Cunning Pathogen Tactics: Neutrophil Movement Influenced by Bacterial Attractants in the Experimental Model
Abstract
To explore the peculiarities of neutrophil motility, two models of chemoattraction were created: a horizontal model, where a container with bacterial chemoattractant was attached laterally to the endotheliocyte monolayer, and a vertical model, simulating a pyemic focus in the lower part of the modified Boyden chamber. Low-molecular weight product secretion and/or degradation of Enterococcus faecalis caused “disorientations” of neutrophil migration with hyperproduction reactive oxygen species (ROS) by immune cells, while Proteus mirabilis inhibited both migration of most neutrophils and the production of ROS by them, while the activity of the remaining uninhibited neutrophils increased. Neutrophils generated ROS during migration, especially actively in the case of a large number of mobile cells (under stimulation with low-molecular weight product secretion and/or degradation of Enterococcus faecalis and Escherichia coli). Using high-resolution microscopy, it was shown that low-activity neutrophils cause changes in the morphology of endothelial cells during migration more than high-activity neutrophils. In the vertical migration model, the morphology of endothelial cells significantly changed during neutrophils diapedesis. It was observed that space between endothelial cells was increased (especially in the case of neutrophil swarming).
1. Introduction
The study of neutrophil migration from the vascular channel to the inflammation zone has recently attracted the attention of a large number of researchers. This is primarily due to the importance of vascular–mesenchymal reactions in the whole genesis of the inflammation development in tissues. All stages of the transendothelial migration process have been studied and described in detail, both in original and review articles [1–4]. The study of neutrophil migration from the vascular channel recently made significant progress. Along with the described processes of paracellular and transcellular migration, a number of interesting phenomena have now been discovered. One of them is cell crawling along the endothelium against blood flow–upstream migration [5], when cells come to the arrest stage on the apical surface of endothelial cells. Such crawling is used both to facilitate transendothelial migration and patrolling of blood vessels. This phenomenon clearly indicates the importance of investigating not only signaling-biochemical and genetically determined stimuli but also biophysical phenomena like the effect of hemodynamic shear stress in the migration process [6]. Another interesting feature associated with neutrophils is the detection of the phenomena of “microleakage” and “macroleakage” of nanoparticles from the bloodstream due to the preinfiltration of the vascular barrier by neutrophils. This property can be actively used in systems of facilitated delivery of antitumor drugs, such as liposomes [7, 8]. These discoveries became possible, first of all, due to the development of new biophysical methods of visualization, in particular, methods of high-resolution microscopy. Since the sequence of migration stages, as well as the speed of neutrophils overcoming the vascular barrier, is crucial for the development of a full-fledged inflammatory response and subsequent tissue repair [9], we developed two models in vitro for studying neutrophil migration along the chemoattractant gradient. The first (horizontal) made it possible to study the dynamics of the migration process. The second (vertical) was based on the transwell principle and allowed us to study neutrophil transendothelial migration activity and their transformation during migration with high resolution [10, 11]. However, standard transwells show the final result of neutrophil migration only. To visualize the dynamics of the neutrophil transendothelial migration process by high-resolution scanning ion-conductance microscopy (SICM), a two-chamber system was developed and a buffer system was selected to provide long-term observations while preserving the viability of all responsible for migration cells (neutrophils, endothelial cells, and bacteria, which were used as a chemoattractant) [12]. This approach provided a reliable gradient of chemoattraction and allowed, for the first time, high-resolution observations of single cell migration and assessment of interactions between neutrophils and endothelial cells during diapedesis. In this study, both horizontal and vertical systems were used to investigate the features of neutrophil migration activity when Gram-positive (Staphylococcus aureus 2879M and Enterococcus faecalis 645-p2) and Gram-negative (Escherichia coli 321 and Proteus mirabilis 649-2) bacteria were used as chemoattractants.
2. Materials and Methods
The study was done with the permission of the Lobachevsky University Bioethics Committee founded on November 11, 2016 (permission Protocol No. 9 was issued on July 17, 2017). The study was in accordance with the standards of the Helsinki Declaration of June 1964. All participants signed informed consent for inclusion in the study before the beginning.
2.1. Bacterial Cells
Two species of Gram-positive bacteria (Staphylococcus aureus 2879M and Enterococcus faecalis 645-p2) and Gram-negative bacteria (Escherichia coli 321 and Proteus mirabilis 649-2) were used as chemoattractants to neutrophil migration in the horizontal and vertical models. The strains were provided by Nizhny Novgorod State Technical University n.a. R.E. Alekseev bacterial strain collection. Strains were grown on dense GRM agar (FBUN State Scientific Center PNB, Russia), resuspended in PBS, and standardized to 1 × 109 cells/mL using spectrophotometer Specs SSP 705 (Spectroscopic systems, Russia). The optical density for E. coli 321 was 0.85; for S. aureus 2879M, it was 0.74; for P. mirabilis 649-2; it was 0.69, and for E. faecalis 645-p2, it was 0.67. Before use, bacterial cells were collected by centrifugation and resuspended in HBSS medium with 2 mM L-glutamin and 10 mM HEPES (PanEco, Russia) with pH 7.4 (further experimental medium). In vertical migration model, bacterial cells were added at MOI 10 concentration to lower compartment of analytical chamber. In the horizontal migration model and in measuring ROS production in bacterial cells, MOI 20 concentration was added in the container containing a dialysis membrane with a pore size 14 kDa.
2.2. Eukaryotic Cells
Peripheral blood of healthy donors from Nizhny Novgorod Regional Blood Center N.Ya. Klimova was used for neutrophil isolation. Blood was stabilized with heparin (50 IU/mL) and diluted 1:1 by PBS. Neutrophils were isolated on a Ficoll-Trazograph double gradient (ρ = 1.077 g/mL, ρ = 1.110 g/mL, 400 g, 40 min). After separation, neutrophils were washed twice with sterile PBS (400 g, 3 min) as described in [13], and red blood cells were lysed with 0.9% NH4Cl (12 min, 0°C). Prepared neutrophils were resuspended in experimental medium and used at a final concentration of 1 × 106 cells/mL. Cell viability was determined by flow cytometry using Cytoflex S (Beckman Coulter, United States) by propidium iodide staining. Neutrophils with at least a 98%–99% viability rate were used.
A monolayer of transformed human umbilical vein endothelial cells EA.hy926 (ATCC) was used to mimic vascular endothelium in models of horizontal and vertical neutrophil migration. The cells were cultured in a Portable Mini NB 203 M CO2 incubator (N-Biotek, Bucheon, South Korea) at 37°C and 5% CO2 in DMEM-F12 medium with the addition of inactivated fetal calf serum (10%), streptomycin (100 μg/mL), penicillin (100 units/mL), L-glutamine (8 mM), HAT (50 μM hypoxanthine, 0.2 μM aminopterin, 8 μM thymidine) (all—PanEco, Russia). Passages according to the conventional technique [14] were carried out every 3–4 days when the monolayer reached 90%–100% confluency. The monolayer was disaggregated by exposure to trypsin-EDTA solution for 3 min (PanEco, Russia). For the horizontal migration model, EA.hy926 cells at 3–25 passages were used at a concentration 2.5 × 105 cells/mL to form a monolayer in a Petri dish (37°C, 5% CO2, 72 h). For the vertical migration model, the EA.hy926 cell monolayer was formed inside 6.5 mm membrane inserts with a 3-μm pore diameter (SPL Lifesciences, South Korea) in the same conditions. Growth control of EA.hy926 cells was performed optically using an AxioVert A1 microscope (Carl Zeiss, Germany).
2.3. Cell Staining and ROS Production Measurement
To visualize the production of reactive oxygen species (ROS) by neutrophils the dye 6-carboxy-H2DCFDA (OOO Lumiprob RUS, Russia) was used. It is a reduced acetylated form of fluorescein that did not affect cell viability after staining (assessed via flow cytometer prior main experiments, results not shown). To stain the neutrophils, 1 μL of the dye solution was added to 1 mL of neutrophil suspension (2 × 106 cells/mL) and incubated for 15 min. For comparative ROS production, EA.hy926 endothelial cells were grown on a 96-well plate, and then, 2 × 105 cells/mL of neutrophil suspension was added to each well (the number of cells was recalculated according to well square), where container with chemoattractant was placed. The release of ROS was analyzed in a BioTek PowerWave HT microplate spectrophotometer (BioTek, United States) discretely each 10 min during 1 h. Endothelial cells were not specifically stained, but if they produced ROS during the experiment, the cells of the line EA.hy926 also showed bright green fluorescence (AxioVert.A1, Carl Zeiss, Germany).
2.4. A System for Horizontal Neutrophil Migration Along a Chemoattraction Gradient
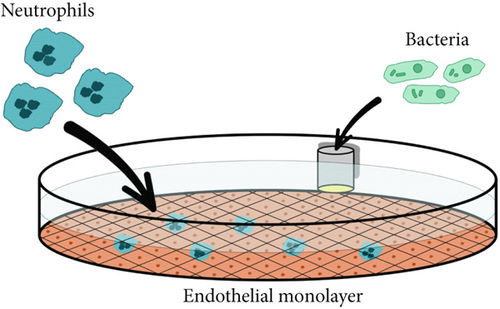
To study the process of neutrophil migration on the surface of endothelial cell monolayer in the horizontal plane in the presence of chemoattraction gradient, an in vitro model was created. A monolayer of EA.hy926 cells was grown inside Petri dish surface (35 mm, Corning, United States) as described above. Before the study, cells were washed by experimental medium. This preserved maximum viability of all three cell types (neutrophils, endothelial cells, and bacteria). To modeling a chemoattraction gradient, we used a polyvinyl chloride container which lower part was a hermetically integrated semipermeable dialysis membrane with a pore diameter of 14 kDa. A container with blank experimental medium was used as a control. Containers with bacterial cell suspensions (in concentrations described above) were used as a source of chemoattractant for experiment. The container was placed in a Petri dish with EA.hy926 cell monolayer, and then, 106 neutrophils were added into the system. Neutrophil migration was monitored by light and fluorescence microscopy (using an Fs 46 filter (excitation BP 500/20, emission BP 535/30) at a magnification of 10× (AxioVert.A1, Carl Zeiss, Germany)) or SICM microscopy (ICAPPIC Ltd., United Kingdom).
2.5. A System for Vertical Neutrophil Migration Along a Chemoattraction Gradient
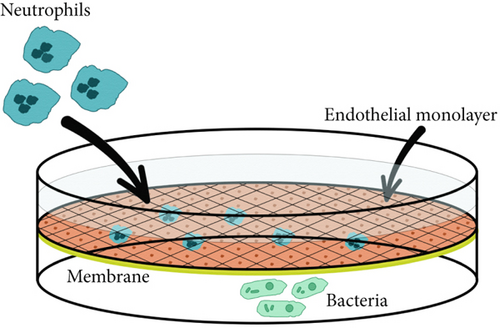
The vertical neutrophil migration model was designed for long-term real time observations using the high-resolution SICM method. The development of a two-section chamber for analysis and the selection of optimal observation parameters for scanning are described in detail previously [12]. The main requirement for selecting conditions was maximum viability of all three cell types (endothelial cells, neutrophils, and bacterial cells) for long-term real-time observation. Briefly, the analytical chamber was fabricated using a QQ-S PRO 3D printer with a direct extruder (FLsun, China) from polyethylene terephthalate glycol plastic and filled with 2.5 mL of experimental medium. A 6.5-mm membrane insert with 3-μm pore diameter (SPL Lifesciences, South Korea), coated with EA.hy926 cell monolayer, was placed in the analytical chamber. A control scan of the monolayer was performed using the SICM method. Then, suspension of bacterial cells was added to the lower compartment of a chamber at a concentration of MOI 10 as a chemoattractant source. Neutrophils were added inside the membrane insert at a number of 105, and SICM microscopy was performed.
2.6. Scanning by the SICM Method
A SICM (ICAPPIC Ltd., United Kingdom) was used for high-resolution cell observation. Scanning was performed in the “hopping mode” with a fall rate of 50–100 nm/ms at a bias potential of 200 mV using nanocapillaries as probes. Nanocapillaries with a characteristic inner tip radius up to 50 nm were obtained on a P-2000 laser puller (Sutter Instruments, United States). The ion current was measured using a MultiClamp 700 B amplifier (Molecular Devices, United Kingdom). Three key factors were taken into account for choosing scanning modes: output image resolution, the ability to follow the migrating neutrophils in the system and the absence of a significant influence of the probe on the system. The pixel resolution for most dynamic images varied from 192 × 192 to 256 × 256 with subsequent upscaling to 512 × 512. The scanning speed was increased by decreasing the delay between probe approaches (up to 3000 ns) and increasing the probe fall speed to 120 nm/ms.
2.7. Statistical and Analytical Systems
Free and open-source Icy Software (Institut Pasteur and France-BioImaging, France) was used to analyze the migration of neutrophil activity in a horizontal model.
Origin 2021b (OriginLab Corparation, United States) was used to construct vector graphics of cell migration activity and for statistical analysis. Boundaries of normal distribution were determined using the Shapiro–Wilk test. The t-test was used since the distributions were normal.
SICMImageViewer software (ICAPPIC Ltd., United Kingdom) was used to scan and analyze SICM images.
3. Results
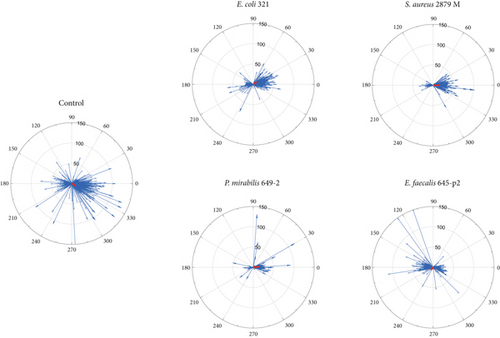
In a horizontal model (Figure 1), we investigated neutrophil migration over the surface of EA.hy926 cells lying on a dense substrate. In this case, a container with bacteria was placed on the side to create a chemoattraction gradient. This gave neutrophils the opportunity to migrate exclusively along the endothelial surface.
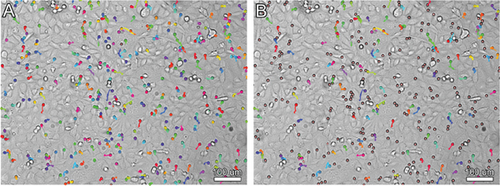
The neutrophil movement tracks were recorded and processed in Icy Software (Institut Pasteur and France-BioImaging, France). An example of migration activity data processing is shown in Figure 2.
Neutrophil migration in the presence of different bacterial chemoattractants was investigated. It was shown that the results of neutrophil migration activity differed significantly depending on the type of bacterial chemoattractant. The control (absence of chemoattractant) was characterized by stochastic migration of neutrophils. The same strategy of neutrophil migration was implemented in the presence of E. coli 321 (with predominant migration vector toward the chemoattractant container) and of E. faecalis 645-p2 (with predominance of the resulting migration vector from the chemoattractant container). A pronounced migration toward the chemoattractant container was observed only for S. aureus 2879M and P. mirabilis 649-2 (Figure 3). However, a comparison of numerical data on migration activity showed statistical significance only for P. mirabilis (Table 1). Interestingly, the greatest number of highly active cells was observed in the case of random stochastic migration in the control and in the presence of the chemoattractant E. faecalis 645-p2. In contrast, directed migration was characterized by either a paucity (P. mirabilis 649-2) or average (S. aureus 2879M) number of highly active cells (Table 1). Additionally, when P. mirabilis 649-2 was used as a chemoattractant, neutrophils migrated the shortest distance toward the chemoattractant container (Figure 3 and Table 1).
| Parameter | Cells migrated more than 10 um (%) | Mean distance for migrated cells (um) | Track of migrating cells (um) | Cells migrated toward the chemoattractant (%) |
|---|---|---|---|---|
| Control | 55.86 ± 17.57 | 50.77 ± 3.46 | 20.22 ± 2.13 | Not applicable |
| Staphylococcus aureus 2879 M | 47.68 ± 10.06 | 51.20 ± 2.30 | 20.51 ± 1.87 | 83.24 ± 12.63 |
| Escherichia coli 321 | 45.36 ± 15.67 | 52.62 ± 7.68 | 19.86 ± 3.79 | 72.67 ± 13.18 |
| Enterococcus faecalis 645-р2 | 62.43 ± 12.57 | 47.33 ± 2.47 | 19.09 ± 1.43 | 51.48 ± 30.72 |
| Proteus mirabilis 649-2 | 25.50 ± 8.83 | 39.25 ± 2.35∗# | 15.71 ± 0.91∗# | 87.24 ± 11.17 |
- Note: Neutrophils from at least three healthy volunteers were used for each experiment.
- ∗Differences are statistically significant compared to the control (p < 0.05), t-test.
- #Differences are statistically significant compared to S. aureus (p < 0.05).
Fluorescence microscopy with 6-carboxy-H2DCFDA (6-carboxy-2 ′,7 ′-dichlorodihydrofluorescein diacetate) dye staining was used to simultaneously monitor cell motility and the production of ROS during neutrophil migration along endothelial cells. Figure 4 shows the results of ROS production by migrating neutrophils in the presence of different chemoattractants.
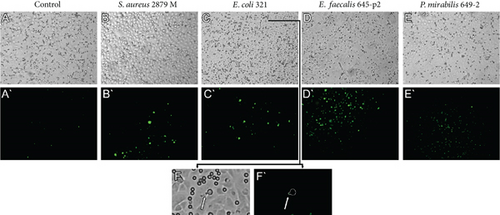
The spectrophotometric method was used for quantitative assessment of ROS production during horizontal migration of neutrophils along endothelial cells. The dynamics of the increase in ROS production in the presence of the chemoattractant are shown in Figure 5. Data shows that migrating neutrophils actively produced ROS (Figures 4 and 5). ROS production was also present in endothelial cells when both neutrophils and the chemoattractant were simultaneously in the system (Figures 4B, 4C, 4D).
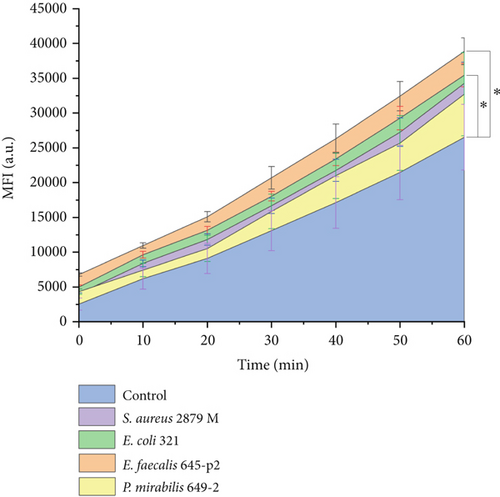
ROS production gradually increased during neutrophil migration. After 1 h of horizontal migration, the production of ROS by neutrophils under E. faecalis 645-p2 and E. coli 321 conditions increased statistically significantly compared to the control (Figure 5). ROS production by migrating neutrophils stimulated by P. mirabilis 649-2 and S. aureus 2879 M did not show statistically significant differences compared to the control (Figure 5). It should be noted that lowest level of ROS production was characteristic when P. mirabilis 649-2 was used as a chemoattractant; the highest was registered under E. faecalis 645-p2 condition. These results correspond to the data on the number of highly active cells (Table 1).
Since migration in the direction of the chemoattractant was observed for neutrophils when S. aureus 2879 M and P. mirabilis 649-2 were used as chemoattractants, we used them for studying the changes in cell morphology in the model of horizontal migration by high-resolution SICM. The results of neutrophil migration toward the container with S. aureus 2879 M or P. mirabilis 649-2 are shown in Figure 6. A vectored migration of neutrophil along endothelial cells with formation of lamellopodia and subsequent “pull-up” of the whole volume of cell were noted. The neutrophils migrated predominantly along the boundaries between two endothelial cells. A similar character of motility was also noted when migration was examined by light microscopy (Figure 4). The morphology of endothelial cells over which the neutrophil crawls also change.
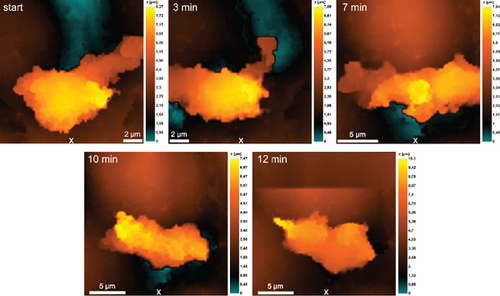
Highly active neutrophils altered the morphology of endothelial cells to much lesser extent (Figure 6b). However, motility of highly active neutrophils induced more significant alteration in the morphology of the neutrophil itself: The cell was no longer “pulled up” to the pre-extended lamellopodia, but “spread out,” as if pushing itself along the line of contact between the two endothelial cells (Figure 6b).
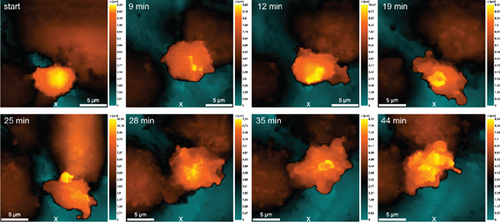
The vertical migration system based on the Boyden chamber design contained a membrane simulated the basal membrane of a vessel covered with a monolayer of endothelial cells. The source of chemoattractant in this case was the lower chamber which simulating a pyemic focus in tissues, where the same strains of S. aureus 2879M (Figure 7) and P. mirabilis 649-2 (Figure 8) were used as chemoattractants. Neutrophils were added to an upper chamber to analyze pattern of transendothelial migration which differs significantly from horizontal model migration. After adhesive contact was established (Figure 6a), the neutrophil extended (Figure 7B) and then moved purposefully into the space between the two endothelial cells (Figure 7C,D). The alterations on the endothelial cell membrane after neutrophil migration are shown in Figure 7E, E ′.
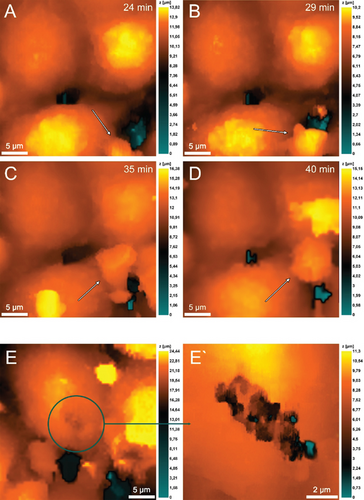
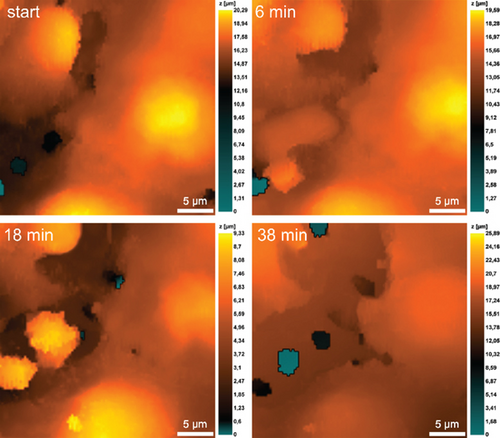
It is also evident that neutrophils significantly expand the space between endothelial cells when they migrate into the lower chamber along the chemoattraction gradient created by P. mirabilis 649-2 (Figure 8). This migration was especially well visualized in the case of neutrophils swarming. Figure 8 at 18 min shows that in the cytoplasmic region of the endothelial cells, increasing granularity can be seen under neutrophil influence. Neutrophils also alter morphology, either elongating or reconcentrating in a limited volume.
4. Discussion
Using a horizontal model of neutrophil migration, we showed that the migration activity of neutrophils significantly depends on the type of microorganism that was used as a chemoattractant. The total migration vector also depended on the bacterial species. Three of the tested bacteria (S. aureus 2879M, P. mirabilis 649-2, and E. coli 321) caused neutrophils to migrate preferably in the chemoattractant source direction. Whereas when E. faecalis 645-p2 was used, neutrophil migration vector was oriented to the opposite direction of the chemoattractant. The maximum migrating distance toward the chemoattractant for a single neutrophil was observed after using as chemoattractants both Gram-positive S. aureus 2879 M and Gram-negative P. mirabilis 649-2. For control (neutrophil migration without chemoattractants) and for experiments with E. faecalis 645-p2 as a chemoattractant, other results were observed: In both cases, the greatest motility and the greatest indeterminacy of neutrophil migration direction were observed, which was expressed by a small displacement of the total vector of movement from the center of coordinates (Figure 3). Previously, Carman et al. [15, 16] showed that before diapedesis, neutrophils form an invasive protrusion which plays the role of a kind of mechanosensor that scans the surface to find a site convenient for migration. Most likely, the mechanism of such “scanning” of space is realized by neutrophils in our horizontal migration model, when each cell was searching for a migration direction. In the case of control, there was no chemoattractant; cells migrated stochastically, trying to compensate for the absence of a pronounced migration vector by high activity. The same high activity of neutrophils with minimal productivity (migration in the opposite direction) was observed in experiments with E. faecalis 645-p2 as a chemoattractant. Previously, the ability of E. faecalis to prevent sensing and migration toward these bacteria was also shown for macrophages [17]. The mechanism by which E. faecalis uses to prevent the sensing and migration of macrophages is not known yet. However, we assume that it also works in the case of “disorientation” of neutrophils, even causing their migration in the opposite direction from the container with E. faecalis. In our study, bacterial chemoattractants were placed in containers with membranes that were permeable only to substances with molecular weights less than 14 kDa. This indicates that E. faecalis 645-p2 pathogenicity factors that prevent the process of normal migration have molecular weights less than these values. However, a larger number of highly active neutrophils were detected in all observations where the summed migration vector was unclear and did not point directly toward the container with the chemoattractant.
A completely different pathogenicity strategy was realized by P. mirabilis 649-2, which did not cause “disorientation” but inhibited neutrophil migration. This was characterized by a decrease of the active neutrophils number, shorter distance of cell migration, and background levels of ROS production by migrating neutrophils, which did not differ statistically significantly from the control. However, the total neutrophil migration vector was directed toward the container with bacteria. The same strategy (decrease in the number of active cells and background levels of ROS production) was observed for S. aureus 2879 M, but the effect was less pronounced compared to P. mirabilis 649-2. It should be noted that in response to the impact of pathogenicity factors of these bacterial strains, neutrophils tried to use a “compensatory strategy” in which cells that retain motility were able migrate to the maximum distance toward the chemoattractant source direction.
In our model, migrating neutrophils produced ROS in the gradually increased levels during an hour. ROS production has been described in many aspects of neutrophil activity. Usually, ROS production is associated with phagocytosis [18] or with some pathological manifestations, for example, death by NETosis mechanism [19]. There are studies suggesting that activation of NADPH-oxidase system and production of ROS negatively regulate actin polymerization and suppress neutrophil polarization and chemotaxis [20]. On the contrary, the production of ROS was observed during rolling, which, among other things, induced an increase of L-selectin expression [21]. The following facts indicate that in our model system, ROS production was associated with cell migration, but not with phagocytosis. Firstly, under sterile control conditions, stochastically migrating neutrophils also produced a high level of ROS. Secondly, according the experimental conditions, the source of chemoattraction (bacterial cells) was isolated in containers. The dialysis membranes of the containers ensured permeability only for bacterial cell subcomponents up to 14 kDa in size. In this case, ROS production cannot be explained by phagocytosis. However, the internalization of secreted subcomponents of gram-negative bacteria as lipid A (less than 14 kDa) by neutrophils cannot be ruled out. Thirdly, we have previously shown that in a single neutrophil and in a neutrophil’s population, the maximum level of ROS production is observed in 20–30 min after contact with stimulate phagocytosis bacteria; after that, it begins to gradually decrease [22]. In this study, a constant increase of ROS level production was observed throughout the observation period (60 min). Previously, using an electrochemical system for determining ROS production inside a single cell, we showed that the simultaneous presence of neutrophils and bacteria induces ROS/RNS production by endothelial cells [23]. A similar result of ROS/RNS production by endothelial cells was observed here in the presence of neutrophils and low-molecular products of bacterial chemoattraction secretions (Figure 4). The high level of ROS/RNS production was shown by endothelial cells stretching in the direction of neutrophil migration (Figure 4f). Another interesting feature is that the maximum ROS production by neutrophils is noted in the case when the bacterial chemoattractants (E. faecalis 645-p2 or E. coli 321) maximally induced stochastic cell migration and/or aggregation of neutrophils.
An interesting phenomenon was revealed by the SICM microscopy method. In the case of migration of low-active neutrophils, the morphology of endothelial cells along which the neutrophil migrates changed more. In the case of migration of highly active neutrophils, no significant changing in the endothelial cells along that the neutrophil migrates were noted. Although the morphology of the migrating neutrophils itself changed very actively.
In the transendothelial migration model, both the character of neutrophil polarization and the morphology of endothelial cells between that the neutrophil migrates changed significantly. To realize the swarming phenomenon, neutrophils expand the space between endothelial cells. In this case, transendothelial migration does not require significant polarization of neutrophils (Figures 7D and 8). The damage of endothelial cell integrity shown in Figure 8 can be attributed to different causes or a complex of them. Damages are formed by pathogenicity factors secreted by bacteria that are located in the lower chamber and act as chemoattractants (e.g., leukocidins in S. aureus [24] or lipopolysaccharide mediated toxic effects in P. mirabilis [25]). Enzymatic activity of neutrophils (e.g., by matrix metallopeptidase 9 activity) can lead to endothelial cell membrane damage [26]. Hyperproduction of ROS/RNS by activated endothelial cell along that neutrophil migrates (Figure 4) can cause cytotoxic effect.
5. Conclusion
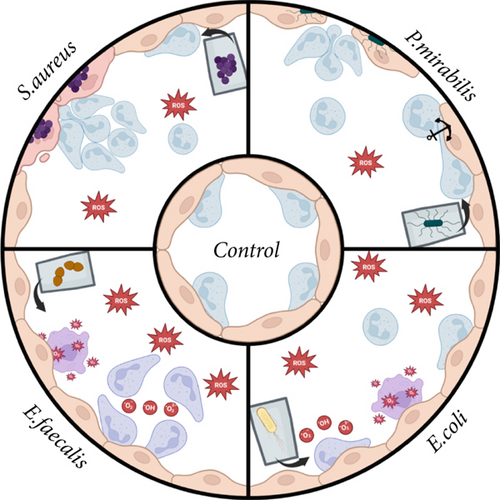
Thus, specific features of neutrophil granulocytes migration depended on the chemoattractants used. This is evidenced by the “disorientation” of cells under the influence of low-molecular weight product secretion and/or degradation of E. faecalis 645-p2 (less than 14 kDa) leading to neutrophil migration in the direction opposite to the chemoattractant and the suppression of neutrophil migration activity by low molecular weight pathogenicity factors of P. mirabilis 649-2. Migrated along the lines connecting endothelial cells, neutrophils actively produced ROS. When both neutrophils and bacterial chemoattractant were present in the system simultaneously, endothelial cells showed active production of ROS/RNS. Maximum production of ROS was detected in neutrophils that migrated stochastically when E. faecalis 645-p2 was used as a chemoattractant. Transendothelial migration in the pyogenic focus model with bacteria showed that migrated neutrophils gradually expanded the intercellular space of endothelial cells to facilitate migration, especially when swarming. Additionally, in the vertical migration model, significant alterations of the neutrophils and endothelial cells morphology and damage of endothelial cells integrity were observed. Results are schematically summarized in Figure 9.
Ethics Statement
The study was done with the permission of the Lobachevsky University Bioethics Committee founded on November 11, 2016 (permission Protocol No. 9 was issued on July 17, 2017). The study was in accordance with the standards of the Helsinki Declaration of June 1964. All participants signed informed agreement for inclusion in the study before the beginning.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research is supported by the Russian Science Foundation (10.13039/501100006769) (23-74-00004).
Acknowledgments
The authors express their sincere gratitude to Prof. S.A. Selkov, Head of the Department of Immunology and Intercellular Interactions, and Dr. D.I. Sokolov, Head of the Laboratory of Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynaecology and Reproduction, for providing the EA.hy926 cell line; and Dr A.S. Erofeev, Head of the MISIS Biophysics Laboratory, and V.S. Kolmogorov, Laboratory Engineer, for providing nanopipettes. The authors thank I.V. Balalaeva, Associate Professor, Department of Biophysics, Lobachevsky State University of Nizhny Novgorod, for assistance with the experiments to measure ROS production.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.