Pseudogene BNIP3P1 Regulates H. pylori–Induced Apoptosis in Gastric Mucosal by Acting on the miR-411-5p/BNIP3 Axis
Abstract
Helicobacter pylori (H. pylori) is one of the major causes of gastric mucosal damage, and infection with H. pylori induces an immune response with gastric mucosal cells, which reduces gastric mucosal damage. The pseudogene BNIP3P1, sharing a remarkable 95.92% similarity with its well-characterized counterpart BNIP3, has largely remained unexplored. To elucidate the role of BNIP3P1 in gastric mucosal damage induced by H. pylori infection, we meticulously constructed both in vivo and in vitro models. Gene chip sequencing, dual-luciferase assays, and cellular phenotyping were detected. We uncovered a compelling positive correlation between the duration of H. pylori infection and BNIP3 overexpression at both the mRNA and protein levels. Intriguingly, overexpression of BNIP3 was found to effectively impede the proliferation and migration of human gastric epithelial cells (GES-1). Furthermore, we identified miR-411-5p as a direct regulator of BNIP3, targeting its 3 ′UTR region and suppressing its expression during H. pylori infection. Notably, BNIP3P1- 3′UTR was observed to competitively bind miR-411-5p, leading to the upregulation of BNIP3 expression. Furthermore, overexpression of BNIP3P1 was associated with a marked decrease in GES-1 cell proliferation and a concomitant acceleration of apoptosis. Our findings suggest that BNIP3P1 functions as a competing endogenous RNA (ceRNA) within the BNIP3/miR-411-5p axis during H. pylori infection, which ultimately hinders cell proliferation and promotes apoptosis in GES-1 cells. This study sheds light on the intricate mechanisms underlying H. pylori infection of GES-1 cells.
1. Introduction
Helicobacter pylori (H. pylori), a highly prevalent bacterium infecting approximately half of the global population, poses a significant threat to gastric health by inducing chronic mucosal damage [1, 2]. The gastric epithelial cell line (GES-1) contributes to the protective barrier against mucus stomach pathogens through mucus secretion [3]. H. pylori disrupts cellular integrity and invades host cells via secreted virulence factors and interactions with host cell surface receptors and then activates multiple intracellular signaling pathways [4, 5]. To establish successful colonization, H. pylori employs strategies to withstand the stomach’s acidity, adhere to host cells, and deploy virulence factors such as BabA, OipA, VacA, CagA, HtrA, NepA, and SabA [6–11]. Chronic H. pylori infection contributes to gastric pathologies, including cell apoptosis, disruption of epithelial barrier function, and diseases like gastric cancer, peptic ulcers, chronic gastritis, and intestinal metaplasia [12].
H. pylori infection triggers the overexpression of diverse regulatory factors in GES-1 cells. Among them, BNIP3 is particularly interesting, implicating cell autophagy and apoptosis. BNIP3, a stress-response protein regulated by HIF-1α, is strongly upregulated under hypoxic conditions [13]. While BNIP3-mediated apoptosis involves mitochondrial outer membrane permeabilization and interactions with antiapoptotic proteins BCL-2 and BCL-XL [14, 15]. The precise role of these in H. pylori–infected GES-1 cells needs further investigation. Notably, HIF-1α-induced BNIP3 overexpression promotes cell survival via the PI3K/Akt/p53 pathway–mediated mitochondrial autophagy under hypoxic conditions [16]. However, the significance of BNIP3 in the context of H. pylori infection and its influence on GES-1 cell immune response remains an understudied area. Moreover, the regulatory potential of the pseudogene BNIP3P1 in H. pylori infection was an even greater enigma.
Pseudogenes, like BNIP3P1, share sequence homology with functional genes but lack conventional protein-coding capacity due to mutations or deletions. Intriguingly, emerging research highlights the regulatory potential of pseudogenes and other noncoding RNAs, particularly long noncoding RNAs (lncRNAs), in various diseases [17, 18]. lncRNAs exert diverse biological functions, including cell cycle regulation, imprinting, neurogenesis, and DNA methylation [17–19]. Meanwhile, microRNAs (miRNAs) are small noncoding RNAs that play crucial roles in posttranscriptional gene regulation. By binding to target mRNAs, miRNAs can induce mRNA degradation or translational repression, thereby modulating gene expression and influencing diverse cellular processes. Importantly, dysregulation of miRNAs has been implicated in various diseases and their significance in pathological processes. However, the specific role of BNIP3P1 within the context of H. pylori infection remains largely unexplored.
This study seeks to elucidate the critical role of BNIP3P1 in H. pylori infection through a combination of gene chip data mining and experiments in vivo and in vitro. Our objective is to uncover a novel BNIP3P1/miR-411-5p/BNIP3 axis that may contribute to the mechanisms of gastric mucosal damage during H. pylori infection.
2. Materials and Methods
2.1. Cell Culture
GES-1, gastric adenocarcinoma (AGS), and mucoepidermoid carcinoma (MGC-803) cell lines were obtained from the Key Laboratory of Biological Resource and Ecological Environment of the Chinese Education Ministry (Chengdu, China) and cultured in either DMEM high glucose or RPMI-1640 medium (Gibco, China) supplemented with 10% fetal bovine serum (FBS, Gibco, China). Cells were maintained in a 5% CO2 incubator at 37°C (Forma Series II 3111, Thermo Scientific, United States) [6].
2.2. Bacterial Strain and Growth Conditions
The H. pylori strain (H. pylori SS1), provided by the Sichuan Provincial People’s Hospital (Chengdu, China), was cultured on Columbia blood agar base medium supplemented with 7% defibrinated sheep blood (Yikang Biological Technology, Zhengzhou, China), 0.84% brain heart infusion broth (Oxoid, United Kingdom), and mixed antibiotics (vancomycin, amphotericin, polymyxin B, trimethoprim, and nalidixic acid, all from Sigma-Aldrich, Dorset, United Kingdom). H. pylori was incubated at 37°C in an anaerobic incubator (E200, GeneScience, United States) under microaerobic conditions (10% CO2, 5% O2, 85% N2) [5, 8].
2.3. Plasmid Construction and Transfection
BNIP3P1 and BNIP3 overexpression plasmids were constructed following the kit instructions using genomic DNA (gDNA) extracted from GES-1 cells (TIANGEN DNA extraction kit, China). PCR amplification of BNIP3P1 and BNIP3 was performed using the gDNA template. PCR products and the pcDNA3.1 vector were digested (BamHI and HindIII, TaKaRa, China) and ligated (T4 DNA ligase, TaKaRa, China). Hsa-miR-411-5p mimics were synthesized commercially (Ruibo Biotechnology, Guangzhou, China) [20].
Dual-luciferase reporter plasmids (psiCHECKTM-2 vector) containing BNIP3-3 ′UTR and BNIP3P1-3 ′UTR sequences were constructed by Guangzhou Ruibo Company using double enzyme digestion (BamHI and HindIII) as described above. The primer sequences are listed in Table S1. Transfections were performed using Lipofectamine 3000 (Invitrogen, United States) with cells incubated for 24 h in a 5% CO2 incubator at 37°C [21].
2.4. H. pylori Infection
H. pylori, cultured for 3–5 days under microaerobic conditions, was harvested. Cells were seeded in six-well plates (8 × 105 cells/well) for 12 h before stimulation with H. pylori at a multiplicity of infection (MOI) of 100:1. Cells were then cultured for 24 h in a serum-free medium within a 5% CO2 incubator at 37°C [8].
2.5. RNA Extraction and RT-qPCR
Total RNAs were extracted using Triazole reagent (Invitrogen, United States) and RNA extraction kit (JIANSHI BIOTECH, China) according to the manufacturer’s protocols. The cDNA synthesis was performed using 1 μg of RNA template and the PrimeScript RT reagent kit with gDNA eraser (TaKaRa, China). RT-qPCR was carried out using TB Green Premix Ex Taq (TaKaRa, China) on the QuantStudio 3 system (Thermo Scientific, United States). Table S1 provides the primer sequences. Relative gene expression was calculated by the 2−ΔΔCt method, normalized to GAPDH as the reference gene [22].
2.6. Western Blot
Protein extraction was performed using RIPA buffer (Biosharp, China) supplemented with 1% protease inhibitors (MedChemExpress, China) and 1% PMSF (Epizyme, China). Protein concentration was determined using a BCA assay kit (Nanjing Jiancheng Bioengineering, China). SDS-PAGE (Bio-Rad, China) was used for protein separation, and the protein was then transferred to PVDF membranes (Millipore, China). PVDF membranes were blocked with 5% skimmed milk (Wako, Japan) and incubated with primary antibodies overnight at 4°C and secondary antibodies for 1 h at 25°C. Detection was performed using the Omni-ECL Femto Light Chemiluminescence Kit (Epizyme, China) and the iBright Imaging System (Invitrogen, United States) [23].
Primary antibodies were as follows: BNIP3 (ab10433, Abcam, China), anti-FLAG (F1804, Sigma, China), and β-actin (T200068-8F10, ZEN-BIOSCIENCE, China). The secondary antibody was as follows: HRP-conjugated affineur goat antimouse (SA00001-1, proteintech, China).
2.7. Gene Chip Mining
Gene expression profiling was performed using the Gene Chip Human Transcriptome Array 2.0 (Affymetrix, Santa Clara, CA, United States). Hybridization was followed by gene expression analysis with the Gene Chip Scanner 3000 (Affymetrix, Santa Clara, CA, United States). After scanning, data normalization and differential expression analysis were performed. Upregulated genes were defined by a fold change ≥ 2 and downregulated genes by a fold change ≤ 0.5 [24].
Gene Ontology (GO: http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG: http://www.kegg.jp/kegg) analyses were conducted to gain biological insights into the host response during the experimental conditions. The immune-related pathway genes were identified using the keyword “immune,” and GO analysis explored the functions, cellular components, and molecular processes. KEGG analysis identified pathways associated with these genes, providing insights into the potential immune response mechanisms [25].
2.8. Cell Proliferation Analysis
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assay (n = 6 per group) following the kit instructions. Cells were seeded in 96-well plates (density: 4 × 103 cells/well for GES-1 and 3.5 × 103 cells/well for AGS and MGC-803) and treated with the relevant DNA constructs, miRNA, or H. pylori for 24 h. CCK-8 reagent (10 μL/well, Biosharp, China) was added to the medium. After 1-h incubation, optical densities at 450 nm (OD450) were measured using a full-wavelength microplate reader (Multiskan GO, Thermo Scientific, United States) [6].
2.9. Flow Cytometry Analysis for Apoptosis
Cells were seeded (2 × 106 cells/well) in six-well plates. Following treatments, GES-1 cells were washed (ice-cold PBS), trypsinized, centrifuged into a cell suspension, and rewashed with binding buffer. Staining was performed with Annexin-V-FITC and PI (5 μL each, Solarbio, China). Following a 15-min dark incubation, 500 μL PBS was added, and flow cytometry analysis was performed on a BD FACScalibur flow cytometer (BD Biosciences, United States) [26].
2.10. Cell Migration Assay
Cell migration was evaluated using a scratch wound assay. GES-1 cells (4 × 105 cells/well) were seeded in 12-well plates and cultured until confluency was reached. Sterile toothpicks were used to create scratches. After being washed with PBS, cells were treated with overexpression plasmids and cultured in a serum-free medium (37°C, 24 h). Images of the wounded areas were acquired and analyzed using ImageJ. Migration was quantified as cell migration percentage (%) = (0–24 h scratch area)/0 h scratch area × 100% [22].
2.11. Animal Experimentation
Ten-week-old, SPF-grade, male BALB/c mice were obtained from the Sichuan University Laboratory Animal Center (Chengdu, China). The study was approved by the Ethics Committee of the School of Life Sciences, Sichuan University (Ethics Approval No. 20201109001), and all procedures adhered to relevant ethical regulations. Mice were acclimatized for 1 week (25 ± 2°C, 12-h light/dark cycle). Following this, they were randomized into two groups (n = 10): the blank and the H. pylori groups. Every 2 days, the H. pylori group was garaged with 0.5 mL H. pylori solution, and the blank group received 0.5 mL saline as a control. Both groups were maintained on the same diet. After 8 weeks of treatment, mice were euthanized via spinal subluxation. Stomachs were excised, longitudinally opened along the greater curvature, washed (physiological saline), and divided into three portions: one for a rapid urease assay, one for RNA extraction (preserved in liquid nitrogen), and one fixed in 4% paraformaldehyde for immunohistochemistry. All animal procedures were aligned with ethical guidelines to ensure animal welfare [27].
2.12. Urease Test
Stomach tissue was placed on the rapid urease test paper. The color change was observed within 5 min, where a deeper shade of red indicated stronger H. pylori presence on the stomach tissue [6, 28].
2.13. Immunohistochemistry Analysis
Mouse gastric tissues were fixed (4% paraformaldehyde), paraffin-embedded, and sectioned (5 μm). Sections were deparaffinized, rehydrated, and blocked (3% bovine serum albumin). Samples were stained with anti-BNIP3 antibodies (ab10433, Abcam, China) for 24 h at 4°C, followed by HRP-labeled goat antimouse IgG (A0216, Beyotime, China) and DAB kit development (Solarbio, China). Finally, slides were washed, counterstained (Hematoxylin), dehydrated (Graded ethanol), cleared (Xylene), mounted (Neutral gum), visualized, and imaged under a microscope [14].
2.14. Luciferase Reporter Assay
GES-1, AGS, and MGC-803 cells were cotransfected with luciferase reporter plasmids and miR-411-5p. Luciferase activity was measured using the Dual-Glo Luciferase reporter kit (Promega, China) on a Synergy H1 microplate reader (BioTek, United States) and normalized to renilla luciferase activity [23].
2.15. Statistical Analysis
Data analysis and plotting were performed using GraphPad Prism 8.0 and Adobe Photoshop CS2. Statistical significance was determined using either one-way analysis of variance (ANOVA) or t-tests, p < 0.05.
3. Results
3.1. H. pylori Infection Upregulates BNIP3 Expression and Promotes Apoptosis In Vitro and In Vivo
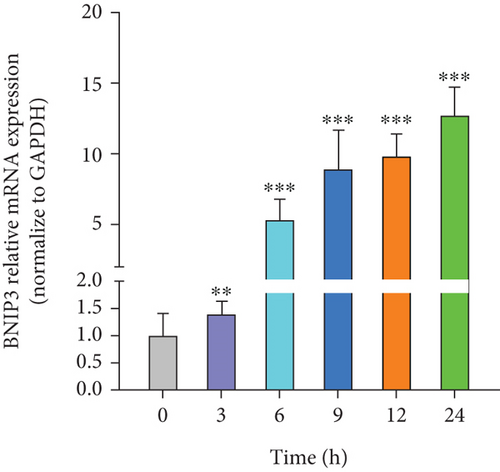
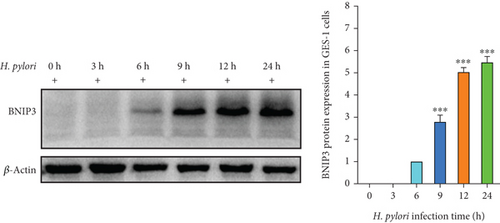
H. pylori, a major contributor to gastric cancer and gastritis, has been implicated in modulating the expression of BNIP3, a gene that plays a critical role in cell survival and death. Our study revealed that H. pylori infection significantly upregulated BNIP3 expression in GES-1 cells. This upregulation of BNIP3 mRNA exhibited a positive correlation with the duration of H. pylori infection, increasing in a time-dependent manner within 24 h (Figure 1a). Western blot analysis corroborated this finding, demonstrating a parallel increase in BNIP3 protein levels following H. pylori infection (Figure 1b). These observations underscored the potential significance of BNIP3 in the context of H. pylori–induced gastric pathology.
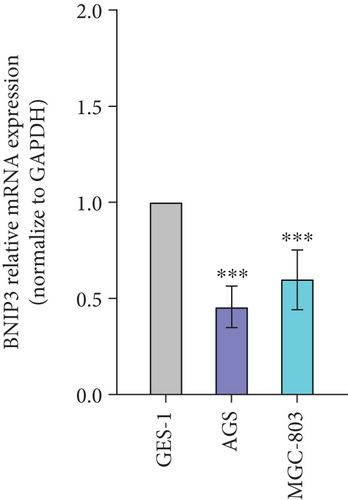
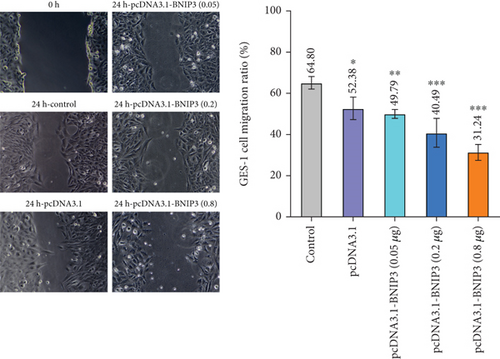
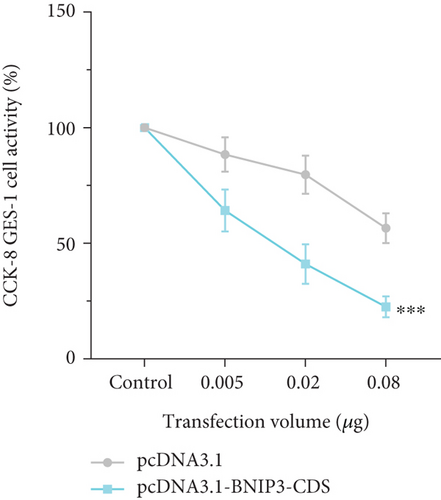
BNIP3 was a multifunctional regulatory gene implicated in wound healing and tumorigenesis. Dysregulated BNIP3 expression could contribute to cellular damage and carcinogenesis. Intriguingly, RT-qPCR assays revealed significantly higher BNIP3 expression in the GES-1 than in gastric cancer cell lines (AGS and MGC-803), suggesting a potential role in wound healing responses (Figure 1c). Furthermore, overexpression of BNIP3 exerted marked effects on GES-1 cell behavior. Increased transfection concentrations led to a dose-dependent reduction in cell migration; GES-1 cell migration was reduced to 31.24% with pcDNA3.1-BNIP3 addition at 0.8 μg (Figure 1d). CCK-8 assays confirmed that BNIP3 overexpression had a dose-dependent inhibitory effect on cell proliferation (Figure 1e). Collectively, these findings suggested that H. pylori infection, by inducing gastric mucosal damage, might drive BNIP3 upregulation, leading to apoptotic responses.
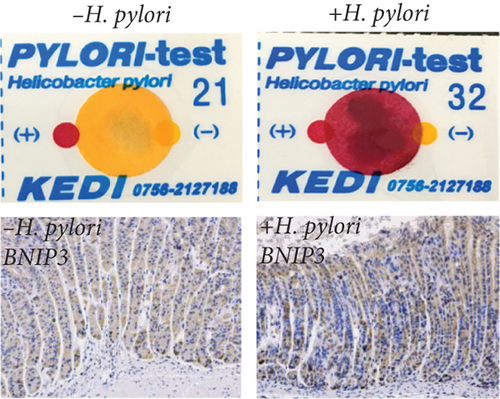
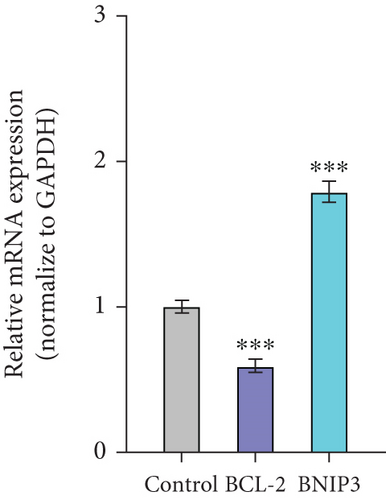
Therefore, we successfully established a mouse model of H. pylori infection, verified by positive urease assay and IHC staining (Figure 1f). Consistent with our in vitro findings, a significant increase in BNIP3 expression was observed within the gastric mucosa of infected mice. RT-qPCR analysis revealed the upregulation of Bnip3 and a corresponding downregulation of the antiapoptotic gene Bcl-2 (Figure 1g). Collectively, our in vivo and in vitro experiments demonstrated that H. pylori infection induced cellular damage, leading to an upregulation of BNIP3 expression, a key component of enhanced apoptotic responses.
3.2. BNIP3P1, A Pseudogene of BNIP3, Is Significantly Upregulated During H. pylori Infection
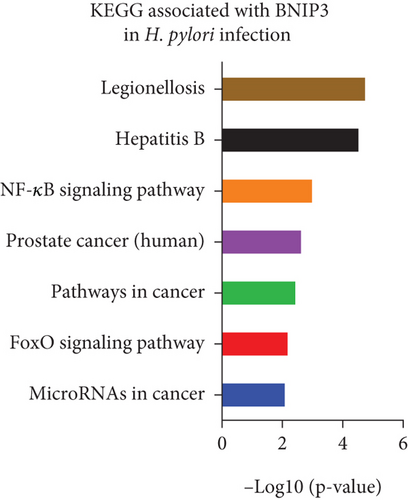
Gene chip sequencing of H. pylori–infected GES-1 cells revealed significant differential gene expression, including a 13.09-fold upregulation of BNIP3 and a notable downregulation of BCL-2 (Figure 2a). This finding aligned with our previous observations, suggesting a link between H. pylori infection and the modulation of apoptotic pathways. GO analysis further highlighted the BNIP3 accumulation within the defense response to the virus following H. pylori infection (Figure 2b). KEGG analysis implicated BNIP3 in the Legionellosis pathway postinfection, providing additional insights into potential mechanisms (Figure 2c).
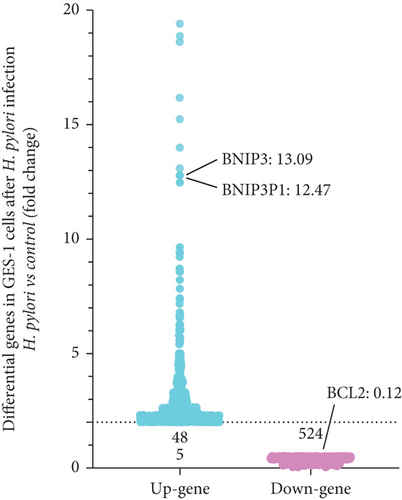
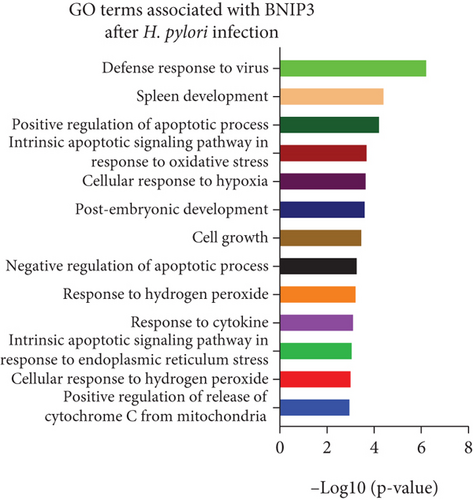
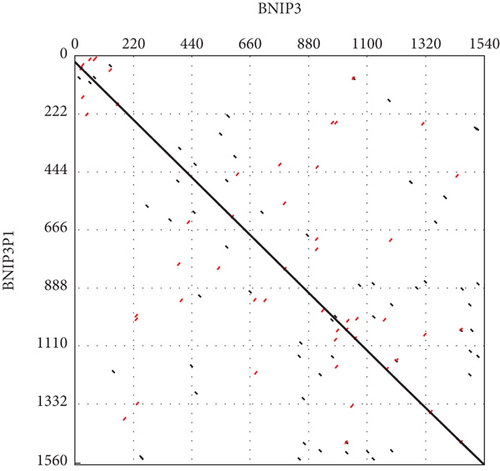
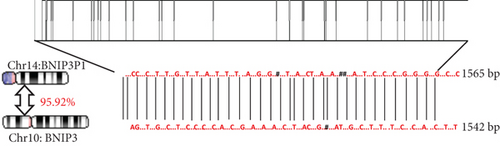
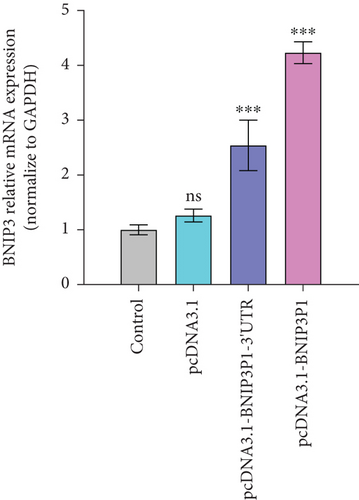
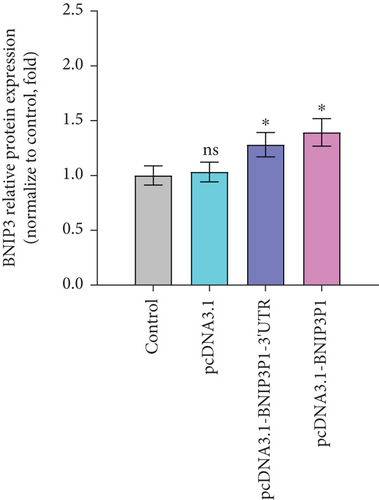
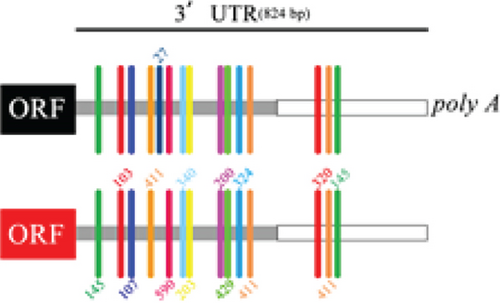
Intriguingly, BNIP3P1, a pseudogene of BNIP3, exhibited a similar upregulation pattern (12.47-fold) during H. pylori infection (Figure 2a). Despite their distinct chromosomal locations (BNIP3 (1542 bp) on chromosome 10 and BNIP3P1 (1565 bp) on chromosome 14), these genes share a remarkably high sequence similarity of 95.62% (Figure 2d,e). While research on BNIP3P1 remained limited, our study revealed a striking parallel: Both BNIP3 and its pseudogene BNIP3P1 exhibited a remarkable upregulation pattern in GES-1 cells responding to H. pylori infection. Furthermore, RT-qPCR assays demonstrated that BNIP3P1 transfection resulted in a 4.23-fold upregulation of BNIP3, and the assumed BNIP3P1 3 ′UTR also moved BNIP3 up 2.54-fold, which suggested that BNIP3P1 promoted the transcription of BNIP3 (Figure 2f). Meanwhile, BNIP3P1 and BNIP3P1-3 ′UTR upregulated the BNIP3 protein content by 1.28- and 1.29-fold, respectively (Figure 2g). In contrast, the protein translation was weaker, and thus, we inferred that there might be a ceRNA regulatory mechanism between BNIP3P1 and BINP3. This observation warranted further investigation into the precise role of BNIP3P1 in the context of H. pylori infection.
3.3. Upregulation of BNIP3P1 Promotes Apoptosis
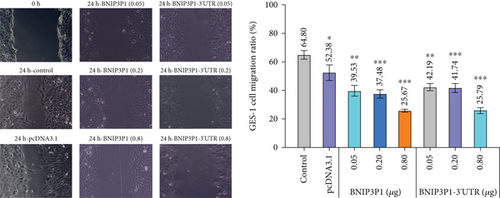
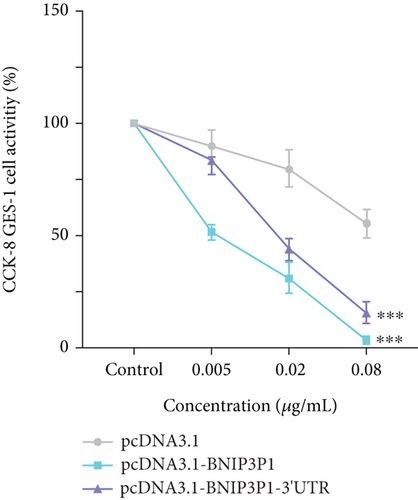
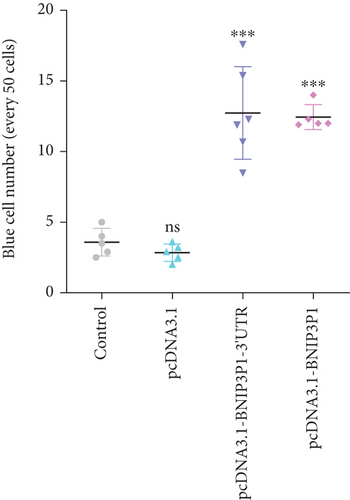
In light of the significant upregulation of BNIP3P1 observed during H. pylori infection of GES-1 cells, we investigated its potential biological function. Overexpression of BNIP3P1 and BNIP3P1-3 ′UTR plasmid were constructed for this purpose. Scratch assays revealed that transfection with either BNIP3P1 or BNIP3P1-3 ′UTR inhibited GES-1 cell migration in a dose-dependent manner, and BNIP3P1 and BNIP3P1-3 ′UTR of 0.8 μg reduced GES-1 migration to 25.67% and 25.79% after 24 h transfection (Figure 3a). This inhibitory effect on cell viability was further corroborated by CCK-8 assays, demonstrating a gradual decline in cell viability with increasing transfection concentrations (Figure 3b). Trypan blue staining assays supported these findings, revealing a reduction in the number of viable cells upon overexpression (Figure 3c).
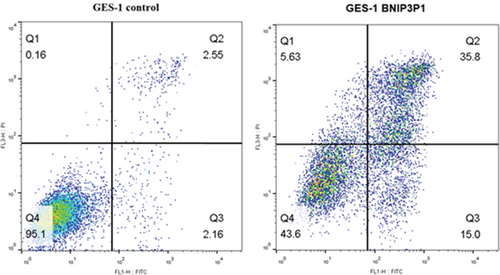
Interestingly, Annexin V-FITC/PI assays demonstrated a significant increase in apoptotic cells following BNIP3P1 transfection, with a late apoptosis level of 35.8%. BNIP3P1 overexpression increased apoptosis compared to the control group (2.55% late apoptosis) (Figure 3d). These observations, consistent with our CCK-8 assay results, strongly suggested that BNIP3P1 overexpression inhibited GES-1 cell proliferation and actively promoted apoptosis.
3.4. miR-411-5p Suppresses BNIP3 Expression During H. pylori Infection
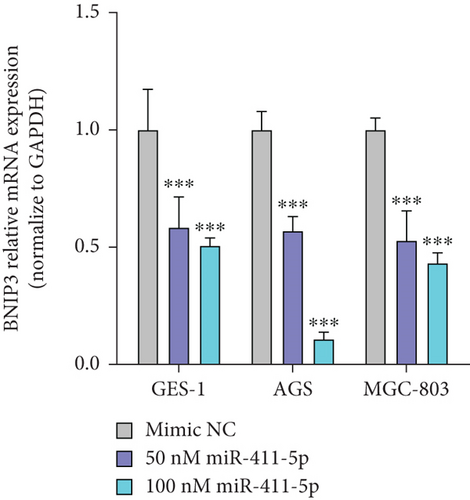
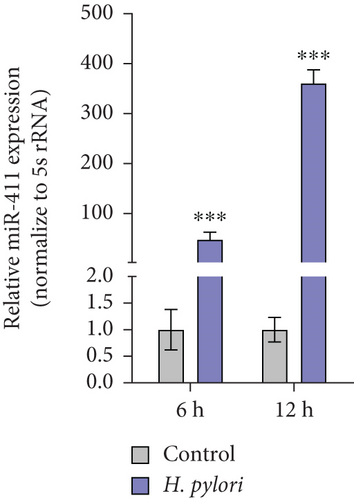
Pseudogenes can exert regulatory functions by acting as ceRNAs, by binding miRNAs, and by modulating the expression of their target genes. Seven miRNAs that can bind BNIP3P1 and BNIP3 were identified by TargetScan and miRanda prediction, of which miR-411-5p has three distinct binding sites on BNIPI3P1 (Figure 4a). Transfection of miR-411-5p (50 and 100 nM) successfully downregulated miR-411-5p expression in GES-1 cells as well as the gastric cancer cell lines AGS and MGC-803, compared to Mimic NC (Controls) (Figure 4b). Intriguingly, we observed a significant upregulation of miR-411-5p following H. pylori infection (Figure 4c), suggesting a potential role in the cellular response to the pathogen.
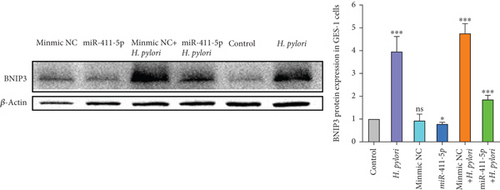
Western blot analysis confirmed that miR-411-5p overexpression decreased BNIP3 protein levels. Importantly, BNIP3 was significantly upregulated in H. pylori infection, but BNIP3 significantly reduced after transfection with miR-411-5p (Figure 4d). These findings collectively demonstrated that miR-411-5p acted as a suppressor of BNIP3 during H. pylori infection, potentially mitigating apoptosis induced by this bacterium. BNIP3P1, as a pseudogene of BNIP3, has been confirmed to be expressed similarly to BNIP3 in microarray data mining and has three common binding sites for miR-411-5p, which suggests that BNIP3P1 may play an important role as a sequence that competes to bind miR-411-5p.
3.5. BNIP3P1 Upregulates BNIP3 Expression by Competitively Binding miR-411-5p
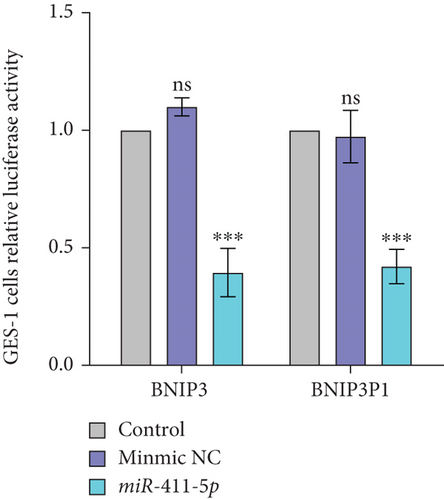
Bioinformatic analysis revealed the common miR-411-5p binding sites within the 3 ′UTR regions between BNIP3P1 and BNIP3. This finding suggested a potential regulatory mechanism that BNIP3P1 could function as a ceRNA, modulating BNIP3 expression. To investigate this hypothesis, we constructed dual-luciferase reporter vectors (psi-BNIP3-3 ′UTR and psi-BNIP3P1-3 ′UTR). Then, they were cotransfected with miR-411-5p, which confirmed the binding of miR-411-5p to both 3 ′UTR regions, as evidenced by a significant decrease in luciferase activity (Figure 5a).
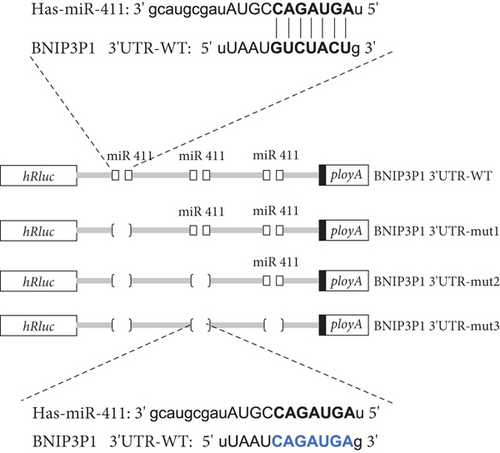
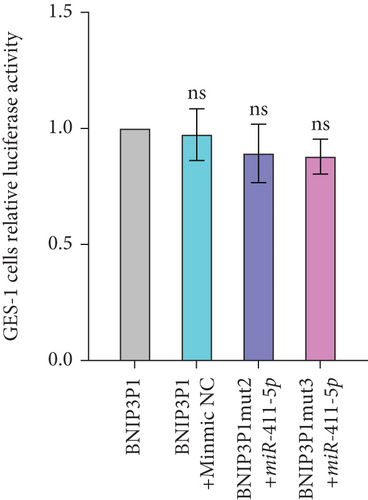
To further validate the specific interaction between miR-411-5p and BNIP3P1, we introduced mutations into the putative miR-411-5p binding sites within BNIP3P1 and generated the vectors psi-BNIP3P1-mut1, psi-BNIP3P1-mut2, and psi-BNIP3P1-mut3 (Figure 5b). In GES-1 cells, cotransfection of these mutated vectors with miR-411-5p revealed a loss of binding activity as there was no change in luciferase intensity (Figure 5c). This finding strongly supported the specificity of the interaction between BNIP3P1 and miR-411-5p.
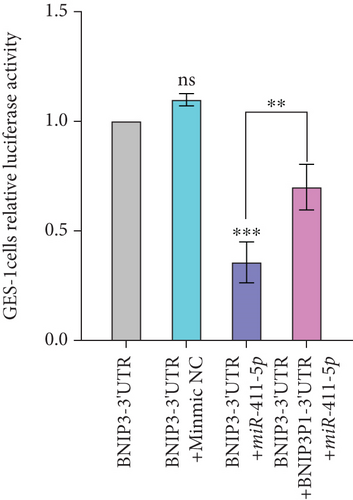
Finally, we developed a transfection model utilizing pcDNA3.1-BNIP3P1-3 ′UTR, psi-BNIP3-3 ′UTR, and miR-411-5p to test the ceRNA mechanism. Overexpression of BNIP3P1-3 ′UTR competitively bound miR-411-5p, leading to a decrease in the binding of BNIP3 and then, a consequent increase in BNIP3 expression (Figure 5d). This result demonstrated that BNIP3P1 functioned as a ceRNA, whose 3 ′UTR region competed with BNIP3 for miR-411-5p binding. This mechanism ultimately modulated the expression of both BNIP3 and BNIP3P1, highlighting the significance of this interaction in the context of H. pylori infection.
4. Discussion
H. pylori, a highly prevalent pathogen, disrupts gastric mucosal integrity through its Type IV secretion system and the release of virulence factors that activate the NF-κB pathway [24, 25, 28]. This activation triggers inflammation, immune responses, and cellular damage [26, 29]. Our study uncovers a novel mechanism underpinning H. pylori–induced cellular injury, which demonstrates the crucial role of the BNIP3P1/miR-411-5p/BNIP3 axis in promoting apoptosis in H. pylori–infected cells.
We demonstrated that BNIP3P1 acted as a ceRNA that bound miR-411-5p, leading to the upregulation of BNIP3 consequently. The upregulation of the BNIP3 protein, which plays established roles in carcinogenesis, has been observed in various tissues [30–32]. Furthermore, increased BNIP3 expression correlates with aggressive tumor phenotypes and poor prognosis [33, 34]. Here, in vitro (GES-1 cells) and in vivo (mouse model) experiments confirmed a significant upregulation of BNIP3 following H. pylori infection. Importantly, the overexpression of BNIP3 inhibited GES-1 cell proliferation and induced apoptosis, highlighting its potential role in H. pylori–mediated pathogenesis.
Intriguingly, bioinformatic analysis (TargetScan, miRanda) revealed multiple miRNA binding sites on BNIP3P1 and BNIP3, including three for miR-411-5p. Western blot experiments validated the inhibitory effect of miR-411-5p on BNIP3 expression during H. pylori infection. Notably, miR-411-5p attenuated GES-1 cell apoptosis by downregulating BNIP3. However, BNIP3P1 competitively bound miR-411-5p, disrupting this regulatory balance. This competition ultimately led to the increase of BNIP3 expression, inhibition of GES-1 cell proliferation, and the promotion of apoptosis.
Collectively, our findings elucidated a novel BNIP3P1/miR-411-5p/BNIP3 axis that represented a crucial cellular response mechanism during H. pylori infection. BNIP3P1 acted as a ceRNA, competitively combining with miR-411-5p and raising BNIP3 expression. The increased BNIP3 triggered proapoptotic pathways that might be a defensive measure to eliminate infected cells and limit the spread of H. pylori. These insights offered new avenues for developing diagnostic markers and therapeutic targets for H. pylori–associated gastric diseases, potentially with a focus on modulating this axis to control cellular responses.
5. Conclusion
Our study reveals a novel mechanism by which H. pylori infection contributes to gastric mucosal damage. We demonstrate that the previously understudied pseudogene, BNIP3P1, functions as a ceRNA for BNIP3. During H. pylori infection, BNIP3P1 consumes miR-411-5p, a key regulator of BNIP3 expression. This competitive binding leads to a significant upregulation of BNIP3, promoting apoptosis within H. pylori–infected cells. Consequently, this proapoptotic response inhibits cell proliferation and may eventually lead to gastric damage. Our findings highlight the BNIP3/miR-411-5p/BNIP3P1 axis as a crucial molecular pathway in the cellular response to H. pylori infection. These insights offer avenues for novel diagnostic development and therapeutic strategies in H. pylori–associated gastric diseases.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Andong Zhang, Xin Yan, and Ningzhe Li contributed to the work equally and should be regarded as co-first authors.
Funding
This work was supported by the Sichuan Science and Technology Program (No. 2023YFS0156, 2023YFSY0055), the Higher Education Personnel Training and Teaching Reform Project of Sichuan University (No. SCU10065), and the Science and Technology Plan Project of Chengdu Longquanyi District (2024LQRD0045).
Acknowledgments
This work was supported by the Sichuan Science and Technology Program (No. 2023YFS0156, 2023YFSY0055), the Higher Education Personnel Training and Teaching Reform Project of Sichuan University (No. SCU10065), and the Science and Technology Plan Project of Chengdu Longquanyi District (2024LQRD0045).
Open Research
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author, [[email protected]], upon reasonable request.