Effect of Exogenous Dietary Protease and a Humic Substance on Growth and Microbial Composition of Nile Tilapia (Oreochromis niloticus) and Romaine Lettuce (Lactuca sativa) in a Biofloc-Integrated Decoupled Aquaponics System
Abstract
Aquaponics and biofloc-based aquaculture systems are environmentally sustainable food production systems. When biofloc technology is combined with hydroponic systems, it expands economic diversity by producing additional value-added products. Dietary additives were incorporated into fish feeds in this trial to improve food production in decoupled aquaponic systems. In this decoupled aquaponic system, the biofloc aquaculture system consisted of nine cylindrical tanks of 3750 L with 170 juvenile Nile tilapia (9.99 ± 0.54 g) stocked in each tank. The fish were randomly assigned to one of three treatment groups: fish fed a commercial feed (Com), commercial diet + protease complex (PC; AG175 by Jefo Nutrition; 175 g 1000 kg−1), and commercial feed + humic substance (HS; MFG50 by Kent Nutrition; 2500 g 1000 kg−1). The feeding trial was conducted for 60 days. A deep-water culture hydroponic system with nine 109 L troughs was used. Each trough corresponded to each tank and contained 15 romaine lettuce plants. The first 4 weeks were fish growth trials, and the last four were fish and plant growth trials. Fish and plant growth parameters showed no differences between treatments. However, the leaf greenness in romaine lettuce plants in the PC treatment had significantly higher chlorophyll content than the other treatments (p = 0.002). When microbial communities were analyzed, significant differences were found in the Shannon diversity indices (fecal, water, and root p < 0.001), Chao1 (fecal p < 0.002, water p < 0.001, and root p < 0.001), and observed species (feca; p = 0.046, water p = 0.017, and root p = 0.015). When beta diversity was analyzed through the Bray–Curtis dissimilarity index, fecal samples (p = 0.001) and root samples (p = 0.002) showed clear differences. The most abundant phyla found in all samples were Pseudomonadota. Overall, this study concluded that these additives did not affect the growth of Nile tilapia and romaine lettuce but influenced the bacterial composition of fish feces, water, and root samples and the chlorophyll content of the plants.
1. Introduction
Aquaculture is one of the fastest-growing food production sectors worldwide. The development and adoption of aquaculture have significantly improved household food security and the nutritional status of people worldwide [1]. Integrated aquaculture systems are recognized as a modern and more sustainable production method; aquaponics is an example [2]. Closed aquacultural systems, like biofloc systems, are one approach to achieving sustainable aquaculture practices [3]. In aquaponic systems, aquaculture effluents are transformed by nitrifying bacteria into bioavailable nutrients that are recycled and utilized by plants in the system, and low volumes of water are used, which reduces the negative environmental impacts usually associated with low-efficiency conventional food production [4]. The central concept behind this technology is maintaining the carbon:nitrogen ratio in an aquaculture system with limited water exchange and stimulating the growth of heterogeneous microbes by adding an external carbon source. These heterogeneous microbes assimilate nitrogenous waste generated to produce microbial biomass that can serve as a food source for the fish species cultured within the biofloc systems [5]. Biofloc systems provide a wide range of benefits to the organisms reared. The microorganisms living in the biofloc systems produce protein-rich microbial biomass that fish can consume. Also, the heterotrophic bacteria in biofloc systems improve and control water quality through denitrification processes [6]. Regarding the plants, those produced in hydroponic systems thrive in aquaponics [7]. The deep water culture (DWC) technique is one way to cultivate plants in aquaponic systems. It involves suspending plant roots in a nutrient-rich, oxygenated water solution without soil. Due to its simplicity and efficiency, this technique is prevalent for growing leafy greens and herbs in aquaponic systems. As oxygen is vital for plant growth and development, regular monitoring of oxygen in DWC hydroponic systems is essential [8].
For aquaponic operations to be successful, aquaculture species must have suitable characteristics for production in intensive recirculation systems. Tilapia can be raised easily in biofloc systems as they are hardy, tolerate high stocking densities, handling, and a wide range of physical–chemical water parameters [9]. Tilapia are a species that is being produced intensively worldwide. Because tilapia feed on low trophic levels in the food chain, they can easily adapt to effectively consume biofloc as a secondary food source. Aquaculture expansion also depends upon developing adequate formulated feed for the cultured aquatic species. Functional feed ingredients, such as immunostimulants, have been evaluated to improve production parameters, including disease management. The industry has traditionally relied upon antibiotic use for disease mitigation, but antibiotic treatments can pose a risk to human health through misuse and antimicrobial resistance [10, 11]. Immunostimulatory compounds are both biological and synthetic. Immunostimulants can be incorporated into the diet to increase the nonspecific cellular and humoral defense mechanisms and curb disease [12, 13]. Additionally, these substances maintain the metabolic processes and are shaped to maintain the structure and growth of organisms [14]. Various exogenous enzymes have been used as feed supplements to increase plant-based nutrient availability, improve digestion, and eliminate anti-nutritional factors. Additionally, humic substances (HSs), such as humic acids and fulvic acids, have the ability to be used as functional fish feed additives. Humic acids can be found in soil, fossil fuels, peat, and brown coal and are regarded as the main organic compounds present in these matter types [15]. Some beneficial effects were found to be present in growth through increasing metabolic processes during the application of HSs in fish feed. Additionally, they improved microbial community diversity [16].
Biofloc systems produce a large amount of sediments during the cultivation of fish species. The most effective method of minimizing sedimentation is the combination of biofloc systems with hydroponic systems, which cultivate plants. The plants can use these effluents produced by biofloc systems as a source of nutrients. This can be a more efficient and environmentally sustainable approach to cultivating both fish and plants [17]. The study of microbial composition of the two main components (fish and plants) in aquaponics systems is relatively unexplored. The microbiome composition of the host can be influenced by various factors, and diet/nutritional status is one of those factors [18]. The different microbial species present in the gastrointestinal tract and other body parts play a vital role in maintaining a homeostatic physiological environment and protecting the host from pathogens [19]. A healthy microbial community in a rearing environment also contributes to maintaining good water quality conditions. Microbial biofilms contain autotrophic nitrifying bacteria such as Nitromonas spp. (ammonium oxidizers) and Nitrospira spp. (nitrite oxidizers) remove toxic ammonia (NH3), producing nitrate (NO3−) in the process [20]. When it comes to plants, the microbiome could help their growth and health through growth stimulation and biocontrol. Taxa, such as the Microbacteriaceae family, can form associations with the plant roots [21].
Given the importance of both functional feed additives and the microbial communities within biofloc system development, more research is needed on the system and microbial interactions and their impact on fish performance. In this study, dietary additives were added to the fish feed to examine their influence on the growth and changes to the microbial communities of Nile tilapia intestines and romaine lettuce reared in biofloc system water.
2. Materials and Methods
2.1. Ethics Statement
All procedures involving the handling and treatment of fish used during this study were approved by the Auburn University Institutional Animal Care and Use Committee (AU-IACUC, Protocol # 2022-1124) prior to initiation. The authors declare no conflict of interest.
2.2. Availability of Data
The data supporting this study’s findings are available from the corresponding author upon reasonable request. The raw 16S rRNA gene sequencing data will be accessible under NCBI BioProject accession number (PRJNA1171849), with individual sample sequencing files available via the Sequence Read Archive (SRA) under accession numbers (SRX26355919 through SRX26356003).
2.3. Fish Culture System
This study was conducted in nine 3.8 m3 cylindrical polypropylene tanks in a greenhouse at the E.W. Shell Fisheries Center at Auburn University (Auburn, AL). Each tank contained two long (120 cm) diffusers (rubber/polyethylene diffuser hose, Pentair Aquatic Eco-Systems, Inc., Apopka, FL) hooked to a common 1.5 hp, Sweetwater regenerative blower (Pentair Aquatic Eco-Systems, Inc., Apopka, FL). Biofloc water to the system was pumped from the adjacent 100 m3 (26,417 gal.) tank that contains biofloc water. The system was recirculated 7 days prior to the start of the trial to attain a similar concentration of biofloc nutrients. To control the collection of solids in the biofloc water, each tank was connected to a conical chamber (113.6 L) receiving water from the main culture tank via a powerhead (Maxi-Jet 110 gph) with settled water returned to the culture tank.
2.4. Fish Growth Trial
Each of the nine culture tanks was stocked with 170 juvenile Nile tilapia averaging 9.99 ± 0.54 g. The growth trial ran for 60 days, and three formulated diets were used to feed the fish. A commercial diet (SouthFresh Feed, Demopolis, AL) containing 28% crude protein (Com) served as the control and the basal diet formulation into which additive treatments were incorporated. The second diet consisted of a protease complex (AG175; Jefo Nutrition Inc., QC, Canada) incorporated into the commercial diet at 175 g 1000 kg−1 (PC). This AG175 is an alkaline serine endopeptidase produced through bacterial fermentation. The third diet was supplemented with a HS at 2500 g 1000 kg−1 (HS; MFG50; Kent Nutrition Muscatine, IA), a broad-spectrum organic acid extract from freshwater reed-sedge peat. The dosage of PC and HS were added to the commercial diets according to the manufacturer’s instructions. The diets were formulated and extruded by a commercial catfish feed mill. The diets were sent to Midwest Laboratories (Omaha, NE) for proximate composition analysis (Table 1). The proximate composition of the diets showed that the control diet (Com), regarded as a 28% protein diet by the manufacturer, contained a lower protein (25.6%) than the PC and HS. Tanks were randomly assigned to one of the three diet groups, and each group was repeated in triplicate. The fish were offered experimental diets at 5% body weight, and the feeding ration was adjusted every 2 weeks based on fish growth and feeding response. The fish were fed twice daily, morning and evening. Fish were sampled every 2 weeks to calculate biomass gains. At 60 days, individual tanks of fish were harvested, group-weighed, and counted. Five fish were sampled on day 0, and five were sampled per tank per treatment at the end of the trial for proximate analysis. Moisture (AOAC 930.15), crude protein (AOAC 990.03), crude fat (AOAC 2003.05), fiber (acid detergent via ANKOM) and ash (AOAC 942.05) were all determined in terms of dry weight. The performance parameters were calculated as follows. Total digestible nutrients (TDNs) and digestible energy (DE) were calculated using the online finished feed matrix software at Midwest Laboratories. For the calculations, the laboratory used a protein factor (Pf) of 0.73, a fat factor (Ff) of 2, an acid detergent fiber factor (ADFf) of 0.58, and a nitrogen-free extract factor (NFEf) of 0.90. The factors are based on unique feedstuff sample types within the software. The software calculations were based on the following equations (P = protein, F = fat, A = ash, ADF = acid detergent fiber):
| Parameter | Com | PC | HS |
|---|---|---|---|
| Crude protein (%) | 25.60 | 30.55 | 30.80 |
| Fat (acid hydrolysis, %) | 4.30 | 4.55 | 4.31 |
| Ash (%) | 7.91 | 6.84 | 6.43 |
| Fiber (acid detergent, %) | 14.15 | 10.60 | 9.85 |
| Sulfur (%) | 0.27 | 0.29 | 0.28 |
| Total phosphorus (%) | 0.88 | 1.01 | 1.01 |
| Total potassium (%) | 1.27 | 1.41 | 1.44 |
| Total magnesium (%) | 0.32 | 0.37 | 0.37 |
| Total calcium (%) | 0.57 | 0.64 | 0.58 |
| Total sodium (%) | 0.05 | 0.08 | 0.07 |
| Total iron (mg kg−1) | 206.50 | 183.00 | 183.50 |
| Total manganese (mg kg−1) | 116.00 | 91.80 | 88.20 |
| Total copper (mg kg−1) | 21.60 | 16.20 | 16.50 |
| Total zinc (mg kg−1) | 165.00 | 110.00 | 120.00 |
| TDN (%) | 68.45 | 72.80 | 71.10 |
| Moisture (%) | 9.28 | 8.32 | 10.51 |
- Note: A commercial feed (Com), a commercial feed with AG175 (PC), or a commercial feed supplemented with a humic substance (HS).
- Abbreviations: HS, humic substance; PC, protease complex; TDN, total digestible nutrients.
2.5. Water Analysis and Solids Management
Water quality parameters, including dissolved oxygen (DO), salinity, and water temperature, were measured twice (AM and PM) daily using a YSI-55 digital oxygen/temperature meter (YSI Corporation, Yellow Springs, OH) and were maintained within acceptable ranges [22]. Total ammonia nitrogen (TAN) and nitrite-N were measured twice weekly using a YSI 9300 photometer (YSI Corporation, Yellow Springs, OH). pH was measured twice weekly using pHTestr30 (Oakton Instrument, Vernon Hills, IL) and was maintained at 6.5–7.0. Lime was added as needed to maintain pH. If the pH was below 6.5, 50 g of hydrated lime was mixed with tank water into a hydrated lime slurry. That slurry was then added around the tank perimeter so that the system water quickly and evenly mixed.
2.6. Plant Culture System
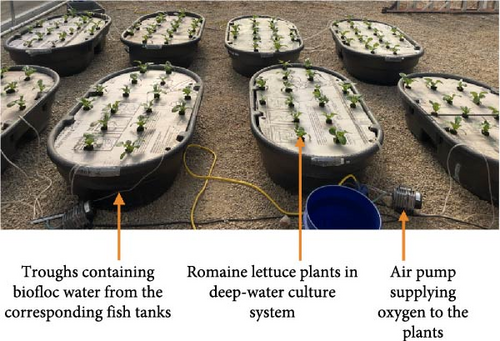
Lactuca sativa “Monte Carlo” (romaine lettuce) seeds were chosen and sown in a 50-cell count tray 3 weeks before the plant growth trial. Seedlings were grown under greenhouse conditions and watered as needed. Fifteen Romaine lettuce seedlings were transplanted into each of nine 208 L holding capacity plant troughs. The plant system employed a complete randomized design in a DWC system. The plant troughs were matched with the fish tank system, with each treatment group represented by three replicates. Water (biofloc sludge + biofloc water) was forced through settling cones connected to each respective tank to ensure a consistent water supply to plant troughs (Figure 1). Continuous aeration to each trough was provided by four air stones (AS4–1.5 × 1.5 inch; Pentair Aquatic Eco-Systems, Inc., Apopka, FL) connected to an air pump (Active Aqua Commercial Air Pump, 60 W, 70 L min−1, Hydrofarm, Shoemakersville, PA). Approximately 20% (40 L) of the water in the plant troughs was replaced with water from the fish system weekly to maintain water quality parameters and optimize conditions for experimental trials while allowing for adequate nutrient buildup in the fish systems. The plant trial was initiated after 4 weeks of the fish feeding trial and continued for another 4 weeks till the romaine lettuce plants reached a marketable size. Water quality was maintained within the target ranges for each parameter (Table 2).
| Parameter | Target range |
|---|---|
| pH | 6.0–7.0 |
| EC | 1.7–2.5 mS cm−1 |
| DO | >5 mg L−1 |
| Salinity | <0.5 ppt |
| NO3− | 10–50 mg L−1 |
| K+ | 30–50 mg L−1 |
| Ca2+ | 40–80 mg L−1 |
| Na+ | <50 mg L−1 |
- Abbreviations: DO, dissolved oxygen; EC, electrical conductivity.
2.7. Data Collection–Plant System
For the microbiome analysis, tissues were taken from five lettuce plants before starting the trial and four root samples each from the trough after the end of the trial.
2.8. Microbial Composition for Fecal Matter and Water Samples
Fish fecal matter microbial composition samples were taken on day 0 (four random fish digesta) and the last day of the trial (three fish from each tank). The fish were killed using 250 mg L−1 of tricaine methane sulfonate (MS-222) buffered to a pH of 7.0–7.5 in culture water. Fish were aseptically dissected, and fecal samples were collected from the distal intestine of fish. The intestine was gently squeezed to collect fecal (digesta) samples into a cryovial. The collected digesta were flash-frozen with liquid nitrogen and stored at −80°C until further processing. Water samples were also collected on day 0 and at the end of the trial. The water samples were processed using CP Select (InnovaPrep, Drexel, MO) with hollow filter pipette tips according to the manufacturer’s recommended settings. The pipette was eluted using an automated wet foam elution process with FluidPrep Elution Can-Tris (InnovaPrep, Drexel, MO). The eluted volume of water (1 mL) was used for microbiome analysis. All samples were stored at −80°C. For root microbial composition, the samples were collected on day 0 and at the end of the plant trial. Root samples were frozen with liquid nitrogen before transferring them to −80°C. DNA extraction on all the samples was performed with a DNeasy PowerSoil Pro Kit (Qiagen, CA). Samples were then sent for library preparations and 16S rRNA sequencing using the ZymoBIOMICS Targeted Sequencing Service (Zymo Research, Irvine, CA).
In brief, the bacterial 16S ribosomal RNA gene sequencing was performed using the Quick 16S NGS Library Prep Kit (Zymo Research, Irvine, CA) with primers designed to amplify the V3–V4 regions of the 16 s rRNA gene. The final PCR products were quantified with qPCR and pooled equimolarly. The final pooled library was cleaned with a Select-a-Size DNA Clean & Concentrator (Zymo Research, Irvine, CA), then quantified with TapeStation (Agilent Technologies, Santa Clara, CA) and Qubit (Thermo Fisher Scientific, Waltham, WA). The final library was sequenced on Illumina Nextseq with a P1 reagent kit (600 cycles). The sequencing was performed with a 30% PhiX spike-in.
2.9. Statistical Analysis
Growth performance data were analyzed using SAS software (Version 9.4, SAS Institute, Cary, NC). A one-way ANOVA was performed, and a Tukey’s multiple comparison test was subsequently applied to assess significant variations among treatment means (p < 0.05). These included growth performance, proximate whole-body composition, mineral composition of fish, romaine lettuce growth parameters, foliar tissue analysis of romaine lettuce, and water quality parameters of the hydroponic system. All percentage data were arcsine transformed. Water quality parameters were also assessed using one-way ANOVA with repeated measures.
Bacterial data were analyzed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Shannon diversity, Chao1, and observed species indices values for the microbial composition of water, fecal samples, and root samples between different treatments were analyzed using Kruskal–Wallis. Microbiota beta diversity of fecal matter, water, and root samples was assessed at the ASV level using unweighted UniFrac, weighted UniFrac, and Bray–Curtis dissimilarity. Beta diversity was also evaluated at the genus level by calculating Bray–Curtis dissimilarity matrices after agglomerating ASVs at the genus level. The vegan package [24] was used to evaluate the homogeneity of dispersion (betadisper). Permutational multivariate analysis of variance (PERMANOVA) (BiodiversityR; [25]) was conducted on all distance metrics to test for shifts in beta diversity centroids between experimental dietary groups.
3. Results
3.1. Fish Growth Performance
Nile tilapia growth performance parameters were not different across treatments (Table 3). The whole-body proximate analysis revealed significant differences in moisture content (Com-76.0%, PC-73.3%, and HS-75.9%) (p = 0.038), but the post-hoc test yielded no differences among treatments. The dry matter content also showed differences between the treatments (Com-23.9%, PC-26.7% and HS-24.2%) (p = 0.032). However, protein, dry weight, fat, and ash showed no differences between treatments (Table 3).
| Parameter | Com | PC | HS | PSE | p-Value |
|---|---|---|---|---|---|
| Growth performance | |||||
| Final biomass (g) | 7503.9 ± 557.7 | 7793.4 ± 861.8 | 8400.6 ± 208.6 | 0.668 | 0.736 |
| Final mean weight (g) | 46.0 ± 2.7 | 46.7 ± 6.1 | 52.7 ± 3.6 | 5.360 | 0.093 |
| Weight gain (g) | 36.0 ± 2.3 | 37.1 ± 5.8 | 42.4 ± 3.2 | 6.130 | 0.278 |
| Weight gain (%) | 358.8 ± 16.2 | 385.0 ± 52.1 | 410.8 ± 15.7 | 0.110 | 0.760 |
| FCR | 1.7 ± 0.0 | 1.6 ± 0.2 | 1.5 ± 0.1 | 0.110 | 0.586 |
| Survival (%) | 95.9 ± 2.0 | 98.2 ± 2.1 | 93.9 ± 4.1 | 4.000 | 0.373 |
| Discharged solids | |||||
| Total solids (kg) | 361.2 ± 248.8 | 472.1 ± 161.1 | 5554.6 ± 38.99 | 59.87 | 0.439 |
| Solids per unit of fish (kg) | 47.8 ± 32.4 | 59.9 ± 17 6 | 66.1 ± 5.4 | 12.40 | 0.601 |
| Solids per unit of feed input (kg) | 37.6 ± 26.1 | 48.4 ± 16.4 | 55.1 ± 5.7 | 10.50 | 0.528 |
| Ash (%) | 83.8 ± 15.7 | 90.8 ± 9.5 | 90.9 ± 12.8 | 7.50 | 0.757 |
| Whole body proximate composition | |||||
| Moisture (%) | 76.0 ± 1.1 | 73.3 ± 1.4 | 75.9 ± 0.8 | 0.322 | 0.038 |
| Dry matter (%) | 23.9 ± 1.1b | 26.7 ± 1.4a | 24.2 ± 0.4ab | 0.305 | 0.032 |
| Protein DW (%) | 62.53 ± 7.3 | 55.3 ± 5.2 | 55.83 ± 0.3 | 0.640 | 0.239 |
| Fat DW (%) | 21.7 ± 1.5 | 24.7 ± 3.6 | 23.3 ± 0.1 | 0.410 | 0.333 |
| Ash DW (%) | 14.83 ± 2.4 | 16.5 ± 2.3 | 12.3 ± 0.1 | 0.393 | 0.093 |
- Note: Different letters denote statistical differences among treatment groups.
- Abbreviations: DW, dry weight; FCR, feed conversion ratio.
3.2. Water Quality
Water quality parameters for the trial are presented for each treatment in Table 4. Evaluation of the data showed no statistical difference between treatments except for temperature (higher than other treatments) in HS-supplemented tanks (p = 0.030). The data analyzed for total solids generated (p = 0.439), solids per unit of fish (kg) (p = 0.601), solids per unit of feed input (kg) (p = 0.528), and ash (%) of the solids (p = 0.757) revealed no significant differences (Table 3).
| Parameter | Com | PC | HS | PSE | p-Value |
|---|---|---|---|---|---|
| DO (mg L−1) | 7.76 ± 0.46 | 7.81 ± 0.46 | 7.82 ± 0.45 | 20.04 | 0.263 |
| Temperature (°C) | 26.26 ± 1.47a | 25.77 ± 1.3b | 25.68 ± 1.3b | 1.46 | 0.030 |
| pH | 7.38 ± 0.50 | 7.40 ± 0.62 | 7.51 ± 0.24 | 0.31 | 0.365 |
| Salinity (mg L−1) | 0.87 ± 0.05 | 0.85 ± 0.09 | 0.89 ± 0.07 | 0.21 | 0.570 |
| Total ammonia nitrogen (mg L−1) | 0.30 ± 0.25 | 0.26 ± 0.25 | 0.2 8 ± 0.27 | 0.15 | 0.853 |
| Nitrite (mg L−1) | 0.36 ± 0.31 | 0.36 ± 0.30 | 0.36 ± 0.25 | 1.13 | 0.994 |
- Note: Values are presented as the mean ± standard deviation. Different letters denote statistical differences among treatment groups.
- Abbreviations: DO, dissolved oxygen; PSE, pooled standard error.
3.3. Romaine Lettuce Growth Parameters
Romaine lettuce growth parameters showed no differences (p > 0.05) except for the perpendicular width of the plants (p = 0.022) and leaf greenness of the plants, measured through chlorophyll content (SPAD values) (p = 0.002) (Table 5). The foliar tissue analysis from the romaine lettuce plants showed that, in all treatments, the macronutrients and some of the micronutrients were within sufficient ranges required by the plant [26]. However, Fe and Cu were deficient in plant leaves across all treatments. Macronutrients Mg (p = 0.011) and Ca (p = 0.001) content and the micronutrient Mn (p = 0.022) in the plants of the Com treatment were significantly higher than the other two treatments (Table 6). The water quality parameters for each treatment are presented in Table 7, but no treatment-related differences were detected for any of the tested parameters.
| Parameter | Com | PC | HS | p-Value |
|---|---|---|---|---|
| Height (cm) | 14.8 ± 2.3 | 16.9 ± 4.0 | 17.3 ± 4.1 | 0.666 |
| Widest width (cm) | 22.3 ± 0.6 | 23.5 ± 1.6 | 23.1 ± 1.0 | 0.460 |
| Perpendicular width (cm) | 21.2 ± 0.5b | 23.9 ± 0.9a | 21.7 ± 1.1ab | 0.022 |
| Leaf count | 20.9 ± 0.8 | 21.1 ± 2.2 | 23.5 ± 0.6 | 0.109 |
| Leaf greenness | 32.2 ± 11.2b | 44.3 ± 3.1a | 38.9 ± 3.1ab | 0.002 |
| Shoot fresh weight (g) | 179.8 ± 30.2 | 273.7 ± 125.8 | 260.0 ± 37.5 | 0.345 |
| Shoot dry weight (g) | 10.5 ± 1.2 | 13.8 ± 4.2 | 13.2 ± 3.9 | 0.493 |
| Root fresh weight (g) | 57.5 ± 23 | 53.9 ± 23.1 | 68.8 ± 18.0 | 0.692 |
| Root dry weight (g) | 4.7 ± 2.3 | 3.5 ± 1.0 | 4.3 ± 4.3 | 0.658 |
| Size index (cm) | 19.4 ± 0.9 | 21.4 ± 1.3 | 20.7 ± 1.6 | 0.310 |
- Note: Values are presented as the mean ± standard deviation. Different letters indicate significant treatment differences.
| Parameter | Com | PC | HS | p-Value |
|---|---|---|---|---|
| Macronutrients | ||||
| N (%) | 3.08 ± 0.42 | 3.38 ± 0.59 | 3.50 ± 0.38 | 0.057 |
| P (%) | 0.41 ± 0.09 | 0.46 ± 0.13 | 0.37 ± 0.05 | 0.058 |
| K (%) | 4.79 ± 0.63 | 5.29 ± 0.74 | 5.33 ± 0.91 | 0.115 |
| Mg (%) | 0.51 ± 0.15a | 0.38 ± 0.06b | 0.48 ± 0.09a | 0.011 |
| Ca (%) | 2.76 ± 0.81a | 1.78 ± 0.42b | 2.37 ± 0.54a | 0.001 |
| S (%) | 0.26 ± 0.05 | 0.28 ± 0.05 | 0.28 ± 0.04 | 0.225 |
| Micronutrients | ||||
| B (mg kg−1) | 29.47 ± 3.62a | 27.50 ± 2.44b | 32.35 ± 4.39a | 0.004 |
| Zn (mg kg−1) | 58.27 ± 22.94 | 71.28 ± 28.95 | 66.78 ± 14.48 | 0.308 |
| Mn (mg kg−1) | 143.27 ± 28.59a | 105.92 ± 29.95b | 136.14 ± 48.31ab | 0.022 |
| Fe (mg kg−1) | 44.74 ± 12.63b | 55.43 ± 12.21ab | 71.85 ± 32.18a | 0.005 |
| Cu (mg kg−1) | 1.94 ± 0.59b | 3.71 ± 1.20a | 3.35 ± 1.21a | <0.001 |
- Note: Values are presented as the mean ± standard deviation. Different letters indicate significant treatment differences.
| Parameter | Com | PC | HS | p-Value |
|---|---|---|---|---|
| pH | 7.81 ± 0.44 | 7.98 ± 0.21 | 8.00 ± 0.50 | 0.250 |
| EC (mS cm−1) | 1.36 ± 0.18 | 1.25 ± 0.17 | 1.36 ± 0.12 | 0.055 |
| DO (mg L−1) | 6.59 ± 1.89 | 6.48 ± 2.24 | 6.39 ± 2.35 | 0.957 |
| NO3 (mg kg−1) | 92.34 ± 110.18 | 133.52 ± 166.73 | 109.85 ± 129.46 | 0.624 |
- Note: Values are presented as the mean ± standard deviation.
- Abbreviations: DO, dissolved oxygen; EC, electrical conductivity.
3.4. Microbial Composition of Fish Fecal Samples, Biofloc Water, and Root Samples
The 16S rRNA gene high-throughput sequencing of the V3–V4 region was conducted by ZymoBIOMICS Targeted Sequencing Service (Zymo Research, Irvine, CA). This resulted in 7,948,428 sequences distributed among 35 fecal samples, 2,396,534 among 10 water samples, and 8,680,186 among 40 root samples. The median read depth for fecal samples was 229,092 ± 25,051, water samples 239,109 ± 18,041, and root samples 219,635 ± 23,657. Reads with large differences in read depth were removed to improve normalization and quality control. Rarefaction analysis curves indicated much of the diversity was adequately captured and reached saturation for downstream analysis.
3.4.1. Fish Fecal Samples
The microbial complexity of fish fecal matter among the three different treatments was estimated as the basis of the Shannon diversity index, Chao1 index, and observed species index. Apparent differences among the treatments were shown in all three indices tested: Shannon diversity index (p < 0.001), Chao1 (p = 0.002), and observed species (p = 0.046). HS-fed fish had the highest Shannon diversity index, and the Com treatment the lowest. The same trend was observed in the other two indices (Table 8).
| Parameters | Com | PC | HS | p-Value |
|---|---|---|---|---|
| Fecal samples | ||||
| Shannon diversity index | 4.9 ± 0.8b | 5.1 ± 0.8a | 5.2 ± 0.8a | <0.001 |
| Chao1 | 294.0 ± 92.9b | 320.8 ± 102.3ab | 334.4 ± 105.0a | 0.002 |
| Observed species | 265.8 ± 94.7b | 292.4 ± 103.6ab | 307.4 ± 107.8a | 0.046 |
| Biofloc water samples | ||||
| Shannon diversity index | 6.3 ± 1.1b | 6.0 ± 0.9ab | 5.7 ± 0.9a | <0.001 |
| Chao1 | 507.9 ± 162.8a | 452.9 ± 143.1ab | 389.0 ± 123.6b | <0.001 |
| Observed species | 453.8 ± 166.2a | 401.6 ± 147.0ab | 350.3 ± 125.8b | 0.017 |
| Root samples | ||||
| Shannon diversity index | 5.7 ± 1.0ab | 4.5 ± 0.7b | 5.9 ± 1.1a | <0.001 |
| Chao1 | 505.6 ± 160.9ab | 460.6 ± 149.3b | 580.3 ± 185.5a | <0.001 |
| Observed species | 459.5 ± 165.7ab | 410.0 ± 152.0b | 522.6 ± 191.2a | 0.015 |
- Note: Values are presented as the mean ± standard deviation. Different letters indicate significant treatment differences.
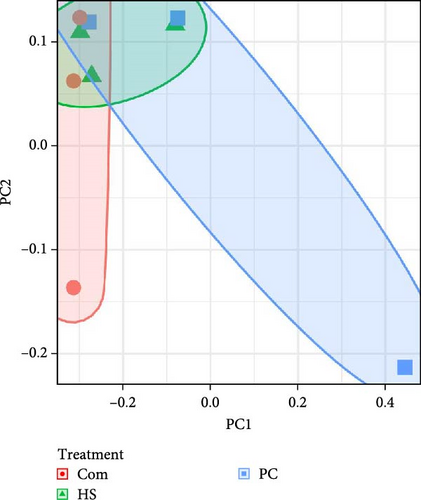
A principal coordinate analysis (PCoA) was used to compare the similarity in the microbial community composition (Figure 2B). The PERMANOVA treatment conducted on Bray–Curtis dissimilarity matrices based on ASV-level data showed statistically significant differences (p = 0.001) in fecal matter samples. Subsequent pairwise-PERMANOVA post hoc analyses indicated that the fecal matter of fish fed with the Com diet significantly differed from those fed the PC diet (p = 0.030) but not those fed with the HS diet. A similar trend was observed when weighted (P = 0.032) and unweighted Unifrac (p = 0.002) values and Bray–Curtis dissimilarity of ASV at the genus level (p = 0.002) was compared between treatments (Table 9). When post hoc tests were performed, there were no significant differences in terms of weighted Unifrac. However, in Unweighted Unifrac, the subsequent pairwise-PERMANOVA post hoc analyses indicated that fish fed with Com diet were different both from PC (p = 0.030) and HS (p = 0.045) diet-fed fish. In Bray–Curtis dissimilarity of ASV at the genus level, the post hoc tests revealed that Com diet fish were significantly different from the PC-fed group (p = 0.030).
| Beta diversity indices | Fecal samples | Water samples | Root samples |
|---|---|---|---|
| Betadisper | |||
| Unweighted UniFrac ASV | p = 0.191 | p = 0.607 | p = 0.100 |
| Weighted UniFrac ASV | p = 0.036 ∗ | p = 0.733 | p = 0.036 ∗ |
| Bray-curtis ASV level | p = 0.098 | p = 0.939 | p = 0.001 ∗ |
| Bray-curtis genus level | p = 0.186 | p = 0.960 | p = 0.001 ∗ |
| PERMANOVA | |||
| Unweighted UniFrac ASV | p = 0.002 ∗ | p = 0.439 | p = 0.001 ∗ |
| Weighted UniFrac ASV | p = 0.032 ∗ | p = 0.972 | p = 0.004 ∗ |
| Bray-curtis ASV level | p = 0.001 ∗ | p = 0.924 | p = 0.002 ∗ |
| Bray-curtis genus level | p = 0.002 ∗ | p = 0.989 | p = 0.002 ∗ |
- Note: Values are presented as the mean ± standard deviation.
- ∗Indicates a significant treatment difference.
The total bacterial abundance in fish fecal samples was classified into 17 phyla, and the most abundant in each treatment is shown in Figure 3A. The most abundant phylum in the Com treatment was Pseudomonadota, accounting for 25.04% of the relative abundance. The most abundant phyla in the PC and HS treatments were Planctomycetes (PC 31.56% and HS 30.30%), followed by Pseudomonadota (26.78% in PC and 25.98% in HC).
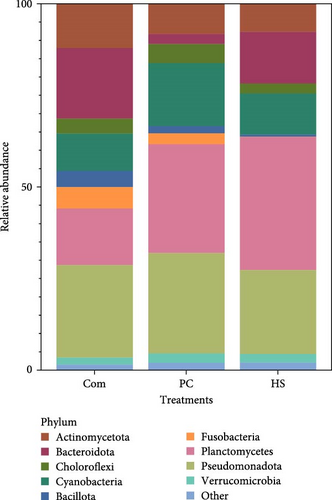
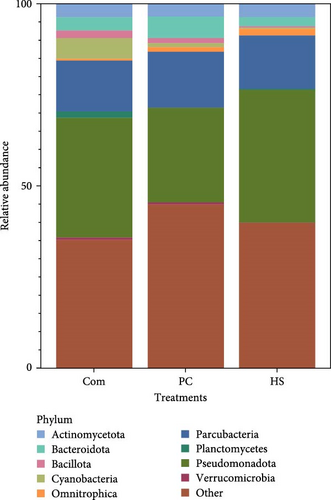
3.4.2. Biofloc Water Samples
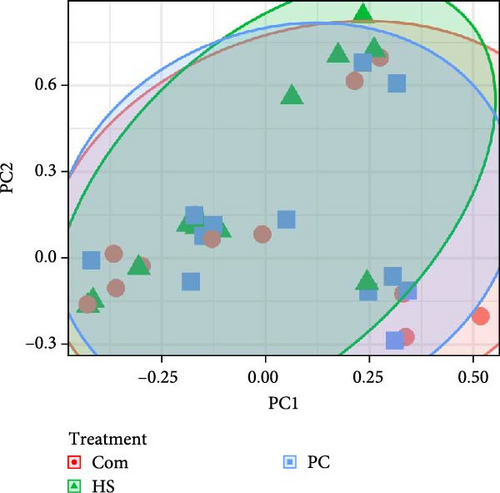
The Shannon diversity index in water samples showed clear differences between the biofloc water in different treatments (p < 0.001). Moreover, differences were observed in the other two indices, Chao1 (p < 0.001) and observed species (p = 0.017). For each of the indices, Shannon, Chao1, and observed species, the highest was reported in biofloc water in tanks receiving the commercial diet (Com), followed by PC and HS (Table 8). In contrast, beta diversity PERMANOVA by treatment conducted on Bray Curtis dissimilarity at ASV (p = 0.924) (Figure 2A) or genus level (p = 0.989) showed no statistical differences. This trend followed when unweighted (p = 0.439) and weighted (p = 0.972) Unifrac values were considered (Table 9). Twenty-eight phyla were observed in all water samples, and the most abundant are shown in Figure 3B. Across all treatments, Pseudomonadota was the most dominant phylum (Com −32.90%, PC −25.93%, HS −36.47%). Apart from that, other phyla, such as Parcubacteria, Actinomycetota, and Bacteroidetes, were present in all treatments.
3.4.3. Lactuca Sativa Root Samples
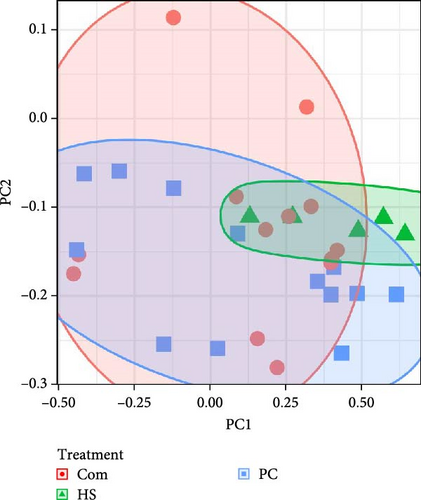
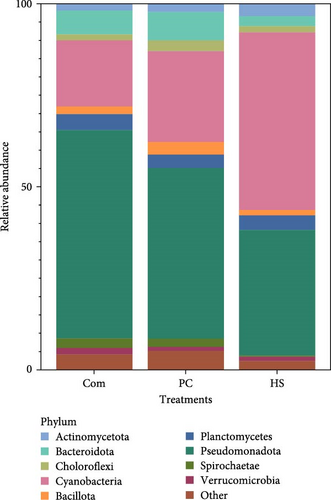
Alpha diversity, as measured through the Shannon diversity index, showed distinct differences between the treatments (p < 0.001). Additionally, Chao1 (p < 0.001) and observed species (p = 0.015) were also different among treatments (Table 8). Significant differences were found between treatments when assessing various beta diversity metrics in root samples. Specifically, upon conducting PERMANOVA by treatment, the Bray–Curtis dissimilarity index at the ASV level showed differences (p = 0.002) (Figure 2C). This trend was similar in the other metrics tested (Unweighted UniFrac-p = 0.001, weighted UniFrac-p = 0.004 and Bray–Curtis genus level-p = 0.002) (Table 9). When subsequent post hoc pairwise comparisons were made, it was found that in all beta diversity metrics, the Com diet-fed fish were significantly different from HS-fed fish (unweighted UniFrac: Com and HS p = 0.015, weighted UniFrac: Com and HS p = 0.015, Bray–Curtis at ASV: Com and HS p = 0.020 and Bray–Curtis at Genus: Com and HS p = 0.010. Among the Com and PC treatments, the most abundant phylum (Pseudomonadota) in root samples (Com 56.95%, PC 46.65%) was similar to water samples in fish production tanks. Cyanobacteria was the second most dominant phyla in these two treatments (Com 18.03%, PC 24.83%). This was reversed in HS-treated plant roots as cyanobacteria (48.55%) was the most abundant, followed by Pseudomonadota (34.41%). The most abundant phyla across all treatments are shown in Figure 3C.
4. Discussion
In this study, we explored the growth performance of Nile tilapia and romaine lettuce in an aquaponic system employing a protease complex and a HS, as dietary treatments. Protease complexes comprising enzymes that break down proteins into peptides and amino acids have gained attention in aquaculture as potential nutritional supplements to enhance nutrient utilization and overall fish growth. Numerous studies have observed that improving nutrient utilization in fish through the incorporation of dietary exogenous enzymes was linked to the solubilization or inhibition of various antinutritional factors, thereby enhancing nutrient absorption [27, 28]. According to Liu et al. [29], protease addition reduced the intestinal muscle layer thickness, thereby increasing the digestibility of nutrients and ultimately promoting the growth of the fish. Furthermore, proteases aid in the digestive breakdown of proteins into peptides and free amino acids. In a recent study conducted by Maryam et al. [30] involving rohu (Labeo rohita), dietary protease supplementation increased the activities of amylases, proteases, and lipases within both intestinal tissues and the hepatopancreas. This enhanced secretion of digestive enzymes contributes to efficient digestion and nutrient utilization. Proteases are also reported to positively affect apparent digestibility coefficients and nutrient retention, thereby increasing fish growth [31]. Furthermore, Wu et al. [32] also reported improved weight gain, feed conversion efficiency, and protein digestibility in tilapia-fed diets containing protease complexes. These findings suggest that the supplementation of proteases contributes to enhanced nutrient absorption and utilization by the fish. Apart from the growth-promoting abilities of protease complexes, they also have inhibitory effects on pathogenic bacteria in fish and may improve the immune responses of aquatic organisms [33].
HSs, derived from decayed organic matter in soil and water, have gained attention for their potential immunostimulatory properties in aquaculture. HSs, such as humic acid, fulvic acid, and humin, have been recognized for their diverse biological activities, including immunostimulatory effects [34]. HSs have many mechanisms that improve the digestive ability of many animals, including fish [35]. For instance, humic acids were found to increase intestinal viscosity, inhibit bacterial translocation from the intestine to the liver, and increase the secretion of digestive enzymes [36]. In addition, the development of viscous gels by dietary compounds such as HSs may create an increased viscosity in the small intestine, which results in a slower rate of feed passage through the gut. In turn, this increases the time of exposure of nutrients to digestive enzymes [35]. These HSs can create protective layers on the epithelial mucous membrane of the digestive tract, preventing the penetration of pathogenic bacteria or their toxins [36]. Digestive enzyme activities, such as pepsin, trypsin, and lipase, have also been associated with feeding humic acids in fish [37]. In past studies, positive outcomes from HS application have been identified in fish, mainly including enhancements in growth, intestinal microflora, lysozyme activity, stress response, and disease resistance. Studies have been mostly limited to common carp [38, 39], sea bass (Dicentrarchus labrax) [40], rainbow trout (Oncorhynchus mykiss) [41, 42], and channel catfish [43]. Musthafa et al. [44] reported increased growth performance and feed utilization of the Mozambique tilapia (Oreochromis mossambicus) when dietary humic acid was added to the feed. HSs may also demonstrate prebiotic effects. Several studies have reported that humic acids can be used as substrates that provide and increase the uptake of nutrients such as organic and inorganic compounds like nitrogen, phosphorus, and vitamins for fungi and bacteria [45]. The acidic groups of HSs also have antioxidant properties, and each humic acid macromolecule contains multiple antioxidant sites [46]. The HSs are characterized by a high content of phenolic units with persistent free radicals and a high electron exchange capacity [47]. Thus, HSs have the ability to improve the health of aquatic organisms by improving nutrient availability, providing antioxidant benefits, and supporting microbial activity by acting as prebiotics.
The utilization of functional dietary additives in tilapia within biofloc and aquaponic systems remains understudied. The present study helped fill this gap by assessing the effects of different dietary treatments on growth parameters in Nile tilapia. The lack of statistical differences in growth parameters among treatments suggests uniform effectiveness in supporting fish development across all three study diets. This information is crucial for developing sustainable aquaculture practices and warrants further exploration of dietary additive interactions in these complex systems. Given the inherent growth-promoting qualities of biofloc systems in isolation [48], the combined application of biofloc systems with PC and HS interactions was anticipated to yield similar results to the Com group. Although the addition of these additives demonstrated positive effects on growth in some reports, results without a major effect on growth performance, similar to the current findings of our study, have also been documented. Similar trends were also reported by Yigit et al. [49] in rainbow trout (O. mykiss), where no differences in feed intake, body weight, and weight gain were observed. Prokešová et al. [16] also discerned that the addition of HSs did not improve growth performance in African catfish (Clarias glariepinus). Another study by Adeoye et al. [50] on exogenous enzyme supplementation also specified no significant differences in specific growth rate and nutrient utilization in Nile tilapia between the protease-supplemented group and the control group with no protease supplementation. Collectively, these studies suggest that while dietary additives like HS and PC may have physiological and digestive benefits, their influence on the growth performance of fish may depend on various other factors such as diet compositions, environmental factors, and the culture environment.
The temperature in HS-supplemented tanks was the only statistically significant difference in water quality parameters between treatments. These temperature variations are likely due to specific conditions in the experimental setup, such as the proximity of tanks to exits. Overall, the mean values for water quality parameters were within acceptable ranges for Nile tilapia, indicating that the different dietary treatments did not affect water quality. Similar findings were found when HSs were added as a prebiotic to a system with Nile tilapia [51] and protease supplementation of Nile tilapia [52]. These results suggest that though the addition of HS or PC will not have significant differences compared to the control, they will not adversely affect the water quality parameters in aquaculture systems. In contrast to these findings of the current study, previous studies done by Gao et al. [53] and Sarkheil et al. [37] have identified that HS addition in aquaculture and aquaponic systems reduced TAN concentrations in the systems and increased nitrate accumulation. These reports have suggested that humic acid acts on microbes and promotes electron transfer efficiency, resulting in a stronger microbial metabolism activity and enhancing the cellular permeability of bacteria as mechanisms to reduce TAN in aquatic systems.
No prior investigations have assessed the impact of dietary supplements used in fish on plant growth parameters in aquaponic systems. While no significant differences in growth parameters were observed in romaine lettuce across various treatments, noteworthy enhancements in perpendicular width and leaf greenness were evident in plants treated with water originating from fish that offered protease complexes. These observed differences could be attributed to the specific nutrient compositions present in the diets. The AG175, which includes dehydrated yeast culture and a dehydrated Streptomyces fermentation soluble extract, may play a pivotal role in mitigating biotic and abiotic stress. This is done by producing various secondary metabolites by Saccharomyces spp. during the fermentation process [54]. Yeast contributes essential phytohormones and amino acids, fostering plant growth and chlorophyll production, consequently intensifying leaf greenness [55]. They explained that humic acid-mediated maintenance of Fe and Zn at sufficient levels is the key factor in elevating chlorophyll content in the leaves. Several studies have investigated the growth of lettuce when humic acids are applied directly. Lettuce growth appears to depend on the dosage of HSs, with significant differences observed in some cases [56, 57]. However, in contrast to these findings, Cimrin and Yilmaz [58] reported no significant differences in lettuce yield and growth. They suggest that this outcome could be attributed to variations in application levels of humic acids.
Aquaculture systems generate substantial quantities of organic compounds and nutrients, such as nitrogen and phosphorus, which necessitate management and proper disposal. These substances, formed through intricate biological processes involving the microbial breakdown of organic matter, encompass a range of components like vitamins, auxins, gibberellins, antibiotics, enzymes, coenzymes, amino acids, organic acids, hormones, and other metabolites. Plants directly absorb and assimilate these compounds, potentially influencing the availability of micronutrients, thereby promoting growth, increasing yields, enhancing vitamin and mineral content, and improving fruit flavor [59]. It was calculated that, over a culture period, fish have the potential to absorb ~11.46% iron (Fe), 13.43% zinc (Zn), 6.81% manganese (Mn), 3.55% copper (Cu), 26.81% calcium (Ca), 20.29% magnesium (Mg), 32.53% nitrogen (N), 7.16% potassium (K), and 15.98% phosphorus (P) from the input feed [60]. In comparison, the sludge settled on hydroponic troughs showed an average absorption rate of 23.93% Fe, 86.05% Mn, 46.17% Zn, 21.49% Cu, 15.71% Ca, 88.87% Mg, 5.55% N, 5.85% K, and 17.90% P from the input feed [61]. These values specify that fish assimilate, and sludge captures different minerals at different levels from the input feed. This also highlights how different components in aquaponic systems contribute to nutrient recycling and removal.
Aquaponic systems that depend exclusively on fish waste for providing nutrients to plants are noted to exhibit limited levels of phosphorus (P), potassium (K), iron (Fe), manganese (Mn), and sulfur (S) [60, 62, 63]. However, in this study, minerals such as phosphorus (P), potassium (K), manganese (Mn), and sulfur (S) were found to be present in adequate quantities in the leaves [26, 64]. Haghighi, Nikbakht, and Pessarakli [65] detailed that the application of humic acid to Gerbera plants in hydroponic systems increased the nutrient uptake of N, P, K, Zn, Ca, and Fe. Yet, in this study, the iron (Fe) and copper (Cu) levels were below the threshold for optimal plant growth. The plant root exudates can modify the chemical structure of HSs, and this process can potentially affect iron chelation, leading to iron uptake interference from the plants [66]. In future research, these micronutrients will need to be supplied for the romaine lettuce plants to optimize development and growth. Maibodi et al. [67] suggested that humic acid enhances nutrient uptake in plants by improving root development and membrane permeability, facilitating the absorption of essential minerals like iron. In the report, it was also explained that this nutrient uptake will depend on the type of plant and the medium in which the plants are grown. The P uptake will be high in soil environments but not in hydroponic systems. Similarly, the application of humic acid showed no differences in the foliar nutrient content of macro elements such as P, K, and Zn.
It is widely known that vertebrate gut microbiota plays vital roles in elucidating immune and better dietary responses [68]. The fish fecal microbiome is greatly influenced by dietary input [69]. When fish fecal samples were analyzed and the microbiome between treatments was compared, it indicated that diet influenced bacterial diversity. The feed additive treatments significantly increased the alpha diversity in the fecal matter compared to fish offered in the Com diet. The highest diversity was recorded in the HS treatment, and similar findings for elevated diversity have been previously reported [40–70]. Louvado et al. [40] observed that humic acid modulation in the diets had a significant increase in the bacterial communities and diversity, and in the study done by Gao et al. [70], an increase in Shannon diversity index was observed in the group with HS supplementation than the control group. Another study investigating Nile tilapia-fed exogenous proteases also described higher Shannon diversity indices than the control diet group [71]. This finding suggests that adding protease complexes and humic acid substances increases the diversity of microorganisms in fish digesta. In this study, AG175 and humic acid substances did not increase the Shannon diversity, Chao1, or observed species indices in biofloc water; however, romaine lettuce cultured in water derived from the HS treatment increased the richness and evenness of their root microbiome compared to the Com treatment. Similarly, Gao et al. [53] reported increased root microbial richness in lettuce plants when humic acid was supplemented in an aquaponic system. Although HS and PC increased bacterial diversity, they failed to produce any observable growth improvements. The nutrient access was adequate for the plants and fish, meaning microbial nutrient uptake improvements did not enhance plant or fish performance. This aligns with the findings of Maibodi et al. [67] where the authors found that HA may have limited effects when plants already receive adequate nutrients. As for beta diversity, a clear divergence between groups was detected, indicating that adding PC and HS would affect the overall microbial communities in the intestine of Nile tilapia and romaine lettuce roots. Similar results were found in the gut microbiome of snakehead fish (Channa argus) when exogenous enzymes were provided as feed additives [72]. For the PERMANOVA analysis, similar differences were also recorded in the intestinal community profiles in Nile tilapia [50].
In the current study, the dietary supplementation of PC and HS manipulated the microbial community in fish fecal matter, biofloc water, and romaine lettuce roots. In both PC and HS-treated fish, Planctomycetes had the highest abundance. Planctomycetes bacteria can transform nitrite and ammonium into dinitrogen gas and water [73]. Thus, these bacteria can simultaneously resolve the buildup of the two most toxic nitrogen species in aquatic animals [74]. Pseudomonadota is one of the most abundant bacterial phyla reported in biofloc systems [75] and, most notably, in aquaponic systems [76]. Psedomonadota, a group of gram-negative bacteria, play an important role in the degradation and fermentation of polysaccharides, proteins, and other organic matter and are the dominant flora in the intestinal microbial composition of many fish [70]. Similarly, it was the most abundant phyla in the Com treatment, including all biofloc water samples and root samples. Pseudomonadota also helps biofiltration in aquaponic systems, by assisting with nitrogen cycling [76]. There is also growing evidence that gut and surrounding microbiota will influence the growth of fish [77, 78]. While bacterial diversity increased due to the application of HS and PC, the overall bacterial community structure in fish intestines, biofloc water, and plant roots remained relatively similar across treatments as the most abundant phyla that can be seen in both water and gut in fish with all treatments is the same, it can be a reason to see no significant differences in growth of the fish. According to the results, the microbial ecosystem in fish intestines and plant roots remained stable, potentially reaching an equilibrium that supports growth but does not enhance it beyond a certain threshold. Another reason for not seeing any growth differences in both fish and plants could be because being more evident at the microbiome level rather than in macroscopic growth outcomes. Microbial changes may contribute to long-term resilience, health, or immune benefits rather than immediate growth improvements.
The bacteria also improved plant growth and were present in the rhizosphere as plant growth-promoting bacteria [79]. The similar bacterial composition between biofloc water and plant root treatments corresponds to similar growth parameters among treatments. Apart from these two main phyla, Bacteriodota and Cyanobacteria also were present in high percentages. Therefore, this study showed that adding PC and HS as dietary supplements affected microbial composition by increasing bacterial diversity and beneficial bacteria in the whole system. It was previously suggested by Islam, Schuhmacher, and Gropp [80] that HS stimulates the growth of potentially probiotic-type bacteria and modifies microbial fermentation in non-ruminant and ruminant animals. This is done through the formation of greater amounts of short-chain fatty acids, which reduces the pH of the medium and inhibits the growth of pathogenic bacteria. Humic acids can also increase the beneficial microbial community in roots. According to García et al. [81], when HS is applied to roots, humic acid fragments accumulate on the root surfaces and cause mild transient stress. Because of this, the plants modulate signaling through reactive oxygen species (ROSs), which induces a physiological state of protection against abiotic stress. They further describe that this stress may be one of the mechanisms that stimulate the shifts in the composition of communities and enrichments of bacteria. Regarding the influence of PC on microbial composition, Dai et al. [72] described that exogenous enzymes like proteases change the environment in the intestine in terms of pH or intestinal substrates. This could explain the increased microbial communities observed in the fish intestine in this study.
Dietary supplementation with HSs and protease complexes has been proposed as a promising approach to enhance immunity and modulate the microbiome in fish, offering protection against diseases. However, several limitations hinder the widespread application of these substances in aquaculture. One major limitation is the lack of comprehensive studies on the mechanisms and pathways through which these substances target specific cells, immune systems, and host–microbial communities [82]. Without a clear understanding of these mechanisms, optimizing their application strategies and use for targeted immune responses remains challenging.
Furthermore, a knowledge gap exists regarding the optimal dosages and duration of exposure necessary to achieve consistent and long-term benefits. Overusing or underusing these bioactive compounds could lead to suboptimal growth and immune responses or unintended microbial imbalances, affecting the system’s overall stability. The long-term effects of these functional additives, particularly in aquaponic and biofloc systems, remain poorly studied [83]. Since these systems primarily rely upon microbial interactions for nutrient cycling and water quality management, any alterations in the microbiome due to these additives could affect the system’s performance. Additionally, the variations in the chemical composition of HSs and protease complexes depending on the source and extraction methods can lead to inconsistencies in their performance. More research is needed to determine the most effective use of humic acids and protease enzymes in commercial aquaculture and aquaponics systems. Standardizing these compounds is also necessary to guarantee the reproducibility of study findings in practical applications [84]. Addressing these limitations through well-designed studies will be crucial in unlocking the full potential of these bioactive compounds in sustainable aquaculture in the future.
5. Conclusion
This study examined the influence of dietary additives on tilapia and romaine lettuce performance and health while reared in a decoupled aquaponic system. Based on these trial results, dietary supplementation with commercial protease complexes and HSs did not influence the growth parameters of Nile tilapia, the water quality of the biofloc system, or the growth of romaine lettuce plants. However, the protease complex significantly enhanced leaf greenness in the Romaine lettuce, so follow-up investigations for plant characteristics with these ingredients are warranted. Changes to the microbial community in terms of richness, evenness, and diversity compared to the control were observed in fish fecal samples, biofloc water, and plant roots. This improvement was shown in Nile tilapia fecal samples and romaine lettuce root samples. These outcomes suggest that applying these specific dietary supplements for fish reared in decoupled aquaponic systems contributes to the advancement of sustainable aquaculture practices by modulating the microbiome. This, in turn, supports the overall health and efficiency of the system by reducing diseases. Moreover, their potential to optimize the health and growth of fish and plants in aquaponic systems could lead to more resource-efficient, environmentally friendly production methods.
Conflicts of Interest
The authors declare no conflicts of interest
Funding
The research was supported by a Non-Assistance Cooperative Agreement between Auburn University and the USDA-Agricultural Research Service (#6010-32000-028-001-S) “Developing sustainable aquaponic production systems.” These studies were also partially supported by USDA National Institute of Food and Agriculture (NIFA) Hatch projects to Bruce (PI) under ALA016-1-19143 and Davis (PI) under ALA016-1-19102.
Acknowledgments
We thank Shrijan Bajracharya, Magida Tabbara, Trinh Ngo, Khanh Nguyen, and Stephanie Velasquez for feeding and trial support. We also thank the staff and students at the E.W. Shell Fisheries Center (Auburn University) for trial assistance.
General Statement
The mention of a trademark or proprietary product does not constitute an endorsement of the product by Auburn University and does not imply its approval to the exclusion of other products that may also be suitable.
Open Research
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request. The raw 16S rRNA gene sequencing data will be accessible under NCBI BioProject accession number (PRJNA1171849), with individual sample sequencing files available via the Sequence Read Archive (SRA) under accession numbers (SRX26355919 through SRX26356003).




