Effects of Varying Salinity Levels on Growth, Antioxidant Defense, and Digestive Enzymes Activity of Whiteleg Shrimp (Litopenaeus vannamei) Postlarvae Reared in the Biofloc System
Abstract
This study evaluated the effects of different salinities on the growth and physiology of whiteleg shrimp (Litopenaeus vannamei) postlarvae reared in the biofloc system. A total of 4200 shrimps (PL20) were randomly distributed in 12 850 L biofloc (BFT) tanks (350 PL20 in each tank). Shrimps divided into four experimental groups in the biofloc system with salinities of 3 (BFT3), 8 (BFT8), 10 (BFT10), and 12 ppt (BFT12). Salinities were made by adding sea salt to tap water. During the rearing period, for the optimal growth and development of biofloc in the tanks, a carbon source (brown sugar and wheat flour in equal proportions) was calculated and added to the tanks to adjust the ratio of carbon to nitrogen (12:1). From the third to the sixth week of rearing, the water nitrate concentration was significantly higher in BFT12 and BFT10 (p < 0.05). The total ammonia nitrogen and nitrite concentration at different sampling times did not show significant differences among experimental groups (p > 0.05). After 6 weeks, salinity had no effect on the growth indices including final weight, weight gain (WG), WG rate, specific growth rate (SGR), and survival (p > 0.05). The highest levels of intestinal amylase (28.97 ± 3.46 U/mg protein) and protease (13.28 ± 2.19 U/mg protein) activities were also observed in the BFT3 group (p < 0.05), while lipase activity did not changed. Muscle glutathione (GSH) and malondialdehyde levels and catalase activity did not show significant differences among the experimental treatments (p > 0.05). On the other hand, the highest superoxide dismutase activity and total antioxidant capacity (TAC) was observed in BFT10 (p < 0.05). Body proximate composition of L. vannamei was not significantly different in experimental groups (p > 0.05). Our findings showed that biofloc system could overcome the reported adverse effects of salinity on L. vannamei growth and possibility of rearing L. vannamei in low salinity in the biofloc system.
1. Introduction
One of the important environmental problems in aquaculture is the production and discharge of a large amount of nutrient-rich wastewater from farms, which causes irreparable damage to the environment. Continuous water replacement also increases the risk of contamination and disease. A practical solution in terms of environmental considerations to increase aquaculture production is the use of dense breeding systems [1]. One of the systems in which shrimp can be grown densely is the biofloc system [2]. Biofloc environment acts as an anti-stress agent [3]. Biofloc technology, which is another name for the cultivation system without water exchange, minimizes the exchange of water. Therefore, its importance is increasing due to biosecurity and environmental advantages [4]. In these systems, some risks, such as the entry of diseases and alien species into the farm and problems related to the discharge of effluent water, which cause environmental pollution, are reduced [5].
Whiteleg shrimp, Litopenaeus vannamei is one of the world’s most important cultivated shrimp species [6]. This species has been transferred to most regions of the world due to its significant breeding advantages [7]. Litopenaeus vannamei has a high growth rate, tolerance to various salinities, and good disease resistance [8]. Due to feeding from natural organisms in the pool and breeding environment, compactness, high survival rate, lower protein requirement than other breeding species, the possibility of storage in small tanks, and quick adaptation to the breeding system, L. vannamei is a desirable species for breeding [7]. The production of L. vannamei in low salinity waters (0.5–5 ppt) is a potential method for the continuous expansion of shrimp farming. However, ion imbalance may affect shrimp physiological processes and productivity [9].
In addition, rearing western Pacific shrimp in low salinity waters is an effective way to expand shrimp production [10]. Different environmental salinities can cause a variety of responses from no change to dramatic fluctuations in exposed aquatic organisms [11, 12]. The effect of salinity on different species of shrimp in the biofloc system has been investigated in previous studies [10, 13, 14]. According to the importance of shrimp farming, introducing biofloc technology to this industry which is particularly important in terms of saving water and land, using less foods and cheaper protein, and organic product production creating a compatible system [15]. It had been shown that salinities 15 and 27 ppt at the start of shrimp culture enhanced the robustness of the white L. vannamei biofloc system [16]. This study was conducted to evaluate the feasibility of cultivating L. vannamei in inland waters with low salinity. Biofloc system was used to improve shrimp performance in this situation. Meanwhile, antioxidant defense as an important part of immune responses in this species was investigated to provide a better understanding of the physiological responses of shrimp.
2. Material and Methods
This research was conducted in Sistan and Baluchistan Science and Technology Park (Zahedan, Iran). Larvae of L. vannamei, PL20, were obtained from Azhdar Fish Company (Chabahar, Iran) and adapted to the experimental conditions for 14 days. Healthy shrimps, by their appearance and behavior, were disinfected with 100 ppm formalin for 30 s and kept in 850 L rectangular fiberglass tanks. During the adaptation period, feeding was done by commercial shrimp feed (Faradane Company, Shahrekord, Iran; 40% protein, 9% fat, 4% fiber, 10% ash) at the rate of 10% of body weight four times per day. The amount of daily water change was about 50% and with aeration, the dissolved oxygen level of the water was maintained above 6 mg/L. Salinities 3, 8, 10, and 12 ppt were used in this study based on the salinity levels of the water in Sistan and Baluchistan, Iran. To adjust experimental salinities, sea salt was added to tap water with a salinity of 3 ppt. After adjusting the salinity, 4200 pieces of healthy shrimp with an average initial weight of about 0.2 g were randomly distributed in 12 biofloc tanks (BFT) of 850 L (water volume: 700 L) with a density of 350 pieces of shrimp in each tank. Each treatment was done in three replicates.
During the rearing period, shrimps were manually fed three times a day (8:30, 12:30, and 16:30) with commercial shrimp feed (Faradane Company) at 4%–6% of body weight for 6 weeks. To calculate the daily feed amount, biometry was done every 2 weeks to determine the total weight of the shrimps in each tank. Weekly water change of 1%–3% of the volume of the tank was performed to compensate for evaporation, water sampling, and collection of excess flocs. The method of water change was that before the morning meal, 1%–3% of the water in the tank was removed and the same amount of water with the same salinity was added. During the period of study, intense aeration in the tanks was done continuously to keep the formed flocs in suspension. The lighting conditions were 12 h of light (600 lux) and 12 h of darkness.
For the optimal growth and development of biofloc in rearing tanks, the carbon source needed to adjust the ratio of carbon to nitrogen 12:1 was calculated daily based on the amount of protein in the diet and the amount of food consumed in each experimental treatment [17]. Then, the carbon source (brown sugar and wheat flour in equal proportions) was well dissolved in a plastic container in some of the water of the experimental tanks and after the second meal, was added evenly throughout the surface of the tank water.
Water temperature, dissolved oxygen, pH, and salinity were measured daily using a portable multiparameter meter (Hach model HQ40d). Alkalinity was determined every 3 days by acid titration method [18]. The concentrations of total ammonia nitrogen, nitrite, and nitrate were measured weekly based on standard methods [19] and using a spectrophotometer (Hach Dr2800). To determine the amount of total suspended solids (TSSs), the tank water sample (50 mL) was filtered using Whatman No. 42 filter paper under vacuum pressure. The filter paper containing the suspended material was weighed and dried in an oven at 105°C and weighed again. Then, the difference in the weight of filter paper before and after being placed in the oven and as a result the amount of TSSs was calculated [20]. To measure the number of settled solids or biofloc volume (BFV), 1 L of shrimp tank water was poured into a cone-shaped graduated funnel and kept constant for 30 min until the suspended solids settled. Then, the amount of deposited sediment was measured in milliliters per liter [1]. If it exceeds the permissible limit, the water in the tank is changed and the excess biofloc is removed [1].
2.1. Growth Parameters
2.2. Sampling
Before sampling, shrimps were deprived of food for 24 h. About 10 min before sampling, to reduce stress, the shrimps were placed in a pan of water containing dry ice at a temperature of 4°C and sampling was done. Shrimps were dissected on ice and muscle and intestine samples were collected from 10 shrimps from each tank to check the activity of antioxidant enzymes [14].
2.3. Biochemical Analysis
The proximate body composition of shrimps including moisture, crude protein, crude fat, and ash was measured using standard methods [22].
For measuring digestive enzymes activity, first, frozen intestinal samples were thawed at 4°C, then, homogenized with a ratio of 1:30 (weight to volume) in cold buffer (50 mM mannitol and 2 mM tris chloride at pH 7) and centrifuged at 3300 rpm for 3 min. They were centrifuged at 1000 rpm. Next, the supernatant was separated to measure the activity of digestive enzymes [23]. The activity of lipase was determined through the hydrolysis of p-nitrophenyl myristate as a substrate [24]. Amylase enzyme activity was measured using 0.3% soluble starch as substrate [25]. The activity of the protease was measured using 1% (w/v) casein as a substrate in 0.2 M phosphate buffer at pH 7 [26].
To extract the muscle enzyme extract, shrimp muscle samples were homogenized with a ratio of 1:10 (volume/weight) in 100 mM potassium phosphate buffer, 100 mM potassium chloride, and 1 mM EDTA with a pH of 7.4. Then, the homogenized samples were centrifuged at 10,000 × g for 30 min at 4°C and the supernatant was kept in a freezer at −80°C until the measurement of antioxidant enzymes activity [27].
Superoxide dismutase activity of shrimp muscle was measured by the method [28], and catalase activity by the method [29] using commercial kits from Randox (England). The amount of glutathione (GSH) was determined by reaction with DTNB and absorbance at 412 nm [30]. Total antioxidant capacity (TAC) was measured using a commercial kit (ZellBio, Veltlinerweg, Germany) according to the manufacturer’s method. The malondialdehyde content of shrimp muscle was also measured colorimetrically [31].
2.4. Statistical Analysis
One-way analysis of variance was used to analyze the data (mean ± standard deviation) and Duncan’s test was used to compare the mean of experimental treatments. Before the analysis, the normality and homogeneity of variance of the data were evaluated with the Shapiro–Wilk and Levene tests, respectively. Statistical analyzes were performed with IBM SPSS Statistics software version 23 and the acceptable level of significance in statistical tests was considered as p < 0.05.
3. Results
3.1. Water Quality
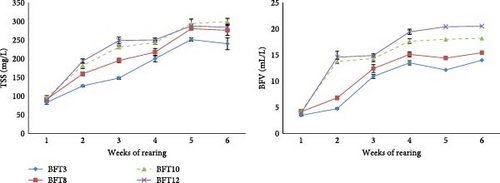
Water quality parameters including water temperature, pH, dissolved oxygen, and alkalinity in four experimental groups during the period of study did not show any significant difference (p > 0.05; Table 1). As seen in Figure 1, in the second and third weeks of the study, with the increase in salinity, the amount of TSSs increased significantly (p < 0.05). In the fourth week, the highest amount of TSSs was observed in the BFT12 and BFT10, while the lowest amount of TSSs was observed in salinity of BFT3 (p < 0.05). In the fifth and sixth weeks, the amount of TSSs in the BFT12, BFT10, and BFT8 was significantly higher than BFT3 (p < 0.05). In the second and third weeks of the study, the highest volume of floc was observed in BFT12 and BFT10 (p < 0.05). In the fourth to sixth weeks, with the increase in salinity, the floc volume increased significantly in the experimental groups (p < 0.05).
| Parameters | BFT3 | BFT8 | BFT10 | BFT12 |
|---|---|---|---|---|
| Temprature (°C) | 27.23 ± 0.68 | 26.93 ± 0.83 | 27.13 ± 0.80 | 26.90 ± 0.81 |
| Salinity (ppt) | 3.03 ± 0.35d | 8.16 ± 0.56c | 10.20 ± 0.75b | 12.13 ± 0.80a |
| pH | 7.95 ± 0.14 | 8.01 ± 0.13 | 7.92 ± 0.10 | 7.68 ± 0.14 |
| Dissolved oxygen (mg/L) | 7.28 ± 0.09 | 7.04 ± 0.39 | 7.08 ± 0.40 | 7.19 ± 0.48 |
| Alkalinity (mg/L CaCo3) | 198.59 ± 16.90 | 204.01 ± 29.21 | 209.01 ± 27.60 | 212.29 ± 6.39 |
- Note: Different letters in each row shows a significant difference.
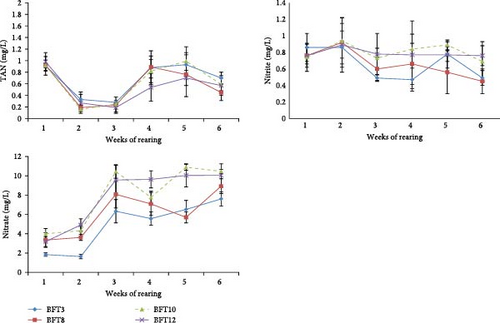
The total ammonia nitrogen and nitrite concentrations in the water were not significantly different between experimental groups at different sampling times (p > 0.05; Figure 2). The water nitrate concentration showed an increasing trend in all experimental groups from the third week of the study. The highest nitrate concentration was observed in BFT12 and BFT10 (p < 0.05).
3.2. Growth Performance
The growth indices including WG and SGR did not show any significant difference in experimental groups (p > 0.05; Table 2). Feed intake, FCR, and PER were not affected by different salinities (p > 0.05).
| Parameters | BFT3 | BFT8 | BFT10 | BFT12 |
|---|---|---|---|---|
| Initial weight (g) | 0.26 ± 0.02 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.01 |
| Final weight (g) | 4.56 ± 0.38 | 4.58 ± 0.70 | 4.76 ± 0.38 | 4.55 ± 0.14 |
| Weight gain (g) | 4.29 ± 0.37 | 4.33 ± 0.70 | 4.51 ± 0.37 | 4.31 ± 0.16 |
| SGR (%/day) | 6.79 ± 0.12 | 6.90 ± 0.38 | 7.08 ± 0.11 | 6.90 ± 0.08 |
| Feed intake (g/shrimp) | 6.42 ± 0.39 | 5.91 ± 0.67 | 5.93 ± 0.65 | 5.85 ± 0.57 |
| FCR | 1.49 ± 0.06 | 1.37 ± 0.12 | 1.31 ± 0.08 | 1.35 ± 0.09 |
| Protein efficiency ratio | 1.67 ± 0.07 | 1.82 ± 0.17 | 1.90 ± 0.12 | 1.84 ± 0.12 |
| Survival (%) | 88.62 ± 1.56 | 87.50 ± 2.78 | 91.81 ± 3.35 | 91.66 ± 2.25 |
3.3. Body Composition
The proximate body composition of shrimp, including moisture, crude fat, crude protein, and ash, did not show any significant difference between different experimental groups as shown in Table 3 (p > 0.05).
| Parameters | BFT3 | BFT8 | BFT10 | BFT12 |
|---|---|---|---|---|
| Moisture (%) | 84.29 ± 1.86 | 82.73 ± 1.29 | 80.76 ± 2.35 | 80.19 ± 1.22 |
| Crude lipid (%) | 4.17 ± 0.15 | 3.85 ± 0.43 | 4.21 ± 0.09 | 4.26 ± 0.19 |
| Crude protein (%) | 13.13 ± 0.37 | 13.42 ± 0.40 | 13.28 ± 0.31 | 13.05 ± 0.35 |
| Ash (%) | 3.85 ± 0.21 | 3.91 ± 0.28 | 4.31 ± 0.49 | 4.41 ± 0.58 |
3.4. Digestive Enzymes Activity
The activity of digestive enzymes had not a significant difference between experimental groups (Table 4; p > 0.05).
| Enzyme | BFT3 | BFT8 | BFT10 | BFT12 |
|---|---|---|---|---|
| Lipase (U/mg protein) | 4.14 ± 0.79 | 3.02 ± 0.28 | 3.17 ± 0.21 | 3.28 ± 0.49 |
| Amylase (U/mg protein) | 28.97 ± 3.46a | 20.72 ± 2.15b | 22.20 ± 1.63b | 20.55 ± 2.21b |
| Protease (U/mg protein) | 13.28 ± 2.19a | 8.02 ± 0.82b | 6.81 ± 1.58b | 6.72 ± 1.88b |
- Note: Different letters in each row shows a significant difference.
3.5. Antioxidant Defense Status
The amount of GSH, malondialdehyde, and catalase activity in muscle showed no significant difference between experimental groups during the period of study (p > 0.05; Table 5). The highest activity of SOD was recorded in BFT10, then, BFT12 (p < 0.05). Also, the TAC of the muscle of L. vannamei in salinities of 10 and 12 ppt was significantly higher than in the two other groups.
| Parameters | BFT3 | BFT8 | BFT10 | BFT12 |
|---|---|---|---|---|
| Glutathione (µmole/mg protein) | 33.78 ± 7.59 | 27.01 ± 3.75 | 26.43 ± 4.78 | 28.69 ± 7.56 |
| Catalase (U/mg protein) | 6.22 ± 1.35 | 5.41 ± 0.24 | 5.95 ± 0.41 | 5.21 ± 1.05 |
| Superoxide dismutase (U/mg protein) | 31.11 ± 1.82c | 26.25 ± 3.39c | 48.50 ± 5.38a | 39.75 ± 2.21b |
| Total antioxidant capacity (µmole/mg protein) | 145.57 ± 6.48b | 139.83 ± 12.32b | 162.08 ± 9.77a | 163.85 ± 8.02a |
| Malondialdehyde (µmole/mg protein) | 1.79 ± 0.66 | 1.33 ± 0.15 | 1.85 ± 0.75 | 1.47 ± 0.30 |
- Note: Different letters in each row shows a significant difference.
4. Discussion
4.1. Water Quality
In the present study, alkalinity was sufficient to promote nitrogen metabolism by floc bacterial communities [1]. An alkalinity level of 150 mg/L calcium carbonate has been suggested for optimal development of biofloc systems [32]. In addition, water quality (including total ammonia, nitrite, and nitrate concentrations) and biofloc (TSSs and BFV) indicators in all groups during the period of study were maintained within the recommended range for L. vannamei [1, 33].
The study found that floc growth and development, indicated by TSSs and BFV, increased across all experimental groups with varying salinities. Additionally, as salinity rose, TSSs significantly increased, with the highest BFVs observed in BFT12 and BFT10. These findings align with previous reports regarding the higher TSSs at elevated salinities [34–36]. These studies have indicated that there is a trend towards increasing suspended particle accumulation and floc size with increasing salinity, although in the study of Esparza-Leal, Amaral Xavier, and Wasielesky [37], this was only evident in the floc volume in salinity 25 ppt. The nitrate concentration in water across all experimental groups consistently increased from the third week of the study, highlighting intensified nitrification processes and nitrifier activity in the biofloc environment [20, 37]. Another reason was the minimal water changes in the tanks. Overall, it was found that heterotrophic bacteria (ammonia absorption) and nitrification in the biofloc system effectively maintained total ammonia nitrogen and nitrite levels within a safe range for L. vannamei across all salinities.
Aquatic animals at low salinity expend more energy to survive [38]. Most likely, in low salinity conditions, nutrients are used by shrimp to maintain their daily metabolism and survival needs rather than growth [38]. In this study, growth indices such as final weight, WG, SGR, and survival rates showed no significant differences among L. vannamei across various experimental groups. Similarly, food intake, FCR, and PER did not differ significantly between experimental groups. It could be concluded that biofloc application could maintain the growth performance, feeding, or survival of L. vannamei at lower salinities. These findings underscore the positive effects of biofloc on L. vannamei performance under suboptimal conditions. Previous studies have also reported the biofloc system’s potential to enhance growth performance and nutritional value in L. vannamei under dense rearing conditions at low salinities [39]. In postlarval Indian shrimp, Fenneropenaeus indicus, the final survival in biofloc system with different salinities (5, 15, 25, 30, and 35 ppt) was significantly higher than the corresponding clear water system [40].
4.2. Body Composition
In the present study, the proximate composition of the body of L. vannamei, including moisture, fat, protein, and ash, did not show a significant difference between the experimental groups. Similarly, Long, Liu, and Lu [14] reported no significant difference in the crude protein or crude fat content of L. vannamei kept in the biofloc system with three different levels of salinity (5, 10, and 15 ppt). Ekasari, Crab, and Verstraete [41] found that the nutritional content of biomass obtained in biofloc systems with different sources of organic carbon and different salinities (0 and 30 ppt) has no effect on crude protein, fat, and n−3 polyunsaturated fatty acids content of floc. It had been showed that salinity had minimal impact on the moisture, protein, and lipid contents of L. vannamei [42].
4.3. Digestive Enzymes Activity
In the current study, the highest activity of digestive amylase and protease activity was seen in the BFT3 group, although the activity of digestive enzyme lipase did not show a significant difference between L. vannamei kept in experimental groups. Among digestive enzymes, amylase and protease play an crucial role in carbohydrate and protein digestion [43]. Amylase activity of L. vannamei in the biofloc system with salinities of 5 and 10 ppt was 141% and 132%, respectively, higher than the biofloc group with salinity of 15 ppt [14], but lipase activity was not significantly different between the groups suggesting that salinity does not affect lipid digestion. In addition, the trypsin activity of the L. vannamei was higher in the biofloc group with the salinity of 5 ppt compared to the biofloc groups with salinities of 10 and 15 ppt [14]. Lower salinity appears to enhance shrimp demand for glucose and amino acids to mitigate salinity-induced stress, as previously showed these substances serve as energy sources to address osmotic challenges [44, 45].
4.4. Antioxidant Defense Status
In the present study, the amount of GSH, malondialdehyde, and muscle catalase activity did not show any significant difference between experimental groups. The highest activity of SOD was observed in BF10. Also, the TAC of the muscle in BFT12 and BFT10 was significantly higher than two other groups. These findings show that biofloc could improve antioxidant status in L. vannamei kept in biofloc system in salinities 12 and 10 ppt compared to 8 and 3 ppt. It has been reported that biofloc system can reduce oxidative stress caused by salinity stress in the Caspian roach (Rutilus caspicus) fry [46]. Some microbial communities in the biofloc system can affect the redox equilibrium in shrimp [47]. Biofloc reduced stress in white shrimp, Litopenaeus setiferus, by stimulating the antioxidant system to maintain redox equilibrium through higher enzyme activity and reduced damage in proteins and lipids of cells [38]. In the present study, the antioxidant capacity of L. vannamei was lower in BFT3 and BFT8 treatments than in other biofloc treatments (higher salinities, 10 and 12 ppt), suggesting that stress can reduce the antioxidant capacity of L. vannamei under low salinity conditions (BFT3 and BFT8 treatments), even though biofloc could promote antioxidant defense. Increase in antioxidant enzymes activity could be considered as an adaptive response to osmotic stress as previously reported [48]. Similarly, Long, Liu, and Lu [14] evaluated the effects of biofloc on the performance of L. vanamei at salinities of 5%, 10%, and 15% and showed that shrimp in the biofloc system with 5 ppt salinity had lower antioxidant and immunity responses.
The study found that heterotrophic and nitrifying bacteria in the biofloc system effectively maintained safe levels of total ammonia nitrogen and nitrite for L. vannamei across salinities of 3, 8, 10, and 12 ppt. Our findings underscore the beneficial effects of biofloc on shrimp performance, even in low-salinity conditions. Overall, the study demonstrates the feasibility of cultivating L. vannamei in low salinity within a biofloc system.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Abbas Alizadeh: data analysis, investigation, methodology. Amir Parviz Salati: preparing drafts, biochemical analysis, supervision. Saeed keyvanshokooh: experimental design, statistical analysis, preparing the final draft. Hamid Mohammadiazarm: investigation, biochemical analysis.
Funding
There is no funding to report.
Acknowledgments
This research was supported by the Khorramshahr University of Marine Science and Technology, Khorramshahr, Iran.
Open Research
Data Availability Statement
The data are available from the corresponding author upon request.




