Partial Replacement of Fish Meal With Shrimp Waste Meal: Effects on Growth, Digestibility, and Immunity in Juvenile Beluga Sturgeon (Huso huso)
Abstract
This study aimed to investigate the effects of replacing different levels of dietary shrimp waste meal (SWM) with fish meal (FM) on growth performance (GP), carcass composition (CC), apparent digestibility (AD), and innate immunity of juvenile beluga sturgeon (Huso huso). A total of 180 juvenile beluga with an average weight of 130.0 ± 6.5 g were assigned to five treatments (with three replicates each): SWM0 (control group), SWM5, SWM10, SWM15, and SWM20, corresponding to 0%, 5%, 10%, 15%, and 20% inclusion of SWM in place of FM in their diets for 8 weeks. The results showed that GP and protein AD declined in the SWM5 group (p < 0.05), while no significant differences were observed in GP and AD across the other treatments compared to the control (p > 0.05). Regarding CC, no significant differences were found among the treatments. The fatty acid (FA) profile of the muscles in juvenile beluga fed with SWM5–20 was similar to that of the control group, with the only notable difference being a decrease in eicosapentaenoic acid (EPA) as dietary SWM levels increased (p < 0.05). The essential amino acid (EAA) content also decreased in the SWM20 group (p < 0.05), although the EAA-to-nonessential amino acid (NEAA) ratio in the SWM5–20 treatments was not significantly different from that of the control group (p > 0.05). The highest levels of serum lysozyme (LYZ) and alternative complement hemolytic (ACH50) activity were observed in the SWM15 group, with the lowest levels found in the control group (p < 0.05). The results showed that substituting FM with SWM at a rate of 15%–20% in the diet had no significant and negative impact on GP, nutritional value, or digestibility. On the contrary, it boosted the immune system of juvenile beluga sturgeon (H. huso). The positive immunostimulation appears to be related to the beneficial effects of amino acid (AA) or imino acids (IAs) and chitin as well as their recognition by mannose/fucose receptors attached to white blood cells.
1. Introduction
The production of aquatic feed for farmed species is projected to increase by 75%, from 49.7 million tons (MT) in 2015 to 87.1 MT by 2025 [1]. Although the use of fish meal (FM) in aquatic diets has decreased in recent years, it remains the primary feed for various carnivorous fish species and crustaceans [2]. Consequently, the substitution of FM with plant-based proteins in aquatic diets has gradually increased [3, 4]. However, plant-based proteins present several challenges, including the presence of antinutritional factors [5], deficiencies in essential amino acids (EAAs) [6], and lower apparent digestibility (AD) [7].
Animal waste proteins, including those derived from aquatic waste, blood meal, poultry waste, and meat and bone meal (MBM), are now considered viable alternatives to FM, with their production rates estimated to be two to three times higher than that of FM [8]. In addition to containing higher protein content and a more favorable amino acid (AA) profile compared to plant-based proteins, these materials are often more cost-effective [9].
Shrimp, in particular, is a highly valued and widely consumed seafood product. Depending on the processing techniques used, 40%–70% of shrimp weight—including the head, carapace, and shell—is typically discarded as waste [10]. Global shrimp production reached 5.03 MT in 2020 and is expected to grow to 7.28 MT by 2025, with an annual growth rate of 6% [11]. During shrimp preparation, around 40%–50% of the shrimp’s weight—which includes the head, shell, and tail—is wasted. Annually, between 6 and 8 MT of crustacean debris are produced globally [12]. Inadequate waste management causes resource waste, disposal issues, and contamination [13]. Notably, shrimp waste (SW) contains important bioactive components, such as protein, chitin, chitosan, pigments, enzymes, lipids, minerals, and even vitamins [14–16]. While there are limited studies on the use of shrimp waste meal (SWM) in animal and aquatic diets, those that exist have demonstrated its benefits as a palatable feed source [17, 18].
Beluga sturgeon (Huso huso L.) is one of the most commercially valuable aquaculture species, known for its high-quality meat and caviar. This species has a high growth rate and strong resistance to various environmental stressors, including low water quality, making it well suited for aquaculture [19]. Given the rising demand for sustainable feed alternatives, exploring replacements for FM in beluga sturgeon diets is of critical importance. A recent study by Sayed Hassani et al. [20] investigated the use of poultry by-products as an FM substitute in juvenile beluga sturgeon diets, highlighting the potential of animal by-products as cost-effective, nutritionally suitable alternatives due to their similar EAA and essential fatty acid (EFA) profiles [21]. Various animal protein sources, including poultry by-products, insect meal, meat meal, bone meal, and blood meal, have been incorporated into formulated aquafeeds, with most studies reporting no adverse effects on growth performance (GP), nutritional value, or digestibility in fish species [22, 23].
While limited research has focused on using animal proteins, particularly SWM, as substitutes for FM in fish diets, studies on species such as Nile tilapia (Oreochromis niloticus) [24, 25], African catfish (Clarias gariepinus) [26], cobia (Rachycentron canadum) [27], goldfish (Carassius auratus) [28], European sea bass (Dicentrarchus labrax) [29], rainbow trout (Oncorhynchus mykiss) [30], and spotted rose snapper (Lutjanus guttatus) [31] have highlighted its strong nutritional value. This study is the first to investigate the effects of dietary SWM as a partial FM replacement in juvenile beluga sturgeon. Specifically, it evaluates the impact of SWM on GP, carcass quality, AD, muscle fatty acid (FA) and AA profiles, and innate immunity. The findings from this research will contribute to understanding the feasibility of SWM as a sustainable and functional ingredient in aquaculture diets, potentially reducing reliance on traditional FM sources.
2. Materials and Methods
2.1. Diet Preparation
To prepare the experimental diets, SWM (Litopenaeus vannamei) was obtained fresh in a Styrofoam box with ice from a packaging and processing center in Babolsar, Mazandaran Province, Iran. The waste (mainly consisted of cephalothorax and abdominal shell) was placed on metal trays and dried in a dryer at 50°C for 24 h before being ground into a powder with a 120-μm mesh [32]. The SWM was then transferred to the food processing laboratory at the Faculty of Natural Resources and Marine Sciences, Tarbiat Modares University (Noor, Iran). The chemical composition of the SWM was determined to be 6.11% moisture, 30.02% crude protein, 13.86% crude fat, and 19.64% ash. Using the LINDO software package (copyright release 6.1, USA), five diets with 45% protein were formulated, ensuring the same levels of energy and nitrogen across all treatments. The composition and analysis of the test diets are shown in Table 1. The treatments were designed to replace FM with SWM at 0% (SWM0) (control), 5% (SWM5), 10% (SWM10), 15% (SWM15), and 20% (SWM20). After thoroughly mixing the dry ingredients, fish oil and lukewarm water were added, and the mixture was combined again. The resulting mixture was pelleted using a meat grinder with a diameter of 4 mm. The pellets were dried on metal trays at 40°C for 24 h and then packed into nylon bags and stored in a freezer at −20°C until the start of the experiment.
| Ingredients (%) | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| Fish meal (FM) | 52. 367 | 49. 961 | 47.555 | 45. 149 | 42.702 |
| Soybean meal | 30 | 30 | 30 | 30 | 30 |
| Wheat flour | 6.363 | 4.769 | 3.175 | 1.581 | 0.018 |
| Shrimp waste meal (SWM) | 0 | 5 | 10 | 15 | 20 |
| Soybean oil | 4 | 3 | 2 | 1 | 0.01 |
| Mineral premixa | 2 | 2 | 2 | 2 | 2 |
| Vitamin premixb | 2 | 2 | 2 | 2 | 2 |
| Antifungals | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Binder (sodium alginate) | 1 | 1 | 1 | 1 | 1 |
| Organic acid | 1 | 1 | 1 | 1 | 1 |
| Monocalcium phosphate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Antioxidants | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Lecithin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Chemical analysis (% DM) | |||||
| Crude protein (CP) | 45.62 | 45.68 | 45.73 | 45.79 | 54.82 |
| Crude lipid (CL) | 7.63 | 7.44 | 7.69 | 8.4 | 7.68 |
| Ash | 15.24 | 15.22 | 15.78 | 16.96 | 17.24 |
| Carbohydrate | 24.01 | 24.17 | 23.3 | 20.75 | 22.56 |
| Energy | 17.91 | 17.88 | 17.84 | 17.69 | 17.73 |
| Moisture | 7.5 | 7.49 | 7.5 | 8.1 | 6.7 |
- Note: Energy calculation based on the coefficients of 23.6, 39.5, and 17.2 (kJ/g) for protein, lipid, and carbohydrate, respectively [33].
- Abbreviations: SWM, shrimp waste meal; SWM0, control (0% SWM); SWM5, 5% SWM instead of FM; SWM10, 10% SWM instead of FM; SWM15, 15% SWM instead of FM; SWM20, 20% SWM instead of FM.
- aMineral premix (mg or g/kg): iron (FeSO4: 20 g), zinc (ZnSO4: 60 g), selenium (400 mg), cobalt (CoSO4: 200 mg), copper (CuSO4: 2 g), manganese (MnSO4: 40 mg), iodine (400 mg).
- bVitamin premix (IU or mg/g): vitamin A, 50,000 IU; vitamin D3, 10,000 IU; vitamin E, 10 mg; vitamin B1, 20 mg.
2.2. Rearing Conditions
This study was conducted at the Shahid Rajaei Caspian Sea Genetic Reserve Conservation and Restoration Center (Sari, Iran). A total of 180 juvenile H. huso with an average weight of 130.0 ± 6.5 g were used in the experiment. The facilities included a rearing hall, tanks, and an aeration system. Initially, the fish were fed a control diet for 2 weeks to allow for acclimation, after which they were randomly distributed into 15,400-Ll tanks, with 12 fish per tank. The water source was a well, and key parameters such as water temperature (17.0°C ± 0.5°C), pH (8.15 ± 0.09), and dissolved oxygen (8.40 ± 0.35 mg L−1) were monitored daily. The fish were fed experimental diets (Table 1) three times a day to satiety for a period of 8 weeks.
The fish were fed to satiation, and any uneaten feed was removed when feces were present to avoid errors in the AD calculations.
2.3. GP and Survival Rate (SR) Assessment
2.4. Analysis of Proximate Composition of Diets and Carcasses
For carcass composition (CC) analysis, three fish were randomly selected from each tank. After removing the intestines and viscera, the carcasses were stored at −20°C until chemical composition analysis. The proximate composition of the carcasses, experimental diets, and SWM was determined following the standard methods of the Association of Official Agricultural Chemists [34], with three replicates. Crude protein was measured using the Kjeldahl method, which involved determining the total nitrogen content and multiplying it by 6.25. Crude fat was extracted using the Soxhlet method with diethyl ether, and moisture was calculated by drying the sample at 105°C and weighing it after cooling. Ash content was determined by incinerating the sample at 550°C for 6 h and then weighing the residue. Carbohydrate content in the diets was calculated by subtracting the combined weight of protein, moisture, fat, and ash from 100 g of the sample.
2.5. FA and AA Analysis in Diets and Muscle Tissue
At the end of the experiment, three fish were randomly selected from each tank and anesthetized for muscle tissue sampling. Muscle tissue from beneath the dorsal fin was homogenized and stored at −80°C.
The total fat was extracted from the muscles, experimental diets, and SWM using a chloroform–ethanol mixture (1:1 ratio), following the method of Folch et al. [35]. The total fat in the samples was then esterified according to Metcalfe and Schmitz [36]. FAs were identified using a gas chromatograph (model: GLC-PU4400, Phillips Scientific Co., Cambridge, UK), equipped with a capillary column (BPX70, 60 × 0.32 mm ID, 0.25 µm film thickness, SGM, Victoria, Australia) and a flame ionization detector (FID). The detector and injection port temperatures were set to 260°C and 230°C, respectively. A 1-μL sample was injected using a Hamilton syringe, with nitrogen (99.999% purity) as the carrier gas. After nitrogen passed through the system and the gradual heating process, the methyl esters of FAs were separated, plotted as a curve, and compared against standard FAs to determine the type and percentage of each FA. FA profile of SWM and experimental diets (% of total FAs) are shown in Table 2.
| Fatty acids (%) | Treatments | |||||
|---|---|---|---|---|---|---|
| SWM | SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| C14 (MA) | 0.35 | 1.43 | 1.27 | 1.1 | 0.39 | 1.49 |
| C16 (PA) | 21.12 | 15.75 | 14.77 | 15.84 | 15.56 | 15.78 |
| C16 1n7 (POA) | 2.34 | 8.32 | 8.4 | 8.37 | 3.12 | 7.48 |
| C18 (SA) | 0.69 | 2.87 | 3.69 | 3.54 | 5.03 | 3.48 |
| C18 1n9 (OA) | 8.08 | 26.92 | 24.87 | 25.42 | 26.63 | 27.45 |
| C18 2n6 (LA) | 26 | 16.54 | 15.54 | 15.38 | 15.4 | 14.14 |
| C18 3n3 (ALA) | 3.38 | 2.73 | 3.05 | 2.82 | 2.68 | 2.23 |
| C20 (AA) | 20.21 | 1.18 | 1.14 | 1.39 | 1.04 | 0.95 |
| C20 1n9 (CA) | 1.61 | 0.29 | 0.24 | 0.11 | 0.22 | 0.34 |
| C20 2n6 (EDA) | 0.64 | 0.03 | 0.03 | 0.03 | 0.05 | 0.02 |
| C20 3n3 (ETA) | 1.64 | 0.28 | 0.36 | 0.44 | 0.89 | 0.37 |
| C20 4n6 (ARA) | 0.89 | 3.57 | 3.82 | 3.34 | 3.85 | 3.89 |
| C20 5n3 (EPA) | 3.74 | 1.43 | 0.26 | 0.57 | 0.53 | 0.94 |
| C22 (BA) | 0.82 | 0.9 | 0.44 | 0.75 | 0.42 | 0.88 |
| C22 1n9 (EA) | 0.43 | 0.87 | 0.25 | 0.72 | 0.72 | 0.72 |
| C22 6n3 (DHA) | 7.94 | 12.32 | 9.44 | 10.56 | 10.26 | 11.69 |
| ∑n-3 | 16.7 | 16.76 | 13.11 | 14.39 | 12.72 | 15.23 |
| ∑n-6 | 27.53 | 20.14 | 19.39 | 18.75 | 19.3 | 18.05 |
| ∑SFAs | 43.19 | 23.56 | 22.58 | 23.72 | 22.44 | 24.07 |
| ∑MUFAs | 12.46 | 36.41 | 33.76 | 34.62 | 32.11 | 35.98 |
| ∑PUFAs | 44.23 | 36.9 | 32.5 | 33.14 | 32.02 | 33.28 |
| ∑HUFAs | 12.57 | 17.32 | 13.52 | 14.47 | 14.64 | 16.52 |
| n3/n6 | 0.6 | 0.83 | 0.67 | 0.76 | 0.65 | 0.84 |
- Note: ∑HUFAs, sum of highly unsaturated fatty acids (C20 4n6 + C20 5n3 + C22 6n3); ∑n-3, sum of omega-3 (n-3) PUFAs; ∑n-6, sum of omega-6 (n-6) PUFAs; ΣMUFAs, sum of monounsaturated fatty acids (C16 1n7 + C18 1n9 + C20 1n9 + C22 1n9); ΣPUFAs, sum of polyunsaturated fatty acids (C18 2n6 + C18 3n3 + C20 2n6 + C20 3n3 + C20 4n6 + C20 5n3 + C22 6n3); ΣSFAs, sum of saturated fatty acids (C14 + C16 + C18 + C20 + C22).
- Abbreviations: AA, arachidic acid; ALA, α-linolenic acid; ARA, arachidonic acid; BA, behenic acid; CA, cetoleic acid; DHA, docosahexaenoic acid; EA, erucic acid; EDA, eicosadienoic acid; EPA, eicosapentaenoic acid; ETA, eicosatrienoic acid; LA, linoleic acid; MA, myristic acid; OA, oleic acid; PA, palmitic acid; POA, palmitoleic acid; SA, stearic acid.
The analysis of AA composition in the muscle tissue, experimental diets, and SWM was performed using a high-performance liquid chromatography (HPLC), following the method of Lindroth and Mopper [37], with slight modifications. For each treatment, 0.1 g of the sample was placed in a digestion tube, and 7.5 mL of 6N hydrochloric acid (HCl) was added. After removing air with nitrogen, the tube was sealed and heated at 110°C for 24 h. The acid was then diluted to 25 mL with distilled water and filtered through a 0.45-µm syringe filter. A 10 µL aliquot was dried under vacuum and stored in a refrigerator. For derivatization, 10 µL of acetate buffer was added to the dried AAs and mixed. Another 490 µL of acetate buffer was added and incubated for 5 min. Afterward, borate buffer and 100 µL of o-phthalaldehyde (OPA) solution were added, and 50 μL of 0.75 M HCl solution was introduced after 2 min to stop the reaction. For AA identification, 20 µL of the mixture was injected into the HPLC system using a syringe. The HPLC column was C18 (250 × 4 mm), operated at 30°C, with fluorescence detection at excitation wavelength 330 nm and emission wavelength 450 nm. The AA content was reported as g/100 g of sample. AA profile of SWM and experimental diets (% of total AAs) are shown in Table 3.
| Amino acids (%) | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| SWM | SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | ||
| Arg (arginine) | 12.2 | 9.47 | 9.76 | 10.05 | 9.91 | 9.61 | |
| His (histidine) | 3.58 | 4 | 4.87 | 5.74 | 6.11 | 6.98 | |
| Iso (isoleucine) | 3.74 | 3.78 | 4.07 | 4.36 | 4.72 | 4.98 | |
| Luc (leucine) | 5.41 | 7.94 | 8.19 | 8.44 | 8.58 | 8.53 | |
| Lys (lysine) | 1.09 | 1.67 | 1.88 | 2.1 | 1.93 | 1.69 | |
| Met (methionine) | 0.72 | 2.43 | 2.65 | 2.88 | 2.67 | 3.03 | |
| Phe (phenylalanine) | 10.33 | 3.23 | 3.52 | 3.82 | 3.91 | 3.79 | |
| Thr (threonine) | 10 | 4.72 | 5.58 | 6.44 | 6.1 | 6.04 | |
| Val (valine) | 7.45 | 5.25 | 5.6 | 5.95 | 6.29 | 6.4 | |
| TEAAs | 54.5 | 42.49 | 46.13 | 49.78 | 50.22 | 51.05 | |
| Ala (alanine) | 8.37 | 2.89 | 3 | 3.11 | 3.46 | 2.12 | |
| Asp (aspartic acid) | 9.62 | 8.61 | 9.32 | 9.86 | 9.28 | 9.68 | |
| Glu (glutamic acid) | 11.97 | 34.14 | 25.45 | 16.85 | 17.54 | 20.56 | |
| Gly (glycine) | 1.74 | 0.4 | 0.48 | 0.57 | 0.61 | 1.1 | |
| Ser (serine) | 10.72 | 8.35 | 13.76 | 17.26 | 16.27 | 13.31 | |
| Tyr (tyrosine) | 3.47 | 0.29 | 0.26 | 0.27 | 0.35 | 0.34 | |
| TNEAAs | 45.89 | 54.68 | 52.23 | 47.92 | 47.51 | 47.18 | |
| EAAs/NEAAs | 1.19 | 0.78 | 0.88 | 1.04 | 1.06 | 1.08 | |
- Note: Bold values for EAAs/NEAAs denote the ratio of total essential amino acids to nonessential amino acids.
- Abbreviations: TEAAs, total essential amino acids; TNEAAs, total nonessential amino acids.
2.6. Measuring AD
2.7. Blood Sampling to Measure Innate Immunity
To assess the innate immunity parameters of serum, specifically lysozyme (LYZ) and alternative complement hemolytic (ACH50) activity, blood samples were carefully collected from the caudal stem using a 5-mL syringe. Three fish from each treatment group were sampled at the end of the 56-day feeding trial after being anesthetized with a clove powder solution. The blood samples were transferred into Eppendorf tubes without anticoagulants. After clotting, the samples were transported to the laboratory, where they were centrifuged at 3000 g for 5 min. The serum was isolated, transferred into microtubes using a sampler, and stored at −20°C for further experiments. The method developed by Clerton et al. [40] was used to determine LYZ activity. For this, 25 μL of serum was added to 96-well plates of the enzyme-linked immunosorbent assay (ELISA) device. Then, 175 μL of Micrococcus lysodeikticus bacterial suspension (Sigma-Aldrich Chemicals, USA), prepared in 0.2M sodium citrate buffer at pH 5.5, was added. Initial optical absorbance was measured at a wavelength of 630 nm. After 90 min at room temperature, absorbance was remeasured. Lyophilized egg white LYZ (Sigma-Aldrich Chemicals, USA) was used to create the standard curve.
ACH50 activity was determined following the method of Milla et al. [41]. Blood serum was diluted 10-fold with gelatin veronal buffer. A 3% suspension of rabbit red blood cells was added to the serum dilutions, and after 100 min of incubation at 27°C, the samples were centrifuged at 1800 rpm for 5 min. The supernatant was collected, and absorbance was measured at 405 nm.
2.8. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics software (version 22, Chicago, USA). The normality of the data was assessed using the Kolmogorov–Smirnov (K-S) test. One-way analysis of variance (ANOVA) was applied to assess the differences between the main parameters in fish treated with different levels of dietary SWM. Mean values were compared using Duncan’s new multiple range test (MRT), with a significance level set at p < 0.05. Data are presented as mean ± standard deviation (SD).
3. Results
3.1. Effects of Experimental Diets on GP and SR
The results for GP and SR, influenced by the substitution of SWM for FM in the diets of juvenile H. huso, are presented in Table 4. The SWM5 treatment (5% SWM) resulted in a significant decrease in FW, WG, and SGR compared to the control and other treatments (p < 0.05). However, the same parameters in the other SWM treatments showed no significant differences compared to the control (p > 0.05). The lowest FCR was found in the control treatment, which was significantly different from the SWM5 and SWM10 treatments (p < 0.05). In contrast, FCR in the SWM15 and SWM20 treatments showed no significant difference compared to the control (p > 0.05). Additionally, there were no significant differences between the control and other treatments regarding HSI and VSI or SR (p > 0.05).
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| IW (g) | 130 ± 6.5 | 130 ± 6.6 | 130 ± 6.7 | 130 ± 6.4 | 130 ± 6.3 |
| FW (g) | 384.27 ± 6.9a | 319.16 ± 6.97b | 361.02 ± 12.8a | 371.24 ± 9.94a | 372.47 ± 9.55a |
| FCR | 1.14 ± 0.04a | 1.76 ± 0.01b | 1.97 ± 0.07b | 1.15 ± 0.04a | 1.27 ± 0.07a |
| WG (g) | 254.27 ± 6.9a | 189.16 ± 6.97b | 231.02 ± 12.8a | 241.24 ± 9.94a | 242.47 ± 5.51a |
| SGR (% d−1) | 2 ± 0.58a | 1.66 ± 0.06b | 1.88 ± 0.11a | 1.94 ± 0.08a | 1.94 ± 0.04a |
| HSI (%) | 1.3 ± 0.03 | 1.28 ± 0.09 | 1.51 ± 0.09 | 1.4 ± 0.19 | 1.46 ± 0.19 |
| VSI (%) | 17.67 ± 1.05 | 16.78 ± 0.56 | 18.71 ± 1.2 | 16.37 ± 1.34 | 18.92 ± 1.39 |
| SR (%) | 94.44 ± 2.78 | 86.1 ± 2.77 | 83.33 ± 0.0 | 94.44 ± 2.78 | 88.88 ± 2.77 |
- Note: The absence of superscript lowercase letters in each row shows that the differences in the mentioned parameters are not significant (p > 0.05). The data are illustrated as mean ± SD of triplicate.
- Abbreviations: FCR, feed conversion ratio; FW, final weight; HSI, hepatosomatic index; IW, initial weight; SGR, specific growth rate; SR, survival rate; SWM, shrimp waste meal; SWM0, control (0% SWM); SWM5, 5% SWM instead of FM; SWM10, 10% SWM instead of FM; SWM15, 15% SWM instead of FM; SWM20, 20% SWM instead of FM; VSI, viscerosomatic index; WG, weight gain.
3.2. Effects of Experimental Diets on CC
The effects of substituting SWM for FM on the CC of juvenile H. huso are summarized in Table 5. Overall, dietary substitution of 5%–20% SWM did not significantly affect the CC values (protein, crude lipid, ash, and moisture) of the juvenile H. huso compared to the control (p > 0.05).
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| Crude protein (%) | 82.49 ± 2.19 | 83.59 ± 2.2 | 85.46 ± 2.49 | 85.73 ± 1.7 | 84.63 ± 2.17 |
| Crude lipid (%) | 10.39 ± 1.88 | 6.35 ± 0.51 | 9.57 ± 1.59 | 9.14 ± 3.49 | 9.5 ± 3.6 |
| Ash (%) | 5.12 ± 0.4 | 5.05 ± 0.3 | 5.15 ± 0.2 | 4.78 ± 0.5 | 4.91 ± 0.14 |
| Moisture (%) | 79.38 ± 0.14 | 80.11 ± 0.67 | 79.21 ± 0.75 | 79.37 ± 0.52 | 78.59 ± 0.38 |
- Note: The absence of letters in each row shows that the differences in the mentioned parameters are not significant (p > 0.05). The data are illustrated as mean ± SD of triplicate.
3.3. Effects of Experimental Diets on FA Profile of Muscles in Juvenile H.huso
The FA profile of muscles in juvenile H. huso fed experimental diets is shown in Table 6. One-way ANOVA revealed significant differences in certain FAs in the muscle tissue of juvenile H. huso, including palmitoleic acid (C16:1n7), eicosatrienoic acid (C20:3n3), eicosapentaenoic acid (EPA) (C20:5n3), and behenic acid (C22:0) (p < 0.05). Specifically, palmitoleic acid (C16:1n7) was significantly lower in the SWM5 group compared to the control (p < 0.05), whereas no significant differences were observed among the other SWM treatments (p > 0.05). Additionally, eicosatrienoic acid (C20:3n3) level increased significantly in the SWM15 and SWM20 groups (p < 0.05), whereas EPA level showed a decreasing trend with increasing SWM inclusion, with significant reductions observed in SWM15 and SWM20 (p < 0.05). Behenic acid (C22:0) level significantly decreased only in the SWM5 treatment compared to the control (p < 0.05), with no significant differences observed in the other SWM treatments (p > 0.05). Overall, total FA content, including saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), highly unsaturated fatty acids (HUFAs), omega-3 and omega-6 FAs, and the omega-3-to-omega-6 ratio, did not significantly differ between treatments (p > 0.05). This indicates that substituting SWM for FM in diets up to 20% can be beneficial without altering the FA profile of the muscle tissue in juvenile H. huso.
| Fatty acids (%) | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| C14 (MA) | 0.69 ± 0.19 | 0.63 ± 0.35 | 0.85 ± 0.22 | 0.56 ± 0.12 | 0,8 ± 0.11 |
| C16 (PA) | 14.58 ± 1.64 | 15.68 ± 2.34 | 15.6 ± 0.89 | 14.91 ± 3.06 | 16.37 ± 0.88 |
| C16 1n7 (POA) | 3.56 ± 0.93a | 1.97 ± 0.77b | 3.54 ± 0.43a | 2.87 ± 0.97ab | 2.64 ± 0.24ab |
| C18 (SA) | 2.93 ± 0.68 | 3.02 ± 0.19 | 2.93 ± 0.09 | 2.87 ± 1.14 | 3.39 ± 0.55 |
| C18 1n9 (OA) | 25.14 ± 2.93 | 23.69 ± 7.08 | 27.51 ± 1.45 | 24.85 ± 4.82 | 27 ± 2.57 |
| C18 2n6 (LA) | 16.66 ± 3.55 | 19.6 ± 4.45 | 17.7 ± 1.3 | 21.22 ± 7.29 | 18.03 ± 0.56 |
| C18 3n3 (ALA) | 2.01 ± 0.96 | 2.02 ± 1.2 | 3.59 ± 0.2 | 3.68 ± 0.1 | 1.78 ± 0.19 |
| C20 (AA) | 1.03 ± 0.54 | 0.65 ± 0.46 | 1.03 ± 0.07 | 0.89 ± 0.62 | 0.93 ± 0.21 |
| C20 1n9 (CA) | 0.87 ± 0.42 | 0.85 ± 0.15 | 0.81 ± 0.05 | 0.89 ± 0.66 | 1 ± 0.29 |
| C20 2n6 (EDA) | 0.67 ± 0.64 | 0.46 ± 0.23 | 0.33 ± 0.07 | 0.71 ± 0.42 | 0.51 ± 0.29 |
| C20 3n3 (ETA) | 0.92 ± 0.37b | 1 ± 0.24ab | 1.21 ± 0.19b | 1.75 ± 0.26a | 1.36 ± 0.11a |
| C20 4n6 (ARA) | 2.06 ± 0.27 | 1.63 ± 0.18 | 1.92 ± 0.13 | 1.8 ± 0.66 | 1.83 ± 0.28 |
| C20 5n3 (EPA) | 4.87 ± 2.03a | 3.43 ± 0.92ab | 2.28 ± 1.06b | 2.45 ± 1.04b | 2.16 ± 1.18b |
| C22 (BA) | 0.66 ± 0.1a | 0.47 ± 0.1b | 0.64 ± 0.01ab | 0.63 ± 1.04ab | 0.59 ± 0.1ab |
| C22 1n9 (EA) | 1.9 ± 0.85 | 1.7 ± 0.63 | 1.75 ± 0.15 | 2.1 ± 1.3 | 1.8 ± 0.42 |
| C22 6n3 (DHA) | 13.4 ± 2.66 | 12.64 ± 1.84 | 13.28 ± 0.15 | 13.84 ± 4.78 | 12.92 ± 1.45 |
| ∑n-3 | 21.21 ± 3.99 | 19.1 ± 2.65 | 18.74 ± 1.22 | 20.46 ± 4.93 | 18.23 ± 2.73 |
| ∑n-6 | 19.4 ± 2.6 | 21.7 ± 4.4 | 19.96 ± 1.1 | 23.74 ± 7.17 | 20.38 ± 0.62 |
| ∑SFAs | 18.82 ± 1.68 | 19.35 ± 2.33 | 19.56 ± 1.05 | 18.68 ± 4.78 | 20.71 ± 1.02 |
| ∑MUFAs | 31.49 ± 3.42 | 28.24 ± 7.57 | 33.61 ± 1.22 | 30.74 ± 2.19 | 32.92 ± 2.15 |
| ∑PUFAs | 40.62 ± 2.21 | 40.8 ± 3.8 | 38.7 ± 2.32 | 44.3 ± 2.84 | 38.61 ± 2.53 |
| ∑HUFAs | 20.34 ± 2.1 | 17.71 ± 2.61 | 17.49 ± 0.96 | 18.09 ± 6.16 | 16.92 ± 2.55 |
| n3/n6 | 1.12 ± 0.37 | 0.91 ± 0.3 | 0.93 ± 0.9 | 0.96 ± 0.53 | 0.89 ± 0.14 |
- Note: The absence of superscript lowercase letters in each row shows that the differences in the mentioned parameters are not significant (p > 0.05). The data are illustrated as mean ± SD of triplicate. ∑HUFAs, sum of highly unsaturated fatty acids (C20 4n6 + C20 5n3 + C22 6n3); ∑n-3, sum of omega-3 (n-3) PUFA; ∑n-6, sum of omega-6 (n-6) PUFAs; ΣMUFAs, sum of monounsaturated fatty acids (C16 1n7 + C18 1n9 + C20 1n9 + C22 1n9); ΣPUFAs, sum of polyunsaturated fatty acids (C18 2n6 + C18 3n3 + C20 2n6 + C20 3n3 + C20 4n6 + C20 5n3 + C22 6n3); ΣSFAs, sum of saturated fatty acids (C14 + C16 + C18 + C20 + C22).
- Abbreviations: AA, arachidic acid; ALA, α-linolenic acid; ARA, arachidonic acid; BA, behenic acid; CA, cetoleic acid; DHA, docosahexaenoic acid; EA, erucic acid; EDA, eicosadienoic acid; EPA, eicosapentaenoic acid; ETA, eicosatrienoic acid; LA, linoleic acid; MA, myristic acid; OA, oleic acid; PA, palmitic acid; POA, palmitoleic acid; SA, stearic acid.
3.4. Effects of Experimental Diets on AA Profile of Muscles in Juvenile H. huso
The AA profile of muscles in juvenile H. huso fed experimental diets is presented in Table 7. Substituting SWM for FM in the diets resulted in a significant difference in the total EAAs (TEAAs) in the muscles (p < 0.05), with the highest TEAAs observed in the control treatment and the lowest in the 20% SWM treatment (SWM20). The other treatments did not show significant differences compared to the control (p > 0.05). There was a significant decrease in the muscle concentration of arginine (Arg) in the SWM5, SWM10, and SWM20 treatments, as well as histidine (His) in the SWM5 treatment, compared to the control (p < 0.05). Additionally, the muscle concentrations of leucine (Luc) and methionine (Met) in the SWM20 significantly decreased compared to the control (p < 0.05). The muscle concentration of valine (Val) in the SWM treatments showed no significant difference compared to the control (p > 0.05). However, the muscle concentration of Val in the SWM5 showed a significant decrease compared to the SWM15 (p < 0.05). The lowest concentrations of Luc and Met were found in the 20% SWM treatment. Moreover, aspartic acid (Asp) concentration in the SWM5 treatment significantly decreased compared to the control and other SWM treatments (p < 0.05). The ratio of EAAs to nonessential AAs (NEAAs) and the total NEAAs did not show significant differences between treatments (p > 0.05).
| Amino acids (%) | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| Arg (arginine) | 10.67 ± 0.4a | 8.73 ± 0.8b | 8.51 ± 0.3b | 9.94 ± 1.1ab | 8.86 ± 0.55b |
| His (histidine) | 6.35 ± 0.7a | 4.64 ± 0.4b | 5.37 ± 1.3ab | 5.16 ± 0.3ab | 5.54 ± 0.5ab |
| Iso (isoleucine) | 4.63 ± 0.3 | 4.3 ± 0.3 | 4.46 ± 0.4 | 4.74 ± 0.1 | 4.11 ± 0.3 |
| Luc (leucine) | 10.06 ± 0.2a | 9.73 ± 0.8a | 9.38 ± 0.8ab | 9.67 ± 0.2a | 7.74 ± 1.8b |
| Lys (lysine) | 3.21 ± 0.8 | 2.29 ± 0.3 | 2.45 ± 0.2 | 3 ± 0.2 | 2.2 ± 0.9 |
| Met (methionine) | 4.23 ± 0.1a | 3.75 ± 0.5ab | 3.66 ± 0.3ab | 3.83 ± 0.1a | 2.8 ± 0.9b |
| Phe (phenylalanine) | 3.86 ± 0.1 | 4.22 ± 1.2 | 3.59 ± 0.2 | 3.68 ± 0.1 | 3.28 ± 0.2 |
| Thr (threonine) | 6.64 ± 0.3 | 6.69 ± 0.7 | 6.4 ± 0.9 | 6.82 ± 0.2 | 6.7 ± 0.2 |
| Val (valine) | 5.54 ± 0.5ab | 4.9 ± 0.5b | 5.62 ± 0.5ab | 6.09 ± 0.1a | 5.56 ± 0.4ab |
| TEAAs | 55.22 ± 0.71a | 49.28 ± 4.7ab | 49.47 ± 2.8ab | 52.5 ± 1.9ab | 46.83 ± 7.21b |
| Ala (alanine) | 3.51 ± 0.2 | 3.85 ± 0.7 | 3.57 ± 0.3 | 3.71 ± 0.6 | 3.25 ± 0.3 |
| Asp (aspartic acid) | 10.65 ± 0.2a | 6.13 ± 0.7b | 10.64 ± 2.1a | 10.22 ± 0.3a | 9.56 ± 1.9a |
| Glu (glutamic acid) | 17.02 ± 1.7 | 17.99 ± 11.64 | 22.79 ± 5.61 | 20.65 ± 2.59 | 19.46 ± 4.9 |
| Gly (glycine) | 1.44 ± 0.1 | 1.42 ± 0.07 | 1.14 ± 0.3 | 1.45 ± 0.1 | 1.47 ± 0.5 |
| Ser (serine) | 10.08 ± 2.2 | 8.18 ± 1.1 | 7.28 ± 0.9 | 8.87 ± 1.6 | 12.75 ± 6.2 |
| Tyr (tyrosine) | 0.38 ± 0.1 | 0.51 ± 0.2 | 0.42 ± 0.1 | 0.39 ± 0.2 | 0.33 ± 0.07 |
| TNEAAs | 43.09 ± 0.29 | 38.08 ± 10.6 | 45.86 ± 4.2 | 45.31 ± 1.53 | 46.83 ± 7.21 |
| EAAs/NEAAs | 1.28 ± 0.02 | 1.36 ± 0.39 | 1.08 ± 0.4 | 1.16 ± 0.08 | 1.01 ± 0.19 |
- Note: The absence of superscript lowercase letters in each row shows that the differences in the mentioned parameters are not significant (p > 0.05). The data are illustrated as mean ± SD of triplicate. Bold values for EAAs/NEAAs denote the ratio of total essential amino acids to nonessential amino acids.
- Abbreviations: TEAAs, total essential amino acids; TNEAAs, total nonessential amino acids.
3.5. Effects of Experimental Diets on AD in Juvenile H. huso
The results for in vivo AD of the experimental diets are shown in Table 8. One-way ANOVA revealed a significant difference in the AD of protein (p < 0.05), but no significant differences were observed in the AD of fat and ash among the treatments (p > 0.05). The highest AD of protein, with a statistically significant difference, was found in the SWM20 treatment, while the lowest was in the SWM5 treatment (p < 0.05). The AD of protein in SWM15 also increased significantly compared to SWM5 (p < 0.05), but no significant differences were found between any SWM treatments and the control (p > 0.05).
| AD (%) | Treatments | ||||
|---|---|---|---|---|---|
| SWM0 (control) | SWM5 | SWM10 | SWM15 | SWM20 | |
| ADCP | 80.26 ± 3.78ab | 75.04 ± 2.37b | 79.62 ± 3.21ab | 83.81 ± 1.2a | 83.86 ± 0.61a |
| ADCL | 82.63 ± 3.56 | 76.71 ± 4.74 | 76.60 ± 4.62 | 75.24 ± 3.19 | 79.28 ± 1.87 |
| ADash | 70.83 ± 2.5 | 72.16 ± 0.8 | 69.46 ± 2.03 | 72.89 ± 1.26 | 73.98 ± 3.93 |
- Note: The absence of superscript lowercase letters in each row shows that the differences in the mentioned parameters are not significant (p > 0.05). The data are illustrated as mean ± SD triplicate.
- Abbreviations: ADash, apparent digestibility of ash; ADCL, apparent digestibility of crude lipid; ADCP, apparent digestibility of crude protein.
3.6. Effects of Experimental Diets on Innate Immunity Indices in Juvenile H. huso
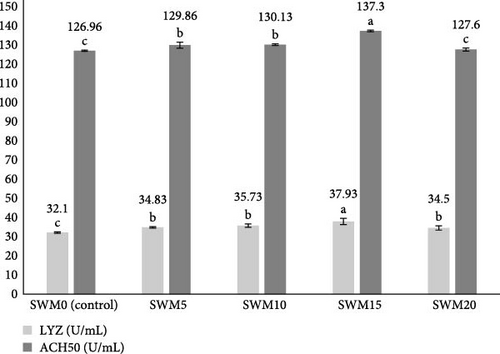
The results for serum LYZ and ACH50 activity are outlined in Figure 1. Significant differences were observed in serum LYZ and ACH50 activity among the treatments (p < 0.05). The highest and lowest serum LYZ activity were observed in the SWM15 and control treatments (p < 0.05), respectively. No significant differences were found among SWM5, SWM10, and SWM20 (p > 0.05), but all showed significant increases compared to the control (p < 0.05). For serum ACH50 activity, the highest value was in the SWM15 treatment, while the lowest was in the control and SWM20 treatments. The SWM15 treatment exhibited a significant increase in ACH50 activity compared to the control and other SWM treatments (p < 0.05). SWM5 and SWM10 did not significantly differ from each other (p > 0.05) but showed significant increases compared to the control and SWM20 treatments (p < 0.05). No statistically significant difference was observed in serum ACH50 activity between the control and SWM20 treatments (p > 0.05).
4. Discussion
The beneficial effects of substituting plant-based protein sources for FM have been documented in several studies involving juvenile H. huso [3, 4]. Research has also explored the use of animal protein sources such as poultry waste [20]. However, no previous studies have investigated the effects of substituting SWM for FM in the diets of juvenile H. huso, a valuable indigenous fish species of the Caspian Sea. This study represents the first attempt to address this issue.
Our results demonstrated that dietary levels of 15% and 20% SWM did not significantly and negatively affect the GP indices including FW, WG, SGR, and FCR. Based on Table 4, there was a significant and negative difference in the GP for the SWM5 group, as well as FCR for SWM10 group. A major problem in employing chitin in aquaculture is its restrictive and inhibitory effects on feed digestibility, which are caused mostly by the lack or reduced activity of the enzyme chitinase (which breaks down chitin) in fish digestive systems [42, 43]. The observed tendency of superior GP with increased SWM inclusion in the diet might be attributed to higher chitin levels. Several studies suggest that chitin and its derivatives, especially chitosan, might improve fish growth, immunology, and disease resistance [44–47]. Chitin’s prebiotic properties make it an ideal substrate for stimulating the growth of beneficial, chitin-degrading bacteria in the gut, ultimately leading to the production of short-chain fatty acids (SCFAs) like acetate, butyrate, and propionate [48, 49]. These SCFAs, as final bacterial fermentation products of chitin, enhance nutrient digestion and absorption in the gut while also promoting fish health [50]. This process may partially explain the higher SGR and lower FCR observed in the SWM15 and SWM20 treatments compared to the other SWM treatments.
In this regard, Abu-Alya et al. [51] reported improved growth and feed efficiency in African catfish with 50% SWM, while Plascencia-Jatomea et al. [24] found that SWM silage at 30% improved growth and nutritional indices in Nile tilapia. The differences in GP could be due to factors such as different levels of substitution, diet composition, chitin content, meal quality, preparation techniques, and species-specific digestive performance [52, 53].
One important characteristic of alternative protein sources is their impact on carcass quality. Hepher [54] noted that internal factors (e.g., size, sex, and life stage) and external factors (e.g., diet composition and temperature) affect CC. In this study, with constant temperature, diet composition was the primary external factor. The carcass quality was evaluated based on high protein and low lipid content, and the results showed that dietary levels of SWM up to 20% did not negatively affect CC.
In this study, the SWM primarily consisted of cephalothorax and abdominal shell, which include residual muscle tissues, hepatopancreas, and exoskeleton. The presence of residual soft tissues contributes to the AA and lipid composition of the meal. While chitin is the dominant polysaccharide in shrimp shells, the hepatopancreas contains a considerable amount of lipids, including PUFAs [15, 16]. Additionally, Cavalheiro et al. [25] have reported that SW-derived meals retain EFAs (e.g., EPA and docosahexaenoic acid [DHA]) from the original shrimp tissue.
The effects of SWM on the nutritional value of muscle FA and AA profiles in fish species are not well-documented. Muscle FA profiles reflect dietary FA content, and improving FA profiles can be achieved through dietary manipulation [55]. PUFAs and HUFAs are crucial for health, and EFAs like arachidonic acid (ARA), EPA, and DHA must be provided through diet. In this study, DHA and ARA levels in the muscles of juvenile H. huso were not significantly affected by SWM treatments, indicating that the experimental diets met the nutritional requirements. Muscle EPA levels showed a noticeable decline, while ETA levels exhibited a significant increase in SWM-fed fish compared to the control group. This pattern can be attributed to the reduction of EPA and the rise in ETA in SWM-based diets relative to FM. Furthermore, EPA levels decreased with higher SWM levels, suggesting that SWM may lack sufficient FAs. Despite the lack of balance in EFAs, the experimental diets did not negatively affect muscle FA profiles. The nonsignificant DHA levels in SWM-fed fish relative to the control can likely be explained by the biological conversion of EPA to DHA. This is due to the selective retention of DHA and the ability of beluga sturgeon to synthesize long-chain HUFAs from shorter precursors, a capability well-documented in many fish species [56].
Thus, the findings of this study indicate that replacing FM with SWM at inclusion levels of 5%–20% does not negatively impact the EFA composition of H. huso. It is possible that increasing the replacement level beyond 20% could further enhance the effectiveness of SWM in meeting the EFA requirements of beluga sturgeon compared to FM.
The relationship between AA concentration in muscle tissue and AA requirements has been well-documented [57, 58]. In this study, the highest and lowest muscle Met were observed in the control and SWM20 treatments, respectively. Diets containing SWM had higher Met levels compared to the FM-based diet. However, a downward trend in the muscle concentration of this EAA was observed in juvenile beluga sturgeon. This decline was statistically significant only in the SWM20 group, which may be attributed to the utilization of muscle Met for cysteine synthesis. As a sulfur-containing AA, cysteine plays a crucial role in the antioxidant defense system [59, 60]. Nevertheless, confirming this hypothesis requires further research on the antioxidant efficiency of replacing FM with SWM in sturgeon diets.
Arg levels were higher in SWM-based diets compared to the FM diet. However, despite this increase in dietary Arg, its concentration in the muscle tissue of juvenile beluga sturgeon declined. However, lysine (Lys) levels did not differ significantly among treatments. This reduction in muscle Arg, along with the unaffected Lys levels in SWM-fed fish, may be explained by a potential inhibition of intestinal Arg absorption due to competition between Arg and Lys transporters in the gut. Since both AAs share the same transport system in the intestine, this competition could lead to reduced Arg uptake [61–63].
In the present study, despite the higher dietary His levels in SWM-based diets, a decrease in muscle His concentration was observed in SWM-fed groups, with a statistically significant reduction occurring only in the SWM5 group. This decline in His levels could be linked to the elevated dietary Arg content in SWM diets. One possible explanation is that muscle His in SWM-fed fish was utilized for carnosine synthesis, given its immunomodulatory and antioxidant properties, which help mitigate oxidative stress in muscle tissue [64]. This mechanism could also explain the observed enhancement of innate immune markers (e.g., LYZ and ACH50 activity) in SWM-fed fish. In the present study, TEAAs were above 45% in all SWM treatments, meeting the protein needs of juvenile H. huso and showing no negative impact on muscle EAAs quality.
The AD of protein, lipid, and ash after substituting SWM for FM in aquatic diets has not been extensively researched. The lowest AD of protein was seen with 5% SWM, significantly lower compared to 15% and 20% SWM. The nutritional value of SWM is influenced by its chitin content, which can change protein availability [65]. Chitin, a polysaccharide, can affect AA balance if not enzymatically hydrolyzed [66]. Chitinase enzymes, particularly β-f-N-acetylhexosaminidase, can hydrolyze chitin [67]. Higher chitin content in diets can enhance chitinase activity [68]. Thus, lower chitin content in 5% SWM diets may have resulted in reduced protein AD and growth, as our results confirm this.
Three key chitinolytic enzymes—chitinase, chitobiase, and LYZ—play a major role in breaking down chitin in the digestive system of fish [69]. The efficiency of chitin digestion in the gastrointestinal tract is directly influenced by the composition of chitinase-producing bacterial communities in the fish’s gut microbiota. Rimoldi et al. [49] reported that crustacean waste meal, compared to FM, led to a higher abundance of chitinolytic bacteria in the intestinal microbiota of rainbow trout, particularly species belonging to the genera Bacillus, Paenibacillus, Brevibacterium, and Actinomyces.
Accordingly, chitinase produced by the gut microbiota enhances digestion by breaking down dietary chitin, thereby improving the availability of digestive enzymes to essential nutrients such as proteins and lipids [50, 70]. This process ultimately contributes to better nutrient absorption and improved gastrointestinal health in fish [71]. This mechanism may explain the increased AD) of dietary protein in the SWM15 and SWM20 groups (which contained higher chitin levels) compared to the SWM5 group.
LYZ activity is an important indicator of innate immunity in fish. LYZ targets both gram-positive and gram-negative bacteria and stimulates phagocytic cells [72]. Complement systems, part of innate and acquired immunity, help eliminate pathogens [73]. This study found higher LYZ and ACH50 activities in juvenile H. huso fed diets with SWM, indicating improved immune function. No previous data on the effects of SWM substitution for FM on immune modulation exist, but the use of chitin-rich shrimp shells may enhance immunity, aligning with studies on chitin and its derivatives in aquatic diets [47, 74, 75]. The observed immune system stimulation may be due to the abundance of carotenoid, imino acids (IAs), chitin, and their interaction with mannose/fructose receptors on white blood cells [76, 77].
5. Conclusion
In this study, SWM was added to the diets of juvenile beluga sturgeon at four different levels: 5%, 10%, 15%, and 20%. The findings revealed that, except for the negative impact of SWM5 on GP and the increase in FCR observed in the SWM10 group, the 15%–20% inclusion levels of SWM did not adversely affect growth, FCR, whole-body composition, muscle concentrations of EFAs (PUFA, HUFA, and n3/n6), or nutrient digestibility. Although the 20% SWM diet resulted in a reduction in the percentage of TEAAs in muscle tissue, this decrease did not indicate a negative effect on muscle AA composition, as TEAAs still accounted for more than 45% in the SWM20 group. Additionally, immune function improved across all SWM inclusion levels (5%–20%). Given these results, future studies should focus on assessing the diversity and abundance of chitinolytic bacterial communities in the gut when crustacean waste meals, such as shrimp by-products, are included in sturgeon diets. This would provide a more precise understanding of the digestive mechanisms, nutrient absorption processes, and overall health of sturgeon. Based on the observed improvements in health indicators and the absence of negative effects on growth and nutritional performance, incorporating SWM at a 15%–20% replacement level can be considered a viable option for juvenile beluga sturgeon diets.
Ethics Statement
All procedures involving animals were done according to the Tarbiat Modares University protocols, which seek to optimize handling and minimize animal suffering following the guidelines adopted from the Declaration of Helsinki (1975) and the Society for Neuroscience Animal Care and Use guidelines (1998) approved for implementation by the Medical Ethics Committee, School of Medical Sciences of the Tarbiat Modares University on April 17, 2006.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Amirsoheil Taheri: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, software, validation, visualization, roles/writing–original draft, writing. Abdolmohammad Abedian Kenari: conceptualization, funding acquisition, project administration, resources, supervision, validation, writing–original draft, writing–review and editing. Maryam Aftabgard: data curation, visualization, writing–original draft, writing–review and editing.
Funding
This research was funded by Tarbiat Modares University (TMU, Iran).
Acknowledgments
The authors would like to give thanks to the Tarbiat Modares University (TMU, Iran) for their financial supports.
Open Research
Data Availability Statement
The data are available upon request.




