Comparative Study on the Growth, Proximate, and Amino Acid Profile of Edible Oysters Cultivated in Indoor and Outdoor Conditions
Abstract
A feeding trial was conducted to assess the impact of a microalgal diet and a natural diet on the growth performance, water quality, nutrition, and survival of the edible oyster, Saccostrea cucullata. Four experimental diets, designated as “Chl” (100% Chlorella vulgaris), “Chaeto” (100% Chaetoceros gracilis), “Chl + Chaeto” (50% C. vulgaris + 50% C. gracilis), and “Outdoor” (natural diet), were used to develop the treatments. The growth performance and survival of oysters were evaluated by measuring initial and final weights and counts, alongside daily water quality monitoring, weekly chemical analysis, and nutritional composition analysis of muscle tissue at the end of the experiment. The treatments fed with microalgae showed significantly better growth performance, higher survival, and improved water quality parameters compared to the outdoor treatment (p < 0.05). Multivariate analysis revealed that salinity, total dissolved solid (TDS), conductivity, and dissolved oxygen (DO) positively influenced oyster growth, while temperature, ammonia, and soluble reactive phosphorous (SRP) showed negative associations. Additionally, protein content (29.1%–30.7%) showed no significant difference among treatments, while lipid (11.1%–18.2%) and carbohydrate (30.6%–36.2%) levels varied significantly (p < 0.05), with the highest values in the “Chl + Chaeto” treatment. Moreover, essential amino acids (EAAs) and non-essential amino acids (NEAAs) varied significantly (p < 0.05), with EAAs higher in monoalgal (“Chl” and “Chaeto”) diet, and NEAAs higher in mixed and outdoor treatments. These findings highlight the potential of microalgal diets, particularly monoalgal formulations, in enhancing the nutritional quality and overall performance of cultured S. cucullata.
1. Introduction
Oyster is the most widely cultured shellfish in the world, which has high nutritional and medicinal value. It is rich in protein, polysaccharides, taurine, peptides, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and other bioactive components, which enhance immunity, reduce blood pressure, and exhibit anti-tumor and other health-promoting functions [1]. Most of the edible bivalves, such as oysters and mussels, which have been popular in developed countries for a long time, are also becoming famous in developing countries [2]. Oysters are influenced by various factors such as water temperature, salinity, and feeding conditions [3].
In many commercial farms, spats are imported from hatcheries located in different regions and then acclimated to environmental conditions from those at the source hatchery. The spat is therefore a critical stage for the industry [4]. Because of changes in the marine environment, including pollution, habitat degradation, and rising sea temperatures, oyster aquaculture industries are facing major challenges associated with infectious diseases that impair growth and threaten sustainability. These diseases are exhibiting severe detrimental effects on oyster production [5, 6]. Moreover, disease-related challenges are further compounded by the limited research on the nutritional requirements and formulated feeds for bivalve mollusks, leaving the oyster aquaculture sector less equipped to enhance resilience through dietary strategies [7].
Microalgae are an important biological bait for shellfish providing essential nutrients for the growth of bivalves and enhancing the bivalve’s natural filtering activity, in order to remove microbial contaminants and other pollutants [8]. A precise knowledge of the composition of microalgal species used in indoor or outdoor facilities is essential to provide juvenile bivalves with the proper diet and enhance growth in hard and soft biomass [9]. Microalgal groups such as chlorophyta, chrysophyta, and bacillariophyta were widely used in shellfish seedling and culture [10]. The most widely grown eukaryotic algae adopted for the study, Chlorella vulgaris contains proteins, polysaccharides, carotenoids, some immunostimulators, vitamins, and minerals [11]. Additionally, Chaetoceros gracilis can be found in brackish or marine water, which is utilized as food for the larvae and juveniles of a variety of aquaculture animals, including bivalve mollusks, shrimp, and the zooplanktons that feed fish and shrimp larvae [12].
According to Pagcatipunan [13], Bangladesh’s coastal waters are home to one species of Saccostrea and three species of Crassostrea. Bangladesh has just taken a step in culturing marine fish and shellfish species where other countries have long-established histories of this sector. Oyster farming began in the United States, Europe, China, and Japan several hundred to thousands of years ago though, India started it a few decades ago [13, 14]. Currently, there is no established method or technology for cultivating oysters for commercial use in an aspect of Bangladesh. The experiment was conducted to evaluate and compare the growth performances, survival, proximate and amino acids of edible oyster, Saccostrea cucullata fed with microalgal diet in indoor and natural diets in outdoor conditions.
2. Materials and Methods
2.1. Collection of Microalgae and Oyster
Oysters were collected from Chowfoldondi, Cox’s Bazar, Bangladesh (21° 30′ 41.83″ N, 92° 00′ 32.94″ E). A total of 300 oysters, ~90–100 days old (mean individual weight 31.93 ± 0.14 g) were used to conduct this feeding trial experiment. Two different strains of marine microalgae, C. vulgaris and C. gracilis were introduced in this experiment. Those were collected from the live feed research corner of the Department of Aquaculture, Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh.
2.2. Mass Culture of Microalgae
Microalgal (C. vulgaris and C. gracilis) mass culture was performed in a 15 L transparent plastic cargo box on a large scale. Conway medium was used for mass culture [15]. For the batch culture, at first 20 mL of microalgal stock was combined with 30 mL medium in the individual flask, and the total volume of culture was 50 mL. After that, the culture volume was gradually expanded to, 250, 500 mL, 1 L, and at last scaled up into 15 L in a transparent plastic box. The cultures were shifted to the next batch during the exponential period of development [16]. After reaching the stationary phase, microalgal cells were directly provided to the oyster culture tanks by using a measuring cylinder. Mass cultivation was continued until sufficient biomass of both algae was attained to conduct the feeding trial [17].
2.3. Experimental Design
The feeding trial for the indoor experiment on edible oysters (S. cucullata) was conducted at the hatchery of Coastal Biodiversity, Marine Fisheries and Wildlife Research Centre, Cox’s Bazar, Bangladesh (21° 23′ 48.66″ N, 92° 0′ 12.85″ E) and outdoor culture was performed in a 100 square feet bamboo raft (10 ft × 10 ft) at Nazirartek, Cox’s Bazar, Bangladesh (21° 28′ 4″ N, 91° 56′ 8″ E). The duration of this feeding trial was 13 months.
Indoor culture involved 12 rectangular clear glass aquariums (24″ × 12″ × 24″) to distribute oysters randomly and encompass sea water up to 70 L. The stocking density was 30 spats/tank within 70 L of water. The outdoor culture incorporated rectangular trays designed to hold 30 spats each. After spats stocking, all the trays were hung under the raft with rope. Before being stocked, oysters were acclimated for 2 days in a big tank under hatchery conditions. No external feed was provided during conditioning—only seawater and aeration were provided to keep the oxygen content adequate. Seawater used in the indoor system was sourced from the Bay of Bengal, Cox’s Bazar, Bangladesh, and filtered through a 0.45 µm membrane before use to ensure removal of suspended particles and microbial contaminants. After conditioning, 30 oysters in each tank were stocked according to the experimental design. Four treatments were designed as “Chl”, “Chaeto”, “Chl + Chaeto” and “Outdoor” with 3 replicates in each. At a rate of 5 × 106 microalgal cells/ml, all three feed categories in indoor treatments were given twice a day, at 8 am and 8 pm. Regular siphoning was used to remove waste and leftover feed from the bottom of each aquarium. Every day, 30% of the culture water in each experimental tank was exchanged.
2.4. Water Quality Parameters
During the feeding experiment, the physicochemical parameters, such as dissolved oxygen (DO), pH, temperature, and salinity on a regular basis and chemical parameters following total ammonia nitrogen (TAN), nitrite nitrogen (NO2-N), and soluble reactive phosphorous (SRP), were examined weekly. To measure the water temperature, pH, DO, and salinity in the culture tanks, the multiparameter digital probe Hanna HI98494 (Hanna instrument, Romania) was used. The chemical method of Parsons et al. [18] was used to measure SRP, NO2-N, and TAN. 30 mL of mixed water sample from each tank was taken and filtered with a Whatman GMF Circles 4.7 cm for analysis.
2.5. Determination of Growth Parameters
2.6. Survival
2.7. Determination of Proximate Composition
Proximate analysis was conducted according to the Association of Official Analytical Chemists’ procedure [19, 20] using triplicate samples to determine the percentages of moisture, ash, crude protein, and lipid on a wet weight basis at the end of the experiment. The sample’s moisture content was determined using Laboratory Drying Oven (Model: BINDER, ED 115). The amount of ash was determined using Muffle Furnace (Model: LHMF 100A, LABNICS Equipment). The protein content was calculated using the Micro Kjeldahl method including digestion (DK20/26, VELP Scientific) and distillation system (Model: UDK 129, VELP scientific). The sample’s lipid content was measured with the help of a Soxhlet apparatus (Model: RD 40, Food ALYT). The carbohydrate contents of oyster muscle tissue were determined by chemical method [21].
2.8. Amino Acid Content Determination
The total amino acid composition was determined using a Shimadzu chromatograph LC-10AT vp high performance liquid chromatography (HPLC) equipped with an ion exchange column, quaternary pump, a 20 µL injection valve, and a fluorescence detector. The results were expressed in terms of g amino acid per 100 g of crude protein [22].
2.9. Statistical Analysis
All the obtained data is presented as mean ± Standard deviation (SD). MS Excel, 21 was used to store and organize all data. All analyses were conducted using R (v4.4.2). Percentage data were transformed using the arcsine square root method. The Shapiro–Wilk test was used to check for normality, and Levene’s test assessed the homogeneity of variances. One-way ANOVA was conducted to confirm the significance (p < 0.05) of data obtained through the experiment, followed by Tukey’s multiple comparison tests. Correlation analysis was performed to explore associations among variables, and principal component analysis (PCA) was conducted, focusing on the first two principal components.
3. Results
3.1. Growth Performance
Growth parameter data of weight increment (WI), SGR, RGR, and SR of edible oysters are depicted in Table 1. It was reported that RGR and SGR of length, length increment, and live weight gain exhibited significant (p < 0.05) differences in all treatments. No significant (p > 0.05) difference was found in RGR and SGR of weight among the treatments. “Chl + Chaeto” demonstrated the highest survivability (76.7% ± 3.33%) while, the “Outdoor” group exhibited the lowest (63.3% ± 8.82%).
| Parameters | Chl | Chaeto | Chl + Chaeto | Outdoor |
|---|---|---|---|---|
| RGR of weight (%) | 107.0 ± 13.0a | 81.9 ± 24.0a | 84.0 ± 15.8a | 118.0 ± 2.86a |
| SGR of weight (%/d) | 0.24 ± 0.02a | 0.198 ± 0.04a | 0.202 ± 0.03a | 0.260 ± 0.004a |
| RGR of SL (%) | 52.5 ± 3.20a | 45.5 ± 4.59a | 44.8 ± 2.69a | 33.2 ± 0.02b |
| SGR of SL (%/d) | 0.14 ± 0.007a | 0.13 ± 0.01a | 0.12 ± 0.006a | 0.096 ± 0.01b |
| Length increment (mm) | 25.7 ± 1.79a | 23.1 ± 1.84a | 23.9 ± 1.92a | 17.6 ± 0.09b |
| Live weight gain (g) | 30.6 ± 1.88b | 24.4 ± 4.18b | 27.9 ± 4.64b | 41.1 ± 2.85a |
| Survival (%) | 75.6 ± 1.92a | 70.0 ± 3.33b | 76.7 ± 3.33a | 63.3 ± 8.82c |
- Note: Mean ± SD along with different small lowercase letters of growth parameters within each column are statistically significant (p < 0.05).
- Abbreviations: RGR, relative growth rate; SGR, specific growth rate; SL, shell length.
3.2. Physico-Chemical Factor and Growth Performance of Edible Oyster
Table 2 describes the physical and chemical parameters of the study. There was no significant (p > 0.05) difference observed in the indoor condition fed with microalgae diets. However, significant (p < 0.05) differences were found between indoor and outdoor conditions in terms of pH, salinity, total dissolved solid (TDS), and conductivity, whereas temperature and DO did not reveal any significant variation (p > 0.05). Values of TAN, NO2-N, and SRP of different treatments exhibited no significant (p > 0.05) alteration.
| Water quality | Chl | Chaeto | Chl + Chaeto | Outdoor |
|---|---|---|---|---|
| Physical parameter | ||||
| Temperature (°C) | 29.2 ± 1.14a | 29.4 ± 1.52a | 29.2 ± 1.54a | 28.3 ± 4.65a |
| Salinity (ppt) | 25.6 ± 2.19a | 26.2 ± 1.93a | 25.7 ± 2.22a | 22.3 ± 4.28b |
| TDS (mg/L) | 19.7 ± 1.31a | 20.1 ± 1.28a | 19.9 ± 1.55a | 17.2 ± 2.46b |
| Conductivity (S/m) | 39.4 ± 2.63a | 40.3 ± 2.57a | 39.7 ± 3.09a | 34.3 ± 4.91b |
| Chemical parameter | ||||
| pH | 8.26 ± 0.07a | 8.29 ± 0.06a | 8.29 ± 0.10a | 8.05 ± 0.29b |
| DO (mg/L) | 5.04 ± 0.66a | 4.95 ± 0.66a | 4.99 ± 0.62a | 4.42 ± 1.28a |
| TAN (mg/L) | 0.03 ± 0.04a | 0.03 ± 0.04a | 0.03 ± 0.04a | 0.01 ± 0.01a |
| NO2-N (mg/L) | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.04 ± 0.03a | 0.11 ± 0.22a |
| SRP (mg/L) | 0.26 ± 0.17a | 0.26 ± 0.19a | 0.33 ± 0.22a | 0.13 ± 0.13a |
- Note: Mean ± SD along with different small lowercase letters within the same column are statistically not significant (p > 0.05).
- Abbreviations: DO, dissolved oxygen; NO2-N, nitrite nitrogen; SRP, soluble reactive phosphorous; TAN, total ammonia nitrogen; TDS, total dissolved solid.
The correlation matrix revealed significant positive relationships among several physicochemical parameters, notably between salinity, TDS, and conductivity (r > 0.85). Temperature showed a strong negative correlation with DO (r = −0.65). Growth parameters (average growth rate (%) of length [AGRL] and specific growth rate of weight [SGRW]) showed moderate correlations with water quality factors including TAN and SRP (Figure 1).
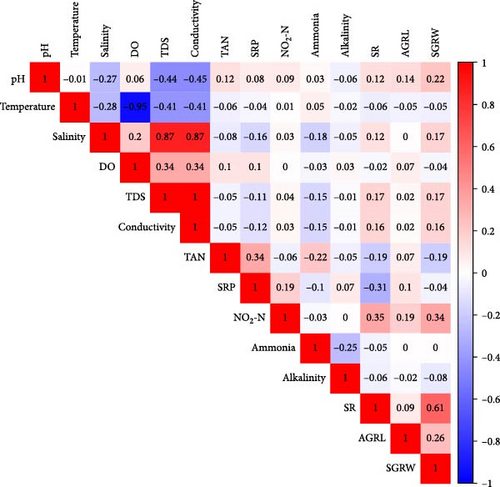
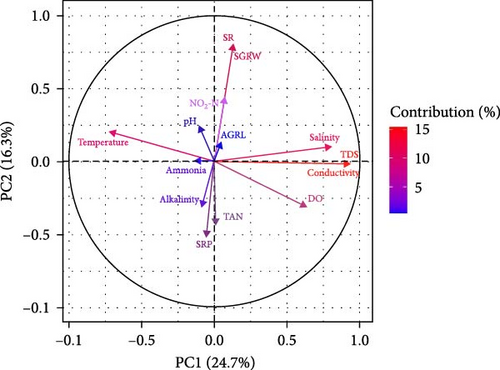
The interrelationship among water quality parameters, growth parameters, and survival of S. cucullata was measured through correlation analysis. A multivariate PCA was performed to understand the linkage among water quality parameters, growth rates, and survival of edible oysters during the culture period (Figure 2). The PCA revealed that the PC1 and PC2 accounted for 41% of the variability in the data (Figure 2). PC1 (explaining 24.7% of the variance) was linked to SGRW by AGRL and was positively correlated with salinity, TDS, conductivity, and DO, while negatively correlated with temperature, pH, ammonia, alkalinity, SRP, and TAN. The PCA findings further indicated that high levels of NO2-N, salinity, DO, TDS, and conductivity positively affected average and SGR.
3.3. Nutritional Composition of Edible Oyster
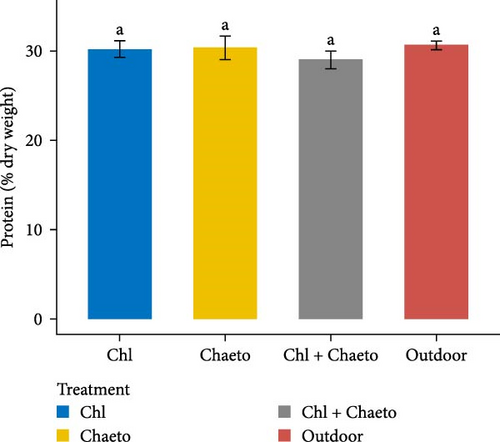
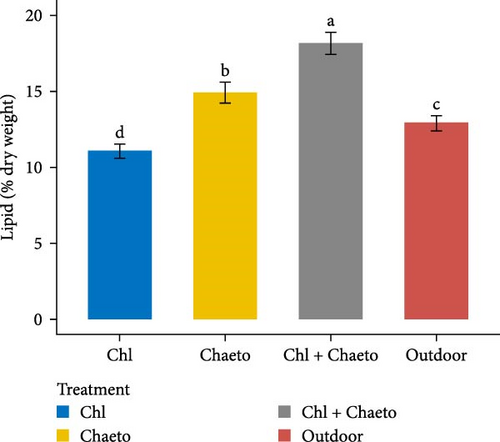
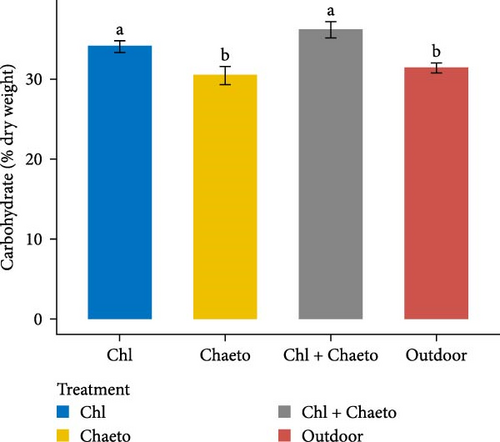
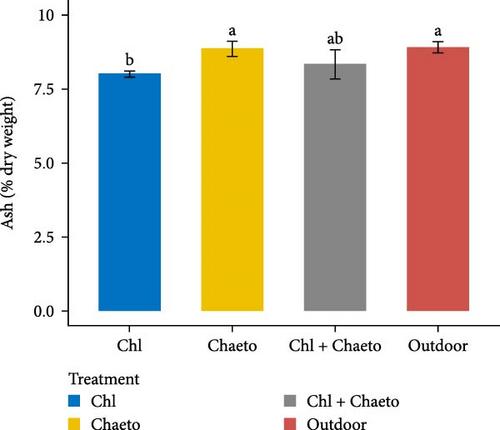
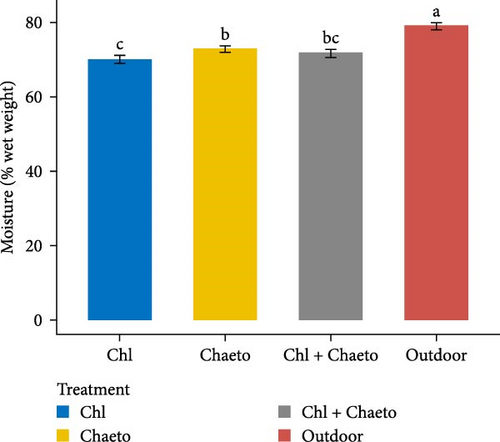
The observed values for protein, lipid, carbohydrate, ash, and moisture ranged from 29.1% to 30.7% (Figure 3A), 11.1% to 18.2% (Figure 3B), 30.6% to 36.2% (Figure 3C), 8.02% to 8.92% (Figure 3D), and 70.1% to 79.2% (Figure 3E) respectively. No significant (p < 0.05) difference was found in protein content among the treatments. On the contrary, carbohydrate, lipid, ash, and moisture showed significant (p < 0.05) differences in all treatments. The highest carbohydrate and lipid were observed in “Chl + Chaeto” treatment compared to other treatments. Similarly, ash (8.92% ± 0.19%) and moisture (79.2% ± 0.98%) content were higher in outdoor treatment.
3.4. Amino Acid Content of Oyster
The amino acid content of cultured oysters is described in Table 3. Essential amino acids (EAAs) and nonessential amino acids (NEAAs) showed significant differences among the treatments (p < 0.05). The EAAs were higher in “Chl” and “Chaeto” treatments compared to mixed and outdoor treatments. NEAAs of mixed (Chl + Chaeto) and outdoor treatment were higher than single microalgae-fed treatment. The amino acid content of oysters is dominated by aspartic acid (9.77% in “Chaeto” to 10.7% in “Chl + Chaeto” and “Outdoor”) and glutamic acid (13.5% in “Chaeto” to 14.7% in “Outdoor”).
| Amino acid (%) | Chl | Chaeto | Chl + Chaeto | Outdoor |
|---|---|---|---|---|
| Aspartate | 10.2 ± 0.17a | 9.77 ± 0.41a | 10.7 ± 1.01a | 10.7 ± 0.21a |
| Threonine | 4.94 ± 0.15a | 4.89 ± 0.20a | 4.97 ± 0.35a | 4.97 ± 0.04a |
| Serine | 5.62 ± 0.29a | 5.59 ± 0.22a | 5.81 ± 0.01a | 6.03 ± 0.19a |
| Glutamine | 14.0 ± 0.19a | 13.5 ± 0.85a | 14.2 ± 0.24a | 14.7 ± 0.48a |
| Glycine | 6.83 ± 0.20a | 6.52 ± 0.07a | 6.40 ± 0.01a | 6.37 ± 0.25a |
| Alanine | 6.85 ± 0.04a | 6.87 ± 0.05a | 6.66 ± 0.03a | 6.53 ± 0.05a |
| Cystine | 4.16 ± 0.56c | 4.37 ± 0.12bc | 4.91 ± 0.33ab | 5.25 ± 0.33a |
| Valine | 3.29 ± 0.18a | 3.30 ± 0.07a | 2.97 ± 0.33a | 3.08 ± 0.18a |
| Methionine | 2.32 ± 0.09b | 2.44 ± 0.17a | 1.99 ± 0.22d | 2.13 ± 0.13c |
| Isoleucine | 2.62 ± 0.23a | 2.75 ± 0.24a | 2.68 ± 0.07a | 2.78 ± 0.06a |
| Leucine | 6.46 ± 0.05a | 6.46 ± 0.03a | 6.62 ± 0.58a | 6.62 ± 0.34a |
| Tyrosine | 3.92 ± 0.03a | 3.95 ± 0.28a | 3.98 ± 0.39a | 4.03 ± 0.08a |
| Phenylalanine | 2.40 ± 0.02b | 2.91 ± 0.18a | 2.74 ± 0.26a | 2.86 ± 0.29a |
| Histidine | 5.69 ± 0.05a | 5.22 ± 0.41b | 4.55 ± 0.36c | 4.45 ± 0.38c |
| Lysine | 8.37 ± 0.34ab | 8.75 ± 0.12a | 8.57 ± 0.05b | 7.90 ± 0.34c |
| Arginine | 7.5 ± 0.22a | 7.66 ± 0.10a | 7.54 ± 0.03a | 6.46 ± 0.27b |
| Proline | 4.81 ± 0.02b | 5.03 ± 0.13a | 4.73 ± 0.26ab | 5.09 ± 0.11a |
| ∑EAA | 36.1 ± 0.29a | 36.7 ± 0.26a | 35.1 ± 0.54b | 34.8 ± 0.25b |
| ∑NEAA | 63.9 ± 0.29b | 63.3 ± 0.26c | 64.9 ± 0.54a | 65.2 ± 0.25a |
- Note: Values are shown as (Mean ± SD). Different lowercase superscript letters indicate significant variation (p < 0.05).
- Abbreviations: EAA, essential amino acid; NEAA, nonessential amino acid.
4. Discussion
This study was conducted to assess the growth performances, survivability, water quality, and nutritional profile of S. cucullata fed with monoalgal (C. vulgaris and C. gracilis), mixed microalgal diet, and natural (outdoor) diet. The hypothesis was that the use of microalgal diets, especially monoalgal or mixed ones, would result in higher growth, better water quality, and enriched nutritional profiles than those obtained with natural feeding in outdoors. The results from this experiment support this hypothesis: indoor-reared oysters fed microalgal diets showed significantly better growth and survival as well as better water quality parameters than oysters reared outdoors with natural diet.
In this current research, the growth performances and survivability showed significant (p < 0.05) differences between the indoor and the outdoor treatments. Similarly, compared to outdoor conditions, various combinations of Cheatoceros sp., Isochrysis galbana, and Tetraselmis chuii resulted in increase of length and height in Caribbean pearl oyster Pinctada imbricata spat [9]. It could have happened because of the variations in the nutritional value of the food in both indoor and outdoor water conditions.
Survival rate of oysters fed on “Chaeto”, and “Chl + Chaeto” mixed diet varied significantly among the treatments (p < 0.05). High survivability of the oysters was achieved due to presence of some bioactive compounds in C. vulgaris, and C. gracilis such as carotenoids, vitamin E, terpenes, and phenolic complexes comprising anthelmintic, cytostatic, antioxidant, antifungal, antiviral, and antibacterial actions, which were absent in seawater and thus, helped to boost the immunity of oysters when fed diet containing microalgae [23]. The findings of this study are supported by previous observations reporting the nutritional advantages of using combined diets compared with unialgal diets, especially if the diet contains the diatom C. gracilis for growing juveniles of the European flat oyster Ostrea edulis [24], and Crassostrea corteziensis [25]. The inclusion of diatoms is beneficial as their silica-rich frustules and high levels of essential fatty acids like EPA, which are essential for bivalve development and immune competence [26]. Similarly, Chlorella sp., high in antioxidants, anti-inflammatory agents, EAAs, and polyunsaturated fatty acids (PUFAs), plays a role in immune function and membrane formation, contributing to overall health in bivalves [27]. Therefore, the combination of the two beneficial microalgal diets in the present study enhanced the SR of oysters.
Oyster culture performance is heavily impacted by some factors such as stocking density, feed quality, immersion time, and water depth as well as water quality [28]. The TAN levels observed in this study align with the optimal range for edible oyster culture reported by Caldini et al. [29]. Furthermore, NO2-N concentration also varied significantly among all the treatments, which was lower than the optimum concentration recommended for aquaculture [30]. In indoor oyster culture, the primary sources of TAN, NO2-N, and SRP are the feed provided, uneaten feed, and oyster-derived feces. Microalgae, an alternative feed ingredient, improves culture water quality due to their water-stabilizing properties. Unlike conventional feeds, uneaten microalgae can survive in the rearing environment without degrading water quality, as they retain essential nutrients like carbon, phosphorus, and nitrogen, preventing eutrophication [7].
The multivariate analysis, including PCA and correlation matrices, provided a comprehensive understanding of the complex interactions among water quality parameters and oyster growth performance. PCA effectively reduced data dimensionality, revealing clear groupings of variables that influenced growth outcomes. Notably, growth parameters (SGR and AGRL) clustered with salinity, DO, TDS, and electrical conductivity, indicating their synergistic role in enhancing growth. Conversely, variables such as ammonia, TAN, and SRP formed distinct groupings, negatively impacting growth. This multivariate approach considered the environmental variables that are important for the oyster growth performance in the indoor system.
According to this study, the “Outdoor” treatment had the highest levels of protein, ash, and moisture, while the “Chl + Chaeto” treatment had the highest levels of fat and carbohydrates. Earlier studies suggested that feeding mixed microalgae is generally preferred over feeding only one species [31]. The scallop Nodipecten subnodosus [9], and the oyster C. corteziensis [32, 33] showed a similar outcome. Some species of Tetraselmis sp. contributed a high nutritional value when included in a binary diet with other Chaetoceros spp., like C. calcitrans or C. muelleri, which resulted in high growth rates [32]. According to Glem et al. [34], the protein levels of the spat given the ternary diet of Chaetoceros-A, Tetraselmis chui, I. galbana were much greater in an indoor laboratory atmosphere compared to the outdoor environment. This result contradicts the findings of the present study. In the indoor environment, natural feed (microalgae) and micronutrients were unavailable due to filtration and UV treatment of seawater, whereas natural environments have higher levels of nutrient enrichment, feed biodiversity, and mineral availability [35].
In the case of the amino acid content of oysters, EAA was found to be higher in single-species diet, but NEAA was found to be greater in a mixed algal diet and outdoor conditions. Indoor culture was typically done in a controlled environment, which allows for stable conditions, such as optimal light, temperature, and nutrient levels. These controlled settings enable single-species diet to prioritize protein synthesis, particularly EAAs [36]. In the outdoors, ecological parameters are not controlled and experience fluctuation in temperature, salinity, light intensity, and nutrient availability, which can alter the level of EAA.
The research highlights the benefits of controlled indoor cultivation of oysters using microalgal diet to improve SRs, nutritional profile, and maintain a consistent yield in comparison with outdoor culture. Overall, the combination of multivariate techniques, PCA, and correlation studies provided an in-depth understanding of the interactions between environmental factors and their effect on the growth performance. These results provide valuable perspectives for refining cultivation settings to improve the quality and stability of the indoor oyster aquaculture.
5. Conclusion
Microalgae species such as C. vulgaris and C. gracilis were deemed promising in terms of growth, water quality, and nutritional composition. Oyster growth, survival, and nutritional makeup are mostly influenced by the diet. The microalgal diet has a good effect on the percentage of protein as well as the deposition of fat and carbohydrates. This study showed evidence of two prospective microalgae that can be used as live feed for edible oyster S. cucullata species, taking into account the positive effects on water quality, SR, and nutritional profile. Future research should include a wider range of microalgal diets to more reliably assess the impact on oyster growth, survival, and tissue composition. In addition, long-term physiological and reproductive impacts of diverse microalgal diets on oyster species under controlled conditions should be investigated. This expanded approach would enhance our understanding of how microalgae influence these key parameters in oysters.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Mohammad Ekramul Haque: Conceptualization, Data Curation, Data Processing, Formal Analysis, Writing-Original Draft, Validation, Revising and Editing. Mahima Ranjan Acharjee: Conceptualization, Data Curation, Data Processing, Formal Analysis, Writing-Original Draft, Validation, Revising and Editing. Subeda Newase: Writing-Original Draft, Revising and Editing. Sifatun Nur, Trina Das, and Sadia Afrin: Data Processing, Revising and Editing. Tashrif Mahmud Minhaz and Zahidul Islam: Validation, Writing-Review & Editing. Helena Khatoon: Conceptualization, Funding Acquisition, Supervision, Resources, Validation, Writing-Review & Editing.
Funding
This study was supported by the Sustainable Coastal and Marine Fisheries Project (SCMFP), Department of Fisheries, Bangladesh Fisheries Matching Grant Facility (BFMGF), WINDOW 1. Project ID: L2W1-04 and Chattogram Veterinary and Animal Sciences University grants through University Grant Commission of Bangladesh.
Acknowledgments
This study was supported by the Sustainable Coastal and Marine Fisheries Project (SCMFP), Department of Fisheries, Bangladesh Fisheries Matching Grant Facility (BFMGF), WINDOW 1. Project ID: L2W1-04 and Chattogram Veterinary and Animal Sciences University grants through University Grant Commission of Bangladesh.
Open Research
Data Availability Statement
Data will be made available on request.




