Nonablative Er:YAG Laser Treatment of Androgenetic Alopecia: A Clinical Observation
Abstract
Background and Aim: Androgenetic alopecia (AGA) is a common hair disorder with a low cure rate. Nonablative Er:YAG laser therapy is a novel treatment modality for AGA, representing an alternative to pharmacological and surgical treatment. To date, several small scale studies employing this laser treatment have demonstrated good results but the evidence is still limited. The aim of this study was to evaluate effectiveness and safety of a nonablative 2940 nm Er:YAG laser for treating AGA in female and male patients with both early and advanced stages of AGA.
Methods: Patients (22 male and 10 female) with active AGA were treated with 2940 nm nonablative Er:YAG laser (SMOOTH™ mode, 7 mm spot size, 7.5–10 J/cm2, and frequency 2.5 Hz). Efficacy of treatment was evaluated clinically on a scale of 0–10 and with blind evaluation of hair appearance as seen in global photographs. Global photographs were taken before treatment and at 1-month follow-up and evaluated on a 5-point scale. Patient satisfaction was evaluated with a questionnaire on a scale from 0 to 3 and pain during treatment was evaluated with a pain scale from 0 to 10.
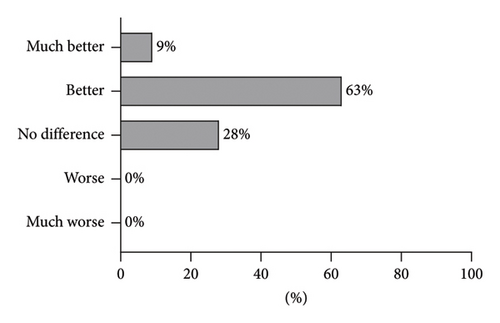
Results: Median clinical improvement was 6 (1–9), median satisfaction score was 3 (0–3), and median pain degree was 3 (0–10). According to blind evaluation, hair appearance at the 1-month FU was better in 63% and much better in 9% of the patients compared with the baseline. No adverse reactions were reported.
Conclusions: The results of this study corroborate the effectiveness of treatment with nonablative Er:YAG laser therapy for AGA. Six biweekly sessions resulted in general patient satisfaction and visible improvement in hair appearance.
1. Background
Androgenetic alopecia (AGA) is a very common hair disorder, characterized by a progressive decrease in hair density. Both men and women are affected, but with different hair loss patterns and prevalence. In China, the male and female prevalence rates are about 21% and 6%, respectively [1]. Typically, hair loss is greatest on the top of patient’s head and less on the sides while hair density in the occipital area remains normal. The cure rate of this disease is low, which seriously affects the quality of daily life of patients. Current clinical treatments of AGA include various oral and topical pharmaceuticals and surgical hair transplantation. These treatment methods can achieve good results but are limited by possible adverse drug reactions, poor patient compliance, and recurrence after drug withdrawal and in case of surgery a poor postoperative hair density and high costs. Stimulation of hair growth by laser therapy represents an alternative treatment that has already demonstrated efficacy in hair restoration [2, 3], including the use of fractional ablative and nonablative Er:YAG lasers in AGA treatment [4, 5]. Despite of a steady increase in research interest on the laser treatment for hair loss [6–8], its mode of action is not precisely known. The presumed method of action involves microcoagulative wounds in the dermis [7], which evoke an immune response and wound repair [6]. Studies on murine models suggest that laser irradiation affects the hair cycle by promoting telogen to anagen transitions via cell signaling [9–11]. The laser may also affect the hair cycle through increased blood flow [12] to the dermal papilla [13], a major site of hair cycle regulation [4]. Effective hair growth stimulation in AGA patients with nonablative fractional laser treatments has already been reported [5, 14, 15], although the published studies are frequently limited by a small number of patients. Therefore, more studies are needed to contribute to the cumulative number of treated patients.
The aim of this study was to evaluate efficacy and safety of a nonablative Er:YAG laser for treating AGA. We employed an Er:YAG laser treatment in SMOOTH™ mode, which consists of trains of subablative laser pulses that result in a larger thermal effect compared with conventional Er:YAG laser settings [16].
2. Patients and Methods
2.1. Patients
Thirty-two patients were recruited from the outpatient department of dermatology of our hospital from May 2020 to January 2021. All patients were between the ages of 20–55, have met the AGA diagnostic criteria, had no history of systemic diseases or special medication history, and had not received any systemic or localized treatment for hair loss. All patients signed the informed consent form after understanding the nature of the trial.
2.2. Procedure
The 2940 nm Er:YAG laser treatment (Fotona, XS Dynamis, PS03X handpiece, SMOOTH mode) was performed every 2 weeks (0 W, 2 W, 4 W, 6 W, 8 W, and 10 W). Each patient received 6 laser treatments, with the following laser parameters: spot size 7 mm, energy 7.5–10 J/cm2, and frequency 2.5 Hz. In each treatment, 3–5 passes of laser beam were applied across the treated areas. Total treatment time was about 15 min. The end point was a slightly red scalp, with a small amount of white scales on the scalp. Efficacy was evaluated after 1 month from the last treatment (1 month follow-up). Patients were monitored for any adverse events.
Global photographs were taken before the first treatment and after 8 sessions at the 1-month FU. Photography was consistent (standardized) in terms of patient positioning, lighting, camera settings, and background, thus representing a standardized photographic patient assessment [17].
2.3. Outcomes
2.3.1. Clinical Improvement
The severity of hair loss was assessed with the basic and specific (BASP) classification [18] at the baseline and at the 1-month follow-up (1-month FU) by 2 doctors who were not involved in the treatment. Clinical improvement at the 1-month FU was evaluated on a scale from 0–10.
2.3.2. Blind Evaluation
Global photographs were evaluated by 5 blinded evaluators. The evaluators received a picture composed of 2 plates (left and right), representing global photographs before treatment and at 1-month FU in a random order, so the evaluators were unaware of which of the pair of photographs was taken before and after treatment. They were asked to choose a score from the following options: (−2) hair appearance on the left plate is much better compared with the right plate, (−1) hair appearance on the left is better compared with the right plate, (0) no difference in hair appearance between the left and right plates, (+1) hair appearance on the right is better compared with the left plate, and (+2) hair appearance on the right is much better compared with the left plate. After the blind evaluation was completed, the collected scores were assigned to a 5-point evaluation scale as follows; (−2) much worse, (−1) worse, (0) no difference, (+1) better, and (+2) much better. Median score of the five evaluators was taken as a final blind evaluation score.
2.3.3. Pain of Procedure
At the end of each treatment, patients were asked to assess pain degree of laser therapy with a pain scale (0–10).
2.3.4. Patient Satisfaction
At the end of each treatment, patients were asked to assess their satisfaction with the treatment on a scale from 0–3 (0 = dissatisfied; 1 = somewhat satisfied; 2 = satisfied; and 3 = very satisfied).
2.4. Statistical Methods
Statistical analysis was performed in Microsoft Excel and GraphPad Prism. Data were checked for normality with the Kolmogorov–Smirnov test. As normality was not met, nonparametric tests were used. Correlation between independent variables was calculated with the Spearman correlation coefficient (rho) with no correction for multiple testing. The chosen level of statistical significance (alpha) was 0.05. Outcome measures were presented with values of median and interquartile ranges in the form of median (Q1–Q3).
3. Results
All of the recruited patients completed the study. Demographic data of patients and outcome measures are presented in Table 1. The average patient age was 32.6 ± 8.3 years, and the average duration of AGA was 2.2 ± 1.0 years. The only adverse reactions detected were mild scaling of the scalp 1 week after treatment.
| Patient no. | Age (year) | Sex | Duration of AGA (year) |
|---|---|---|---|
| 1 | 37 | F | 2 |
| 2 | 26 | M | 1 |
| 3 | 46 | M | 3 |
| 4 | 33 | F | 2 |
| 5 | 30 | M | 2 |
| 6 | 28 | M | 1 |
| 7 | 28 | M | 3 |
| 8 | 27 | M | 4 |
| 9 | 43 | F | 2 |
| 10 | 29 | M | 1 |
| 11 | 23 | F | 3 |
| 12 | 27 | M | 4 |
| 13 | 31 | M | 2 |
| 14 | 27 | M | 3 |
| 15 | 26 | M | 1 |
| 16 | 25 | M | 4 |
| 17 | 28 | M | 3 |
| 18 | 27 | F | 1 |
| 19 | 40 | F | 1 |
| 20 | 34 | M | 2 |
| 21 | 32 | M | 3 |
| 22 | 39 | F | 2 |
| 23 | 40 | F | 1 |
| 24 | 33 | M | 1 |
| 25 | 25 | M | 3 |
| 26 | 45 | M | 4 |
| 27 | 32 | M | 2 |
| 28 | 41 | F | 1 |
| 29 | 60 | M | 2 |
| 30 | 26 | M | 2 |
| 31 | 34 | F | 1 |
| 32 | 20 | M | 2 |
The median clinical improvement was 6 (range 1–9), the median patient satisfaction (0–3) was 3 (range 2-3), and the median pain degree (0–10) was 3.0 (range 2.3–4.0).
Clinical improvement was not significantly correlated with patient age (rho = 0.06, p = 0.718), patient satisfaction (rho = 0.21, p = 0.237), or duration of AGA (rho = −0.27, p = 0.131). The difference in average clinical improvement between female and male patients (−0.83 ± 0.96) was not significant (t (30) = 0.87, p = 0.39).
4. Discussion
Laser treatment evaluated in this study was effective in treating AGA, demonstrated by a relatively high clinical improvement. The median observed clinical improvement after 6 biweekly sessions was 6 on a scale of 0–10 (Figure 1(a)). This is in agreement with other studies employing nonablative laser monotherapy for hair restoration. It was reported that 12 sessions of thulium laser therapy resulted in up to 50% increase in hair density [14], while 10 sessions with erbium glass laser resulted in hair density increase ranging from 12.3% [19] to 57% [20]. It is important to note that these studies reported a worsening of hair density at later follow-up visits, which nevertheless remained higher compared with baseline values. No comparison can be made with the Er:YAG laser used in this study, as no additional follow-up assessments were performed. The most recent systematic review of laser therapy for AGA [21] concluded that nonablative laser monotherapy significantly improved hair count and/or shaft diameter and that this effect was durable on subgroup analysis for both erbium glass 1550 nm and thulium 1927 nm laser. Both erbium glass and thulium lasers are nonablative lasers, which work by inducing a thermal injury to the dermis [22]. Given that the Er:YAG laser used in our study was also used in nonablative mode, it is reasonable to anticipate a similar tissue effect as in the case of erbium glass and thulium lasers and consequently a similar clinical improvement; however, further data are required to corroborate this claim. Although the mechanisms of hair regrowth are not fully understood, it has been shown that lasers of different types induce Wnt and β-catenin expression pathways which are implicated in hair growth [23]. Increased vascularity and the microenvironment of healing wounds likely play a role through stimulating hair follicle stem cells, causing an increase of anagen induction [23].
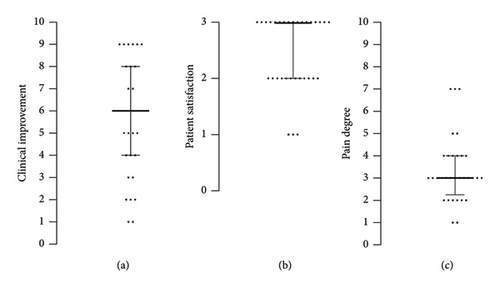
Notably, the present study included patients with relatively short duration of AGA (Table 1). The detected clinical improvement (Table 2) corroborates the general notion that the earlier the treatment of AGA is commenced, the better the outcome will be. Nevertheless, some studies [5, 18] report significant improvement in patients with advanced stages of AGA as well. Furthermore, one study evaluating the same laser modality as used in our study has demonstrated significant improvement of AGA in patient with long-term AGA condition as well but mostly in patients that received treatment in combination with topical minoxidil and oral pharmaceuticals [5]. The present study is important as it demonstrates the efficacy of the Er:YAG nonablative laser in monotherapy. The treatment is tolerated well, which is demonstrated by low pain scores (Figure 1(c)) reported by the patients. The only adverse reactions detected were mild scaling of the scalp 1 week after treatment. Clinical improvement after treatment is in line with the high patient satisfaction (Figure 1(b)) and the blind evaluation of global photographs (Figures 2 and 3). No difference was observed in average clinical improvement between female and male patients. According to blind evaluation, hair appearance at the 1-month FU compared with the baseline was better in 63% and much better in 9% of all patients (Figure 4).
| Patient no. | BASP classification at Baseline | BASP classification at 1-month FU | Clinical improvement at 1-month FU (0–10) | Pain during treatment (0–10) | Patient satisfaction at 1-month FU (0–3) |
|---|---|---|---|---|---|
| 1 | M0F2 | M0F1 | 8 | 7 | 2 |
| 2 | M2F3 | M0F1 | 8 | 4 | 3 |
| 3 | U1F3 | U1F2 | 6 | 2 | 3 |
| 4 | M0F2 | M0F1 | 6 | 3 | 2 |
| 5 | M1V3 | M0V1 | 9 | 2 | 3 |
| 6 | M2F3 | M2F2 | 8 | 3 | 3 |
| 7 | M2V3 | M2V3 | 2 | 3 | 2 |
| 8 | M2F3 | M2F3 | 3 | 7 | 1 |
| 9 | M0F1 | M0F1 | 6 | 3 | 3 |
| 10 | M0F2 | M0F2 | 6 | 4 | 3 |
| 11 | M0F1 | M0F1 | 9 | 3 | 2 |
| 12 | M0F2 | M3F3 | 5 | 3 | 1 |
| 13 | M0F1 | M0F1 | 9 | 2 | 3 |
| 14 | M2F3 | M2F2 | 7 | 3 | 2 |
| 15 | M1V1 | M1V1 | 4 | 2 | 3 |
| 16 | M3F2 | M3F2 | 4 | 4 | 1 |
| 17 | M1F1 | M1F1 | 5 | 5 | 3 |
| 18 | M0F2 | M0F1 | 4 | 7 | 3 |
| 19 | M0F1 | M0F1 | 5 | 3 | 2 |
| 20 | M1V2 | M1V1 | 9 | 4 | 3 |
| 21 | M2F3 | M2F3 | 6 | 2 | 2 |
| 22 | M0V1 | M0V1 | 6 | 3 | 3 |
| 23 | M0F1 | M0F1 | 5 | 4 | 2 |
| 24 | M2F3 | M2F3 | 9 | 3 | 3 |
| 25 | M1V2 | M1V1 | 2 | 2 | 2 |
| 26 | M2F3 | M2F3 | 3 | 1 | 3 |
| 27 | M2F3 | M2F3 | 1 | 1 | 2 |
| 28 | M0F1 | M0F1 | 6 | 3 | 2 |
| 29 | M0F2 | M0F1 | 2 | 3 | 3 |
| 30 | M1F1 | M1F1 | 9 | 4 | 3 |
| 31 | M0F2 | M0F1 | 1 | 5 | 2 |
| 32 | M2F3 | M2F3 | 7 | 3 | 3 |
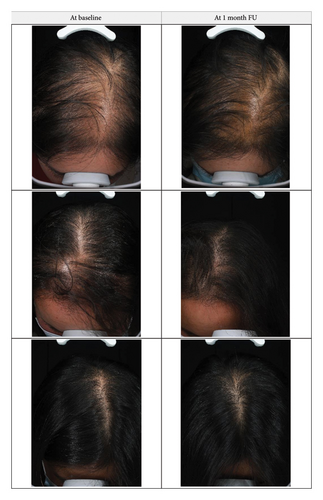
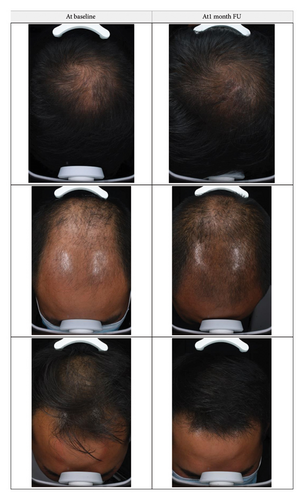
Although AGA does not affect physical health, it may significantly affect patients’ mental health and quality of life because the progressive worsening of symptoms culminates in baldness. Early and long-term treatment is very important, as it can significantly delay the progression of hair loss and thereby improve the quality of life of patients. The present study revealed high satisfaction scores, which indicates a good balance of efficacy and tolerability of this particular treatment protocol. Understanding patient satisfaction can help healthcare providers tailor treatments to individual needs and provide insights into how the treatment affects their daily lives beyond clinical measures. The efficacy of new hair growth with current US FDA-approved therapies can be unsatisfactory, and significant improvement is not always observed [7]. Given that AGA is a common age-dependent trait in men and women, with its frequency and severity increasing with age [24], the interest for hair restoration treatments, including laser therapy, is expected to increase and further research into this topic is warranted.
5. Conclusion
Based on the results of this study, we can conclude that nonablative Er:YAG laser therapy is an efficient treatment modality for AGA, resulting in general patient satisfaction and visible improvement in hair appearance. It may be used as an adjuvant treatment or as monotherapy in AGA patients that seek minimally invasive and nonpharmaceutical treatment options. The novelty of this research predominantly lies in the unique laser modality and a focus on monotherapy. Overall, it contributes valuable new insights into the field of laser therapy for hair restoration, specifically through its focus on the Er:YAG laser in nonablative mode as a stand-alone treatment and its implications for early intervention in AGA management. Furthermore, this study demonstrates patient comfort and adherence to treatment which are critical for long-term success. An important limitation of the present study is a lack of an objective outcome and a lack of longer follow-up that would enable an assessment of longevity of treatment results. Due to a relatively small sample size, the statistical power of this study is low. Another major limitation of this study is the absence of a placebo or sham control, which limits the strength of our conclusion regarding the effectiveness of the intervention. Randomized controlled trials with objective outcome measures such as trichoscopy are needed to corroborate the results of the present study.
Consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
Iva Talaber is an employee of Fotona d.o.o., the manufacturer of a medical device used in the study. The authors declare no conflicts of interest.
Author Contributions
All authors contributed equally to this work.
Funding
This study did not receive any funding in any form.
Open Research
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding author.




