Utilization of Algal Turf Scrubber Biomass in Sea Urchin (Lytechinus variegatus) Diets
Abstract
This study evaluated the growth indices in sea urchins fed varying levels of algal turf scrubber (ATS) biomass for the partial replacement of dietary menhaden fishmeal. Juvenile Lytechinus variegatus were fed four formulated diets with differentiating levels of ATS biomass (10%, 10%, 20%, and 50%). Biomass was harvested from two ATS systems, one receiving treated wastewater effluent and the other incorporated into a hydroponics system. A 12-week growth trial was conducted, and each sea urchin (n = 100) was weighed and fed daily. Diets were formulated using Agri-Data Systems Pro 5 (Version 2.41, Agri-Data Systems). Four isonitrogenous and isocaloric diets were prepared. At the end of the growth trial, no significant differences were found in final weight, final diameter, and survival across all diets. All treatments performed well based on condition level and higher levels of ATS biomass did not indicate reduced growth or dietary utilization. Final weights of dry test, and dry gut, were not significantly different between treatments, except for dry gonad compared with menhaden fish meal. These findings indicate that in sea urchin diets, partial replacement of fish meal with ATS biomass is well tolerated.
1. Introduction
The sea urchin, Lytechinus variegatus, stands as a prominent inhabitant along the eastern coast of the United States, extending its range from North Carolina to the northern coast of Brazil, with a significant presence in the Gulf of Mexico [1]. Recognized as one of the most widely studied model systems, these organisms have also found application in aquaculture production, as their global appeal as a culinary delicacy has depleted wild stocks in many areas. In 1995, an estimated 120,000 metric tons in whole weight of sea urchins were harvested with the majority of consumption in Chile, Japan, and the United States [2].
L. variegatus predominantly occupies seagrass meadows and often resides on hard sites covered with algae, rock, or sand. L. variegatus also remains in regions that have elevated tidal currents and densities vary widely between habitats [3]. Factors including salinity, temperature, wave activity, and phenology can heavily impact densities [4]. Shallow waters, areas with low oxygen concentration, and pollutants such as copper and phosphates can also potentially influence densities as they cause mass mortalities and impact sea urchin maturation [5]. Across multiple studies, densities have been recorded ranging from 0.4 individuals per square meter to 636 individuals per square meter[5, 6].
Typical food sources for the sea urchin include seagrasses such as Thalassia testudinum, Syringodium filiforme, Cymodotus, and organic material in the sand [7]. Predators of the sea urchin include coastal birds, such as herring gulls. These birds have been seen dropping the sea urchins onto rock surfaces to open them and consume their roe [5]. Wading birds, including the Louisiana heron, little blue heron, and common egret, have also been seen preying on L. variegatus. Other predators of L. variegatus include fish, gastropods, and crustaceans [8]. Contact with toxic algae or consumption of specific fungi can lead to L. variegatus death [5]. Wild populations of L. variegatus play an essential role in their habitat [5]. The sea urchins’ feces and ammonium contribute valuable nutrients to the environment and function as meals for detritivores. Gametes from these organisms also function as food for fish [9].
L. variegatus possesses considerable nutritional value, as studies have shown that their mass can comprise up to 57.8% protein, 9.36% fat, and 1.86% ash content. Most of the fat content comprises polyunsaturated fat and omega-3 fatty acids [10], which can result in reduced cholesterol and blood pressure levels in humans. The inclusion of sea urchins in diets has also been demonstrated to provide significant medical and human health benefits [11]. The embryos and sea urchin roe have previously exhibited anticancer, antioxidant, antimicrobial, anti-inflammation, and antihemolytic properties [12]. However, the thriving demand for sea urchin roe has ushered in challenges, particularly a decline in production attributed to the escalating demand and the overexploitation of sea urchin stocks globally [2]. The pressures on natural sea urchin populations have prompted a critical examination of sustainable alternatives to meet this culinary demand.
Sea urchin aquaculture has increased as a potential solution to counteract demand and elevated stress on wild populations. Cultured sea urchins require daily nutrition which typically contains a feed that utilizes fish meal as a source of dietary protein [13]. Fish meal is not a sustainable protein source and efforts have been underway to reduce its use in aquaculture [14]. The algal turf scrubber (ATS) may provide a promising biomass source to help alleviate the use of fish meal in sea urchin diets. Functioning on the principle of utilizing attached algae to efficiently remove inorganic nutrients from water, the ATS not only aids in water quality management but also produces harvestable algal biomass [15]. The core mechanism of the ATS system involves the cultivation of attached algae on screens within a shallow raceway. Nutrient-laden water is gently pumped onto these screens, allowing the algal community to perform photosynthesis and, in the process, uptake inorganic compounds [15]. These algae function as purification agents, removing nutrients through biological uptake and assimilating them during biomass production [16]. At the raceway’s terminus, the now-cleaner water is released back into the waterway, bearing a significantly reduced nutrient load compared to when it first entered the system [15]. Moreover, the rapid growth rate of algae within the ATS facilitates the swift removal of nutrients, underlining the importance of timely harvesting. Despite these advantages, the challenge lies in the high ash content typically associated with ATS biomass, rendering its integration into fish and crustacean aquaculture feeds problematic.
This study evaluated the growth indices in green sea urchins (L. variegatus) fed diets containing varying levels of ATS biomass for the partial replacement of dietary menhaden fish meal. The unique dietary requirements of L. variegatus, characterized by a naturally high ash content, present an intriguing opportunity to explore the potential of incorporating ATS biomass into their diets [17]. The present study aims to evaluate the feasibility of utilizing harvested biomass from two distinct ATS systems—one receiving treated wastewater effluent and the other integrated into a hydroponics system as a replacement for fishmeal and ash in nutritionally complete diets for L. variegatus. The successful incorporation of ATS biomass into L. variegatus diets not only addresses the challenges posed by the high ash content but also contributes to the sustainability of aquaculture feeds.
2. Materials and Methods
2.1. Experimental Diet Formulation, Preparation, and Analysis
Diets were formulated using Agri-Data Systems Pro 5 (Version 2.41, Agri-Data Systems). Prior to formulation, all dietary ingredients to be used in the experimental diets were analyzed for protein [18], lipid [19], fiber [20], and ash [19]. For the growth trial, five isonitrogenous and isocaloric diets were prepared: control diet (22% protein, 5% lipid, as-fed) and four diets (22% protein, 5% lipid, as-fed) containing 10% replacement of Special Select menhaden fish meal with algal turf biomass grown on an ATS receiving treated wastewater and 10%, 20% and 50% replacement of Special Select menhaden fish meal with algal turf biomass grown on an ATS receiving aquaponics system water (Table 1). Proximate composition of the ATS biomass was determined prior to formulation for protein [18], lipid [19], fiber [20], and ash [19] (Table 2). Diets were produced through cold extrusion using a commercial mixer (KitchenAid, Benton Harbor, MI) fitted with a meat chopper attachment. The dry ingredients were placed within a food mixer and mixed until homogenous. Once homogenous, alginate, and deionized water were mixed with a hand mixer for 45 s and added to the dry ingredients until the correct consistency for extrusion had been achieved. A meat chopper attachment fitted with a 3 mm die was used to extrude the mixture into pellets, which were then air dried until a moisture content of 8%–10% was reached. Once dried, the pellets were ground into a 2–4 mm pellet and stored at -20°C until needed.
Ingredient (g kg−1) |
Control | Fish meal replacement level | |||
|---|---|---|---|---|---|
| 10%b | 10%c | 20%c | 50%c | ||
| Alginated | 10 | 10 | 10 | 10 | 10 |
| Cellulosee | 40 | 34.84 | 36.24 | 32.49 | 21.24 |
| Diatomaceous Earthf | 233.08 | 140.68 | 213.17 | 193.22 | 133 |
| Fish mealg | 344.72 | 308.45 | 308.45 | 274.18 | 171.36 |
| Fish oilh | 17.3 | 19.09 | 16.42 | 15.54 | 12.9 |
| Vitamin premixi | 15 | 15 | 15 | 15 | 15 |
| Mineral premixj | 10 | 10 | 10 | 10 | 10 |
| Wheat starchk | 330.9 | 331.23 | 316.04 | 301.23 | 257.13 |
| Vitamin Cl | 1 | 1 | 1 | 1 | 1 |
| ATS biomassm | 0 | 129.71 | 0 | 0 | 0 |
| ATS biomassn | 0 | 0 | 73.68 | 143.75 | 368.37 |
- aDiets contained 22% crude protein, 5% crude lipid, 4.56% fiber, and a P:E ratio of 70 on as-fed basis, calculated using Agri-Data Systems Pro 5 (Version 2.41, Agri-Data Systems).
- bAn additional diet was evaluated at 20% replacement with WWTP ATS biomass; however, upon proximate analysis, it was revealed that the diet was incorrectly manufactured and removed from analysis.
- cAlgal turf scrubber biomass produced using aquaponics system water.
- dSigma–Aldrich, St. Louis, MO.
- eSigma–Aldrich, St. Louis, MO.
- fP.F. Harris Manufacturing Company, Cartersville, GA.
- gOmega Protein Corporation, Houston, Texas.
- hSigma–Aldrich, St. Louis, MO.
- iVitamin premix contains on a per kilogram basis: vitamin A (retinol) 650,000 IU, vitamin D (cholecalciferol) 500,000 IU, vitamin E (tocopherol) 25,000 mg, vitamin K (menadione) 2500 mg, vitamin B1 (thiamine) 5000 mg, vitamin B2 (riboflavin) 10,000 mg, vitamin B6 (pyridoxine) 20,000 mg, niacin 12,500 mg, pantothenic acid 12,500 mg, biotin 187 mg, folic acid 5000 mg, and vitamin B12 (cyanobalamine) 37.5 mg.
- jMineral premix contains: calcium 14.73%, phosphorous 11.39%, sodium 2.91%, potassium 10.76%, magnesium 11.45%, iron 994 ppm, zinc 876 ppm, 1678 ppm, copper 667 ppm, selenium 4 ppm, chloride 101 ppm, and iodine 8 ppm.
- kSadaf Foods, Los Angeles, CA.
- lSigma–Aldrich, St. Louis, MO.
- mAlgal turf scrubber biomass produced using treated wastewater effluent.
- nAlgal turf scrubber biomass produced using aquaponics system water.
| Biomass | Protein (%) | Lipid (%) | Fiber (%) | Ash (%) |
|---|---|---|---|---|
| ATS biomassa | 16.5 | 1.07 | 4.4 | 66.0 |
| ATS biomassb | 32.5 | 5.9 | 6.0 | 43.1 |
- Abbreviation: ATS, algal turf scrubber.
- aAlgal turf scrubber biomass produced using treated wastewater effluent.
- bAlgal turf scrubber biomass produced using aquaponics system water.
2.2. Experimental Setup
The experimental setup consisted of 30, 60.6-L polyethylene tanks. Each tank contained four mesh cages (7.5 cm diameter × 30 cm height) comprised of 3 mm polypropylene open mesh secured by plastic cable ties. System tanks were connected to a recirculating system with a recirculation rate of 1.9 lpm per tank. This recirculating system consisted of one high-rate sand filter (Pentair Aquatic Ecosystems, Apopka, FL) filled with 0.085 m3 of Perma Beads (Perma Beads, Vista, CA), a 40-W UV sterilizer (Pentair Aquatic Ecosystems, Apopka, FL), a biological filter filled with 20 L of Kaldness k1 media, and a 651-L sump. System water temperature was maintained at 28±1°C using a chiller (Aqua Logic, San Diego, CA) and submersible heater (Hygger, Shanghai, China). Temperature, pH, dissolved oxygen, turbidity, and ORP were measured daily with a Horiba multiprobe meter (Horiba, Kyoto, Japan), while ammonia (Nitrogen, Ammonia; Method 8155), nitrite (USEPA Diazotization Method; Method 8507), and nitrate (Cadmium reduction method, Method 8039) were measured weekly using a Hach 3900 spectrophotometer. The collected water quality parameters fell within an acceptable range for optimal sea urchin growth [21]. A 12:12 photoperiod was used to maintain diurnal regularity and normal feeding behavior for the sea urchins.
2.3. Feeding Growth Trial
Juvenile sea urchins (L. variegatus), weighing ~23 g, were collected from Port St. Joe, Florida, and were allowed to acclimate to the system for 3 weeks before trials began. Sea urchins were fed our control diet (crude protein 22%, crude lipid 5%) during the acclimation period. Prior to the start of the experiment, five urchins were randomly selected, and stored frozen at −82°C for later analysis. Groups of four sea urchins (one per mesh cage, four cages per tank) with an average initial weight of 23.97 g were assigned randomly to each of 30 tanks. Prior to the start of the trial a one-way ANOVA was performed to verify no significant differences existed between initial treatment weights.
Each diet was randomly assigned to a set of five tanks and fed once daily by hand at a rate approaching satiation with preweighed rations. The daily feed ration was 3% of total body weight per tank per day. Any uneaten food and feces were removed daily before the next feeding.
2.4. Evaluation Parameters
After 12 weeks, all sea urchins were individually weighed and photographed. For each tank, percent weight gain ([(final weight, g − initial weight, g)/initial weight, g] × 100) and survival ([final number of organisms/initial number of organisms] ×100) were calculated. Sea urchins were individually dissected to determine average gonad, gut, lantern, and test weight for each tank. Test diameter was determined for each urchin using Image J (NIH, 1.54 h, 2023) to determine average test diameter per tank.
2.5. Statistical Analysis
Before statistical analysis was done, normality and homogeneity of variance were tested. Initial diameter was log10 transformed prior to ANOVA. Initial weight, final weight, and final diameter were analyzed using the Kruskal–Wallis nonparametric test. All other analyzed data met the normality and homogeneity test and were analyzed using a one-way ANOVA. A Tukey’s HSD test was used as a mean separation procedure when a significant difference was observed (ANOVA p value < 0.05). Outliers were determined through quartile fences. All statistical analyses were performed using SPSS version 29.0.1.1.
3. Results
3.1. Feeding Trial
After 12 weeks, there were no significant differences between the final weights of the different treatments (p value = 0.697). Survival was 100% for all diets (Table 3).
| Control | 10% FMR WWTPa | 10% FMR aPb | 20% FMR aPb | 50% FMR aPb | |
|---|---|---|---|---|---|
| Initial weight (g) | 23.99 ± 3.20 | 24.02 ± 2.77 | 23.01 ± 2.083.01 | 24.14± | 24.36 ± 3.20 |
| Initial diameter (mm) | 53.74 ± 3.88 | 52.71 ± 3.97 | 52.44 ± 3.64 | 51.92 ± 3.45 | 53.69 ± 3.18 |
| Final weight (g) | 29.11 ± 5.60 | 31.90 ± 7.90 | 34.13 ± 6.85 | 31.2 ± 5.92 | 33.95 ± 6.35 |
| Final diameter (mm) | 66.7 ± 4.02 | 68.02 ± 4.15 | 66.40 ± 14.34 | 68.36 ± 4.72 | 71.54 ± 4.75 |
| Protein efficiency ratio (PER) | 1.95 | 3.00 | 4.24 | 2.69 | 3.65 |
| Gonadal somatic Index | 19.18 ± 4.62 | 17.73 ± 5.57 | 16.73 ± 4.38 | 61.88 ± 5.55 | 16.89 ± 3.58 |
| Survival rate % | 100 | 100 | 100 | 100 | 100 |
| Meana ± STD (g) | — | — | — | — | — |
- aATS biomass composite, Statesboro Wastewater Treatment Plant.
- bATS biomass composite, SARC aquaponics system.
The sea urchins displayed a final average weight of 32.06 (g) and a final average diameter of 67.71 (mm). An average of 8.10 (g) increase was observed across all individuals (Table 3). The protein efficiency ratio (PER) was also not significant between dietary treatments (p value = 0.124). Internal composition of the L. variegatus presented no significant differences between any parameters, except for the dry gonad (Figure 1). Additionally, there were no significant differences between the test, gut, and lantern (Figure 1).
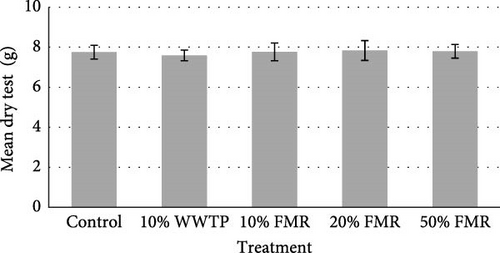
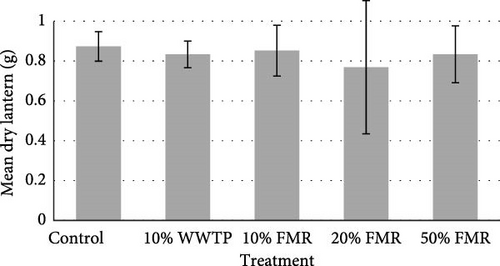
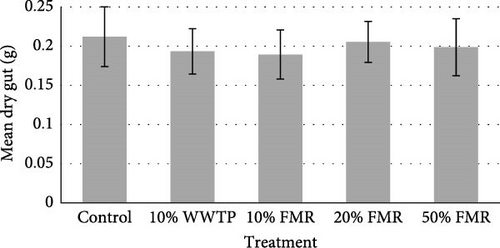
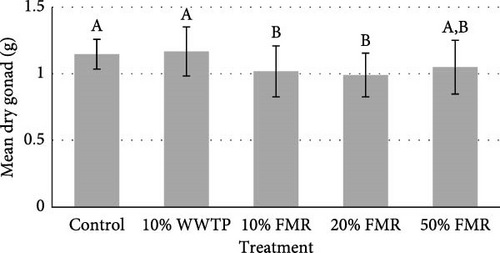
4. Discussion
The primary purpose of this study was to determine the growth response of L. variegatus diets containing varying levels of ATS biomass for partial replacement of dietary menhaden fishmeal. ATS biomass is highly sustainable, utilizing waste nutrients for production and achieving a high productivity which was a key focus for this study. Findings indicate that all dietary treatments, including those with higher levels of ATS biomass, performed comparably in terms of final weight, final diameter, survival rate, and PER. There were no significant differences between the final weights of the different treatments (p value = 0.645). Survival was 100% for all diets. This suggests that the partial replacement of fishmeal with ATS biomass is well tolerated by sea urchins, without compromising growth or diet utilization.
Research in the last decade has been directed toward enhancing the effectiveness and sustainability of fish meal usage in aquaculture. However, shortcomings in both sustainability and cost efficiency associated with fishmeal-based diets are evident. Numerous studies have explored the viability of algae as an alternative to fish meal, yielding positive outcomes across various species. ATSs present a unique opportunity for aquaculture and alternative feed practices. Mustafa et al. [22] demonstrated that replacing 5% of fish meal with macroalgae led to increased body weight, feed efficiency, and muscle protein deposition of red sea bream fingerlings. Similarly, Hussein et al. [23] reported beneficial effects on growth in Nile tilapia with up to 50% replacement of corn gluten by biofuel algae. Traditional microalgae production also presents a high economic cost. The expenses linked to microalgae production stem from technologies required for cultivation, harvesting, and processing. Currently, the traditional microalgal market yields approximately 5 kt annually, with production costs averaging around $25,000 per ton of biomass, predominantly sourced from five taxa [24]. However, since the primary focus of ATS technology is on nutrient removal, particularly targeting nitrogen and phosphorus, utilization of the biomass, which is typically regarded as a waste product, would not pose the same high economic costs compared to the methods previously discussed.
While costs are a major concern for any dietary ingredient, stable proximate composition and adequate supply are also heavily considered. Throughout the period of 1 year, a consistent level of protein, lipid, ash, and fiber was observed in the ATS biomass, despite significant variations in productivity both seasonally and, in some instances, weekly. Dietary ash remained high likely attributed to heightened diatom activity observed during the summer and fall, aligning with findings reported by Li et al. [25]. Diatoms were identified as the predominant algae species in nonpoint source periphyton communities, as highlighted in studies by [26, 27]. Marella et al. [27] specifically reported a sustained 90% diatom activity in a wastewater treatment ATS raceway. Despite the distinctions observed between the biomass flumes of the wastewater treatment plant and aquaponics systems, the composition and proximate analysis of the biomass remained remarkably stable throughout the duration of the study. In the biomass from the wastewater treatment plant, a diverse array of species including Compsopogon caeruleus and Pithophora constituted much of the turf biomass, with the remaining portion composed of diatoms. Conversely, the aquaponics system predominantly produced biomass composed of diatoms. A notable difference between the two systems was the presence of exogenous ash contamination, which was more prevalent in the biomass obtained from the wastewater treatment plant. This discrepancy is likely attributable to the location of the aquaponics system within an indoor greenhouse, which afforded it greater protection from external sources of contaminant ash compared to the more exposed environment of the wastewater treatment plant.
Through least-cost feed formulation, we were able to determine the percent fishmeal replacement of both aquaponics and wastewater produced ATS biomass in sea urchin, L. variegatus sp., feed using proximate composition data presented in Table 2. Diet formulations suggest that ATS biomass can replace up to 50% fishmeal, as a protein alternative, in a practical sea urchin diet. Despite concerns regarding the potential negative effects of elevated ash levels on growth and digestibility, the study’s findings indicate no significant adverse effects. Full replacement was hindered by low protein concentrations especially given the high ash content of the ATS biomass. Algal colonies from single species have been previously employed to develop feeds, allowing for the full replacement of up to 100% fishmeal, aiming to minimize human impact on resources [28, 29]. Research on the utilization of ATS biomass as a feed remains limited, likely attributed to factors including variations in composition and inconsistent productivity.
The lack of significant differences in body composition between dietary treatments suggests that ATS biomass was well utilized by the sea urchins. Given that we saw an average of 8.10 (g) increase across all individuals (Table 3), and the PER was also not significant between dietary treatments (p value = 0.072), the data suggest high productivity within the trial. Despite the overall positive outcomes, several limitations and areas for improvement should be acknowledged. First, the study’s reliance on a 12-week growth trial may not capture the long-term effects of dietary treatments on L. variegatus performance and digestibility. A more comprehensive understanding of the implications of dietary composition and longitudinal analysis could be beneficial. Additionally, the study’s focus on proximate composition analysis provides valuable insights into the nutritional content of ATS biomass but does not account for potential variations in bioavailability and nutrient utilization. Future research incorporating digestibility trials and metabolic studies could elucidate the mechanisms underlying the observed growth responses and optimize feed formulations for enhanced performance. Furthermore, while the study controls for confounding factors such as initial weight and environmental conditions, inherent variability in biological responses among individual sea urchins may introduce sources of error. Increasing sample sizes and implementing robust statistical analyses can help mitigate these sources of variability and enhance the reliability of findings.
5. Conclusions and Future Directions
In conclusion, this study demonstrates the feasibility of incorporating ATS biomass into the diets of L. variegatus sea urchins as a partial replacement for dietary menhaden fishmeal. The findings indicate that sea urchins fed diets containing varying levels of ATS biomass exhibited comparable growth, survival rates, and PERs to those fed commercially available diets. Despite concerns regarding the potential negative effects of elevated ash levels on growth and feed utilization, no significant adverse effects were observed. Through least-cost feed formulation, we determined that ATS biomass can replace up to 50% of fishmeal as a protein alternative in practical sea urchin diets. However, full replacement was hindered by low protein concentrations, particularly given the high ash content of the ATS biomass. The lack of significant differences in body composition between dietary treatments suggests that ATS biomass was well utilized by the sea urchins, as evidenced by the average weight increase across all individuals and the consistent PERs. Further research is needed to validate these findings, optimize feed formulations, and address remaining knowledge gaps to support the sustainable growth of sea urchin aquaculture industry.
Disclosure
The abstract was submitted and presented at the World Aquaculture Society (WAS) annual conference in San Antonio, TX, USA (February 20, 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was supported by the National Science Foundation REU (grant number 2244232) and internal funding from Georgia Southern University through the Vertically Integrated Projects Consortium.
Acknowledgments
This study was supported in part by the Virtually Integrated Projects (VIP) program headquartered at Georgia Institute of Technology. Funding for this project was provided by Georgia Southern University.
Open Research
Data Availability Statement
The data will be made available to the public through Digital Commons@Georgia Southern University (http://digitalcommons.georgiasouthern.edu) within 3 months of publication and can be obtained before that by contacting the corresponding author.




