician global assessment (PGA) scores and patient satisfaction scores. Evaluation was performed before injection and 2, 4, 8, and 12 weeks later by two blind dermatologists who were not involved in the injection procedure and were unaware of the treatment codes using the MASI score. Also, the melasma improvement was evaluated by PGA scores at 2, 4, 8, and 12 weeks after injection. The assessment of the MASI score is based on area involvement, darkness, and homogeneity. The PGA score was graded from 0–6: 0, clear; 1, almost clear, very significant clearance (90%); 2, marked improvement, significant improvement (75%); 3, moderate improvement, intermediate between slight and marked improvement (50%); 4, slight improvement, some improvement (25%); 5, no improvement; and 6, worse. Patient satisfaction was graded from 0% to 100%.
2.1.3. Statistical Analysis
To study the trend of MASI and PGA measured repeatedly over time, linear mixed-effects models with random effect for person and face side were selected to analyze longitudinal data. The likelihood ratio test was done to choose the most suitable model. The most suitable model was the model with interaction terms between the drug and time for the MASI and PGA outcomes. To study patient satisfaction scores measured repeatedly over time, linear mixed-effects models with random effect for person were selected to analyze longitudinal data. The pairwise comparison was done with the Scheffe’s method for all outcomes. The intraclass correlation coefficient was checked between the two reviewers for all results.
Data were analyzed according to intention-to-treat (ITT) analysis using STATA/IC 15.1 software packages (StataCorp, College Station, TX, USA).
2.2. In Vitro Study
2.2.1. Cell Culture
The cells used in this study were the B16F10 mouse melanoma cell line (ATCC, MD, USA). B16F10 cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 units/mL)/streptomycin (100 μg/mL) and maintained at 37°C in a humidified incubator containing 5% CO2/95% air.
2.2.2. Cytotoxicity Test of BoNT-A on B16F10 Cells
Cytotoxicity was determined using the MTT (3- (4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide) assay. This colorimetric assay measured the reduction of yellow MTT to purple formazan by mitochondrial succinate dehydrogenase in living cells to assess viability at any concentration of BoNT-A up to 100 U/mL. B16F10 cells were treated with BoNT-A for 24 h. After washing the cells with PBS, the plates were incubated with medium containing 0.2 mg/mL of MTT at 37°C in a 5% CO2 incubator for 1 h. Then, 200 μL of DMSO was used to solubilize the purple formazan in each well. The plates were gently shaken for 10 min. The optical density was then measured at 595 nm using a spectrophotometer. Viable cells were expressed as a percentage of the control (100%, non-UVA irradiated and untreated cells).
2.2.3. Treatment of Cells With BoNT-A and UVA Irradiation
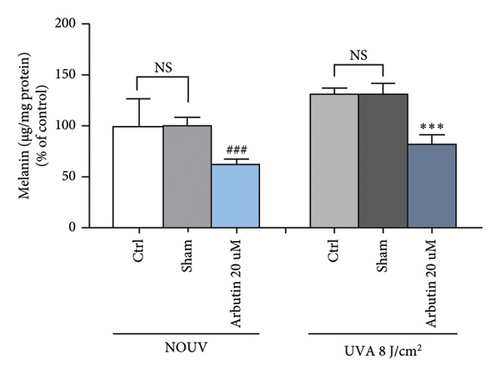
Cells were exposed to BoNT-A at selected concentrations of 7.5, 15, 30, and 60 U/mL for 30 min in phosphate-buffered saline (PBS). After BoNT-A treatment, cells were exposed to UVA radiation dose of 8 J/cm2 (xenon arc lamp, Dermalight ultrA1). Subsequently, cells were harvested for measurement of melanin content and tyrosinase activity. Non-UVA-irradiated and non-BoNT-treated cells were used as the negative control. Arbutin 20 μM was used as the positive control.
2.2.4. Melanin Content Assay
B16F10 melanoma cells were seeded at a density of 500,000 (0.5 × 106 cells) per well in a 6-well plate for the assessment of cellular melanin content. Cells were pretreated with BoNT-A (7.5–60 U/mL) in PBS for 30 min before exposure to UVA (8 J/cm2). The total melanin contents were then extracted by 200 μL of 1N NaOH at 60°C for 1 h and the lysate was collected in microtubes. Subsequently, 100 μL of lysis buffer (50 mM Trisma base; 10 mM EDTA; 1% (V/V) Triton X-100; 0.57 μM pepstatin A 2 μM leupeptin) was added to extract protein and subsequently centrifuged at 10,000 rpm at 4°C for 10 min. The supernatant was collected by centrifugation, and 100 μL melanin lysate was added to a 96-well plate to be measured by spectrophotometry at 475 nm. The melanin content (μg/mg protein) was calculated by comparison to a standard curve derived using synthetic melanin of non-UVA-irradiated and non-BoNT-treated cells (100%).
2.2.5. Tyrosinase Activity Assay
B16F10 melanoma cells were seeded at a density of 500,000 (0.5 × 106 cells) per well in a 6-well plate for the assessment of cellular tyrosinase activity. Cells were pretreated with BoNT-A (7.5–60 U/mL) in PBS for 30 min before exposure to UVA (8 J/cm2). Cellular tyrosinase activity was determined by measuring the rate of oxidation of L-DOPA. Cells were lysed with 100 μL of lysis buffer (0.1 M phosphate buffer pH 6.8, 0.1% Triton X-100) and incubated for 10 min at 4°C with gentle shaking. The cell lysates were then clarified by centrifugation at 10,000 rpm for 10 min at 4°C. The supernatant was collected and incubated with 10 μL of substrate solution (20 mM L-DOPA) and 90 μL of cell lysates were assayed on a 96-well plate at 37°C. Subsequently, the DOPAchrome production rate, reflecting tyrosinase activity, was measured by spectrophotometry at 475 nm for 1 h at 37°C. The results of tyrosinase activity (unit/mg protein) were calculated by comparison with a standard curve derived using purified mushroom tyrosinase (2034 units/mg) of non-UVA-irradiated and non-BoNT-treated cells (100%).
2.2.6. Statistical Analysis
The statistical significance of the differences between the BoNT-A-treated groups or UVA-irradiated groups and the control was evaluated by one-way ANOVA followed by Dunnett’s multiple comparison post-test using GraphPad Prism5 statistical analytic software (GraphPad Software Inc). The mean and standard deviation were adopted to express the data for each group. Statistically significant differences from basal or control values were classified as ∗, #p < 0.05; ∗∗, ##p < 0.01; and ∗∗∗, ###p < 0.001.
3. Results
3.1. Demographic and Clinical Data
Twelve female participants were enrolled. The mean age was 39.8 (SD = 6.9) years. The average duration of melasma was 6.1 (SD = 6.2) years. There were 7 patients (58.3%) who had a history of BoNT injection. The average duration of the latest BoNT injection is 0.90 (SD = 1.0) years. The family history of melasma is 41.7%. The average duration of sun exposure is 1.02 (SD = 1.3) hours per day. Most of the patients did not do outdoor activities with sun exposure (91.7%). For sunscreen usage, 11 patients (91.7%) used sunscreen with SPF 50 and 8 patients (66.7%) used a sufficient amount of sunscreen (Table 1).
| Characteristics | N | Percentage or mean (SD) |
|---|---|---|
| Age (years) | 39.8 (6.9) | |
| Gender, female | 12 | 100 |
| Duration of melasma (years) | 6.1 (6.2) | |
| History of botulinum toxin injection | 7 | 58.3 |
| Latest botulinum toxin injection (years) | 0.90 (1.0) | |
| Family history of melasma | 5 | 41.7 |
| Duration of sun exposure (hours/day) | 1.02 (1.3) | |
| Outdoor activities | 1 | 8.3 |
| Sunscreen SPF ≥ 50 usage | 11 | 91.7 |
| Sufficient sunscreen usage | 8 | 66.7 |
- Abbreviations: N, number; SD, standard deviation; SPF, sun protection factor.
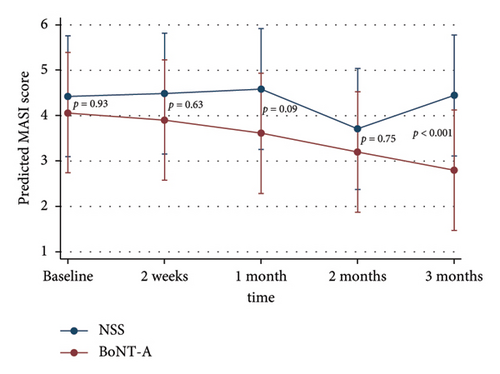
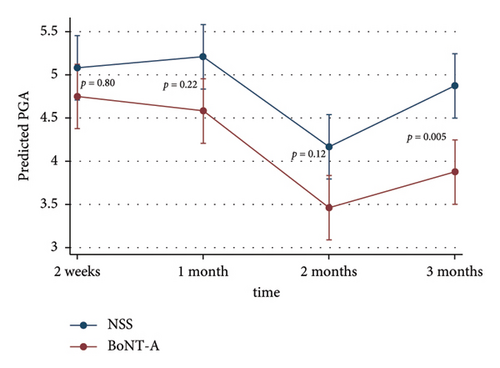
The adjusted MASI scores on the BoNT-A side were significantly lower than the control at 3 months, 2.8 versus 4.5 (p < 0.001), respectively. The BoNT-A side showed a significant decrease in MASI scores at 2 and 3 months compared with the baseline from 4.1 to 3.2 (−22%) (p = 0.01) and to 2.8 (−31.7%) (p < 0.001), respectively (Table 2) (Figures 2(a) and 3). The adjusted PGA scores on the BoNT-A side were significantly less than the control at 3 months, 3.9 versus 4.9 (p = 0.005), respectively (Table 2) (Figure 2(b)). The intraclass correlation coefficient between the two reviewers for the MASI and the PGA scores had a moderate correlation of 0.73 and 0.43, respectively.
| Time | MASI (unadjusted) | MASI (adjusted) | pvalue | ||||
|---|---|---|---|---|---|---|---|
| BoNT-A group | Control | BoNT-A group | 95% CI | Control | 95% CI | ||
| Baseline | 4.1 ± 2.5 | 4.4 ± 2.7 | 4.1 | (2.7, 5.4) | 4.4 | (3.1, 5.8) | 0.93 |
| 2 weeks | 3.9 ± 2.6 | 4.5 ± 2.5 | 3.9 | (2.6, 5.2) | 4.5 | (3.2, 5.8) | 0.63 |
| 1 month | 3.6 ± 2.2 | 4.6 ± 2.4 | 3.6 | (2.3, 4.9) | 4.6 | (3.3, 5.9) | 0.09 |
| 2 months | 3.2 ± 2.4 | 3.7 ± 2.5 | 3.2 | (1.9, 4.5) | 3.7 | (2.4, 5.0) | 0.75 |
| 3 months | 2.8 ± 2.1 | 4.5 ± 2.6 | 2.8 | (1.5, 4.1) | 4.5 | (3.1, 5.8) | < 0.001 |
| Time | PGA (unadjusted) | PGA (adjusted) | pvalue | ||||
| BoNT-A group | Control | BoNT-A group | 95% CI | Control | 95% CI | ||
| 2 weeks | 4.8 ± 0.7 | 5.1 ± 0.3 | 4.8 | (4.4, 5.1) | 5.1 | (4.7, 5.5) | 0.80 |
| 1 month | 4.6 ± 0.5 | 5.2 ± 0.4 | 4.6 | (4.2, 5.0) | 5.2 | (4.8, 5.6) | 0.22 |
| 2 months | 3.5 ± 0.9 | 4.2 ± 0.7 | 3.5 | (3.1, 3.8) | 4.2 | (3.8, 4.5) | 0.12 |
| 3 months | 3.9 ± 0.9 | 4.9 ± 0.9 | 3.9 | (3.5, 4.2) | 4.9 | (4.5, 5.2) | 0.005 |
| Time | Satisfaction scores (unadjusted) | Satisfaction scores (adjusted) | pvalue | ||||
| BoNT-A group | Control | BoNT-A group | 95% CI | Control | 95% CI | ||
| 2 weeks | 20.8 ± 9.7 | 15.0 ± 14.5 | 22.8 | (16.6, 29.0) | 13.1 | (6.9, 19.3) | < 0.001 |
| 1 month | 25.4 ± 11.2 | 14.2 ± 13.1 | 24.6 | (18.4, 30.8) | 14.9 | (8.7, 21.2) | < 0.001 |
| 2 months | 22.9 ± 11.0 | 13.3 ± 13.0 | 23.0 | (16.8, 29.2) | 13.3 | (7.1, 19.5) | < 0.001 |
| 3 months | 27.1 ± 15.1 | 15.0 ± 16.2 | 25.9 | (19.7, 32.1) | 16.2 | (10.0, 22.4) | < 0.001 |
- Abbreviations: BoNT-A, botulinum toxin A; CI, confidence interval; MASI, melasma area severity index; PGA, physician global assessment.
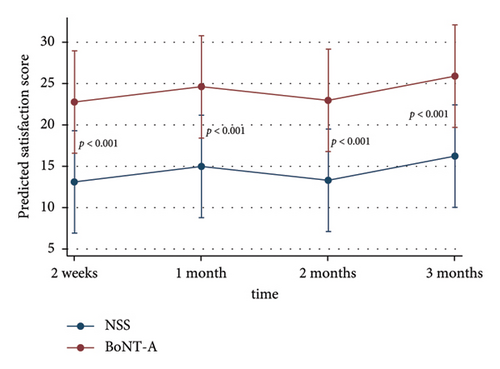
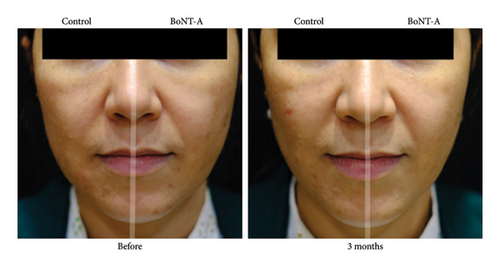
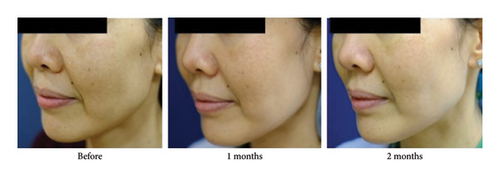
The adjusted patients’ satisfaction scores on the treated side were significantly higher than the control at all time points after injection (p < 0.001) (Table 2) (Figure 2(c)). The adverse event was erythema for a few days in 33% of all patients without significant difference between the groups.
3.2. In vitro Study of the Effect of BoNT-A on Cellular Melanogenesis Induced by UV Radiation
3.2.1. Cytotoxicity
For the cytotoxicity test of BoNT-A in B16F10 cells, all concentrations of BoNT-A from 0.39 to 100 U/mL did not cause significant cell death (Figure 4(a)).


3.2.2. BoNT-A Inhibited UVA-Induced Melanin Content in B16F10 Cells
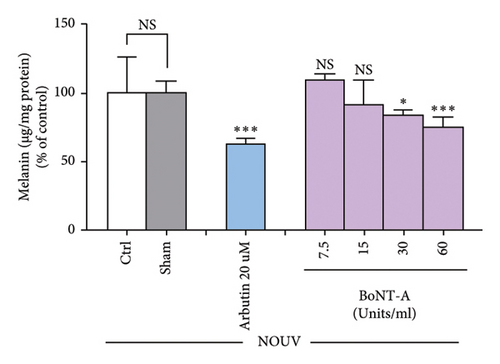
BoNT-A at the concentration of 30 and 60 U/mL significantly inhibited the melanin content in unirradiated B16F10 cells in a dose-dependent manner. Untreated B16F10 cells irradiated by UVA (8 J/cm2) significantly induced the melanin content compared to unirradiated control cells. BoNT-A at the concentration of 15, 30, and 60 U/mL significantly inhibited the melanin content in UVA-irradiated cells in a dose-dependent manner. At 60 U/mL BoNT-A, the inhibitory effect was 50.3% ( ∗∗∗p < 0.001), without significant difference from the positive control (Figures 4(b), 4(c), and 4(d))
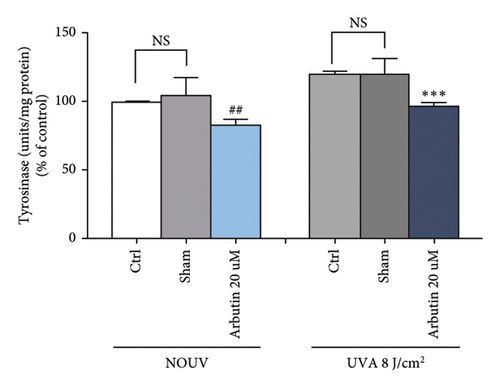
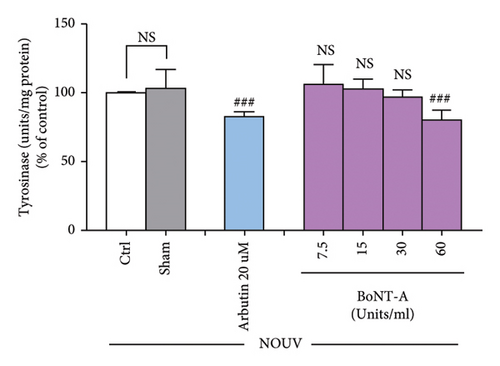
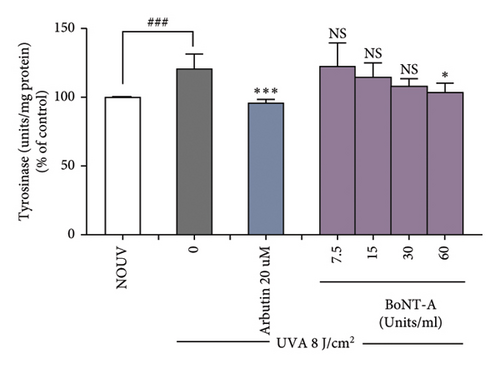
3.2.3. BoNT-A Inhibited UVA-Induced Tyrosinase Activity in B16F10 Cells
The tyrosinase activity assay showed that BoNT-A at the concentration of 60 unit/mL significantly inhibited tyrosinase enzyme in unirradiated B16F10 cells. Untreated B16F10 cells irradiated by UVA (8 J/cm2) significantly induced tyrosinase enzyme when compared with unirradiated control cells. BoNT-A at the concentration of 60 unit/mL significantly inhibited tyrosinase enzyme in UV-irradiated cells. The inhibitory effect was 16.9% ( ∗p < 0.05), without significant difference from the positive control (Figure 4(e), 4(f), and 4(g))
3.2.4. Summary of the In Vitro Study
The melanin content in UVA-irradiated B16F10 cells was significantly reduced by BoNT-A in a dose-dependent manner (15–60 U/mL) without causing cytotoxicity. Also, tyrosinase activity in UVA-irradiated B16F10 cells was significantly reduced by BoNT-A at 60 U/mL without causing cytotoxicity.
4. Discussion
Melasma is a hyperpigmentation disorder that detrimentally affects individuals as an ongoing cosmetic concern [2, 6]. Treatment of melasma is challenging, with high rates of relapse or worsening of the disease still persisting [10]. The complex and not fully understood pathogenesis of melasma requires continued exploration to develop new treatment strategies. Novel therapeutic strategies focus on several targets such as the melanogenesis pathway, tyrosinase enzymes and tyrosinase-related proteins, hyperactive melanocytes, reactive oxygen species, neovascularization, and increased mast cells [11].
Our study showed that intradermal BoNT-A injection significantly reduced the MASI score at 2 and 3 months compared with the baseline and significantly decreased the MASI score and the PGA score at 3 months compared with the control. The in vitro study revealed that BoNT-A significantly inhibited the melanin content and the tyrosinase activity in both UVA-irradiated and unirradiated cells in a dose-dependent manner without causing cytotoxicity. To the best of our knowledge, this is the first study to show that intradermal BoNT-A injection significantly improved melasma lesions. Also, it is the first study to demonstrate that BoNT-A inhibited cellular melanogenesis and UVA-induced melanogenesis in a dose-dependent manner. A very recent study using onabotulinumtoxinA (BOTOX) injection for the treatment of wrinkles on the upper face in healthy skin also revealed the lightening effect of BoNT-A as early as 15 days after injection in all regions with significant differences on the forehead and upper face [9].
Some anecdotal reports found white discoloration of the skin after using BoNT-A (BOTOX) injection for medical treatment [12, 13]. A report revealed permanent white discoloration of bilateral periocular skin after repeated high-dose BoNT-A injections for medical purposes until 18 months of follow-up. The authors hypothesized a connection among BoNT-A, skin melanocytes, and tyrosinase activity [12]. More recent publications also reported the lightening effect of BoNT-A on periorbital skin pigmentation. Even lentigines were reported to improve with BoNT-A injection compared with the control until 26 weeks follow-up [13].
Our in vitro study found that BoNT-A has a dose-dependent effect on the inhibition of melanin contents and tyrosinase activity. This implied that the higher concentration of BoNT-A injection may cause a greater improvement in skin hyperpigmentation. This finding is supported by two clinical studies of incobotulinumtoxinA injection 7 days after UVB irradiation. The highest concentration of BoNT-A at 1:2.5 dilution caused the best improvement in hyperpigmentation compared with lower concentrations [7, 14]. Among BoNT-A, we used abobotulinumtoxin in melasma due to its greater area of diffusion compared with onabotulinumtoxin and incobotulinumtoxin [15]. Furthermore, because pathological changes in melasma are found in epidermis and dermis [16], intradermal injection was used.
In terms of duration of efficacy, our study showed that the effects last at least 3 months. This is concurrent with the duration of effect of BoNT-A [8, 17]. Interestingly, the peak effects of BoNT-A were not at the usual 2 weeks [8], but the MASI score on the BoNT-A side gradually decreased to reach the peak effect at 3 months. This may be due to the different mechanisms of action of BoNT-A in improving melasma versus the antiwrinkle effect. Since the exact mechanism is still unknown and the trend of MASI score continued to decline at 3 months, the effects of BoNT-A on melasma may be longer than expected.
Although the exact mechanism of the effects of BoNT-A on skin pigmentation is still unknown, our study found that BoNT-A has an inhibitory effect on tyrosinase activity, the key enzyme in cellular melanogenesis, from UVA-induced melanogenesis. According to Jung et al., the melanin content in human epidermal melanocytes was also significantly reduced by BoNT-A under UVB irradiation. In addition, the melanin content and tyrosinase activity in UVB-irradiated mouse skin were significantly decreased by BoNT-A [6]. As the tyrosinase enzyme plays an important role in melanogenesis, it is proposed that BoNT-A to directly affects melanin production. Moreover, a recent human study showed that intradermal injection of BoNT-A has a protective effect on UVB-induced skin hyperpigmentation [7]. Together with our results with UVA, these strongly indicate the beneficial effects of BoNT-A against both UVA- and UVB-induced hyperpigmentation and thus their potential applications in melasma treatment.
Other proposed mechanisms are as follows. Melanocytes have an ectodermal origin from neural crest cells similar to neurons. As BoNT-A inhibits the release of acetylcholine at the neuromuscular junction, BoNT-A may have some effects on the melanin pathway [6]. Furthermore, the ability of BoNT-A was shown to reduce UV-induced hyperpigmentation through its anti-inflammatory effects by inhibiting three inflammatory cytokines for UV-mediated pigmentation related to melasma pathogenesis, including decreasing the level of prostaglandin E2, interleukin-1, and basic fibroblast growth factor [7, 18, 19]. In addition, BoNT-A was shown to reduce angiogenesis, which is one of the dermal changes in melasma, by inhibiting the expression of vascular endothelial growth factor (VEGF) [20, 21]. Our additional proposed mechanism of BoNT-A in melasma is related to mast cells. Mast cells and their chemical mediators have been proposed to be part of the pathogenesis of melasma [16]. Studies demonstrated that BoNT-A inhibited mast cell counts and degranulation in human and murine skin in a dose-dependent manner [22, 23]. Moreover, since recent evidence showed that senescent fibroblasts are involved in the pathophysiology of melasma, studies also demonstrated the beneficial effects of BoNT-A on dermal fibroblasts [16, 24, 25]. An in vitro study demonstrated the antiphotoaging potential of BoNT-A by anti-UVB-induced premature senescence of human dermal fibroblasts by decreasing senescent-related proteins [24]. Another in vitro study also showed that BoNT-A promotes fibroblast activity without causing fibroblast toxicity. It increased the level of type I carboxy-terminal peptide of procollagen, upregulated the expression of type I collagen, and decreased the production of some form of matrix metalloproteinases (MMPs) [25].
Our study has some limitations. First, the lack of objective measurement methods to quantitatively assess the degree of melasma improvement while we have complemented our clinical findings with an in vitro study to confirm the antimelanogenic effects of botulinum toxin in melanoma skin cells. Second, a longer follow-up is required to see the maintained effects of BoNT-A in melasma. Third, higher concentrations of BoNT-A should be evaluated in vitro. However, the dose-dependent relationship observed in our in vitro findings implied that higher concentrations of BoNT-A might be more effective for melasma.
5. Conclusions
Melasma is a recalcitrant pigmentary disorder with no definitive treatment solution. Our study showed favorable results in the treatment of melasma with BoNT-A, particularly in its ability to suppress cellular melanogenesis. However, several important areas require further investigation. Future research should focus on elucidating the molecular mechanisms by which botulinum toxin exerts its effects on melasma, particularly its influence on key signaling pathways involved in melanogenesis. In addition, studies are needed to determine the optimal dose, treatment frequency, and the long-term efficacy of BoNT-A for sustained improvement in melasma. Furthermore, investigating combination therapies, such as BoNT-A with other depigmenting agents, may also enhance treatment outcomes.
Nomenclature
-
- BoNT-A
-
- Botulinum toxin A
-
- NOUV
-
- No ultraviolet radiation
-
- Ctrl
-
- Control
Ethics Statement
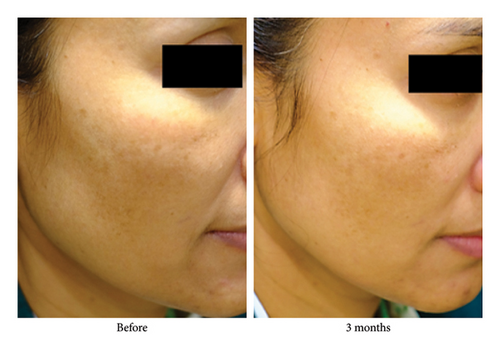
Written informed consent was obtained from the patient for use of her anonymized photographs in this manuscript. The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
Consent
The authors have already obtained such permission for the patient’s photographs.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Study concept and performance were developed and organized by W.T. and N.S.. Dermatologists who performed botulinum toxin injection and provided the data were W.T. and N.S.. The in vitro study was done by U.P. and S.T.. Essential data monitoring and statistical analysis were performed by T.S. and N.S.. Paper original draft writing was done by N.S.. Writing review and editing was done by W.T. and U.P.. Visualization was provided by T.S. and N.S.. All authors had access to the data and reviewed and approved the final manuscript.
Funding
No funds, grants, or other support were received.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




