Determining the Best Weaning Age to Dry Feeds and the Influence of Phospholipids and Long Chain Polyunsaturated Fatty Acids (LC-PUFAs) on Digestive Enzyme Activity and Growth Performance of African Bony-Tongue (Heterotis niloticus) Fry
Abstract
Limited information exists on the dietary requirements of the African bony-tongue (Heterotis niloticus) fry as well as the suitable age to wean fry from live prey to formulated diets. These have been the major challenges to the commercial culture of the species. This study assessed the optimal weaning age of fry as well as the effect of varying phospholipid (PL) content and long chain polyunsaturated fatty acids (LC-PUFAs) on survival, performance, and digestive enzyme activity of H. niloticus fry. The two weaning diets used were formulated to be isonitrogenous, isolipidic, and isoenergetic (49% CP, 20% CL, and 22.7 MJ/kg). The diets differed only in the content of PLs and LC-PUFAs; eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Soy lecithin (PLs) and Algatrium DHA70 (a glyceride product high in DHA and EPA) were included at two levels. The diets were, respectively, labeled low phospholipid (LPP) (formulated to contain: 3.16% PLs, LC PUFAs: 0.66% DHA 0.10% EPA) and high phospholipid (HPP) (formulated to contain: 12.96% PLs, LC PUFAs: 3.04% DHA, 0.75% EPA). African bony-tongue fry were fed Artemia nauplii until 15, 25, 35, 45, 65, or 85 days after hatching (DAH), respectively, then cofed with Artemia and one of the two weaning diets for an additional 7 days after which Artemia feeding was completely withdrawn. Hereafter, the fry were maintained solely on the experimental diets for 10 days. A control group was kept on Artemia nauplii throughout the experiment. The results showed that the survival rate of fish on the LPP and HPP diets increased significantly (p < 0.05) with weaning age; from 0% (LPP and HPP) for fry weaned at 15 DAH to a survival rate of 69.4% ± 3.9% (LPP) and 80.0% ± 9.4% (HPP) at 65 and 85 DAH, respectively. This was, however, lower than the survival rate obtained for the control (Artemia) which was 93.3% ± 3.33% at 85 DAH. The final mean weight, weight gain, and survival rate improved significantly (p < 0.05) for fry fed the HPP diet compared to the LPP diet. All digestive enzyme activity decreased significantly with increasing fish age (p > 0.05). Based on the results, it is recommended, that H. niloticus fry is maintained on live Artemia nauplii and provided with dry feeds high in LC-PUFAs (33.98 mg g−1 diet) and PLs (12.96 mg g−1 diet) from <65 DAH to obtain higher survival rates.
1. Introduction
The African bony-tongue (Heterotis niloticus) is an omnivorous freshwater fish native to Africa. It has attracted a great deal of interest as an aquaculture candidate due to its fast growth, tolerance to culture conditions, high demand, and price [1]. A species’ capacity for culture is contingent upon factors, such as the ease with which fingerlings are produced for commercial culture [1–3]. Fish farmers mostly acquire wild Heterotis fingerlings from fishermen to stock their ponds due to limited success with captive breeding [4, 5]. According to Moreau [6] and Monentcham et al. [1], raising larvae and fry usually results in extremely high mortality rates, ranging from 80% to 100% within 2 months. Earlier studies on H. niloticus reported that a whole batch of fry may die out between 5 and 7 days after hatching (DAH) [3, 7], which in part has been attributed to starvation [5]. Feeding must begin prior or shortly after the yolk reserves are depleted to increase survival rates [5], as observed in the larvae of freshwater and marine fish species [8]. Fish larvae are typically fed with live prey and later dry feeds with a high content of essential amino acids and fatty acids (FAs) [9].
Live prey remains an important component of most hatchery operations, as it contributes to improved larval growth and survival. However, live prey is expensive and the cost of producing it (i.e., including microalgae, rotifer, and Artemia production) can represent as much as 50% of the total costs of production and may result in variable nutritional quality for the different batches produced [10, 11]. Thus, to reduce costs in hatcheries, it is important to shorten the period of utilization of live prey and introduce nutritionally balanced and palatable formulated diets that enhance survival. H. niloticus has been identified as a species with a low acceptance of formulated diets during early weaning and preferring live prey, making the initial feeding of artificial diets among the most challenging tasks during its larvae and fry culture [1, 3, 5]. The transition of fry from live prey to dry feeds has a large impact on its survival and development. In replacing live prey with a dry inert feeds, the “cofeeding strategy” which involves starting with a combination feeding and progressively withdrawing the quantity of live prey until the dry diet is solely offered, is typically the preferred methodology (i.e., weaning method) and is known to improve survival and growth [12–14]. H. niloticus fry are typically weaned to dry feeds around day 40 or at an older age in order to enhance survival, even though the fry at this age is relatively large (0.60 ± 0.17 g) and considered well-developed [15]. A low survival of H. niloticus fry with earlier weaning to dry feeds (i.e.,11, 13, 15, 24, and 26 DAH) has been reported [3]. The low survival observed was attributed to several factors including feeding behavior, low-quality ingredients, dietary composition, unavailability of attractants, and palatability [3]. Additionally, inadequate understanding of the development matureness of its digestive system and physiology and the transition from larvae to fingerling stage are other explanations [3, 5].
Formulated feeds have the advantage of being easily accessible, simple to store, feed, and often more affordable than live preys. However, the success of a feed depends on its acceptance, digestibility, and assimilation by the target species [16]. Several indices exist for measuring these variables but among the best ways to measure acceptance and use of feeds is to monitor the digestive enzymes of the target species. Digestive enzymes have an important role in the digestive system of a larvae/fry; their appearance, abundance, and activity levels in the gut are indicators of larval age and development and may determine the optimal composition of feeds and the most appropriate age for weaning [17, 18]. Simple morphology of the digestive tract typically correlates with a low production of enzymes, which can make formulated diets difficult to digest and absorb [19, 20]. Research on the digestive enzyme activity of H. niloticus is limited but trypsin, amylase, and lipase activity have been confirmed during early larvae ontogeny [5].
Nutritional inadequacies of essential long chain polyunsaturated fatty acids (LC-PUFAs) in diets can be a critical contributing factor to poor larval development in both freshwater and marine fish species [21, 22]. LC-PUFAs are essential for several key functions in the fish body including immune system development [23, 24], pigmentation [23–25], cell membrane integrity [26, 27], and ultimately affecting growth performance and survival [23, 27–30]. These essential fatty acids (EFAs) must be acquired from feeds rich in LC-PUFAs, as fish larvae are mostly unable to synthesize them from FAs precursors. EFA deficiency or insufficiency in fish feeds can cause poor fish growth, low feed efficiency, a variety of pathological problems, and even death [23, 31, 32]. Furthermore, it is well-known that many fish and crustacean larvae require dietary phospholipids (PLs) for optimal development; growth, stress tolerance, and survival [33–35]. Sufficient dietary PLs have been shown to enhance growth performance, improve the development of the digestive system, increase antioxidant capacity, improve stress resistance, as well as improve uptake of lipids and thus LC-PUFAs [18, 36–38]. Whether H. niloticus larvae or fry have a dietary requirement for LC-PUFAs and PL is at present not known. Research has demonstrated that as a freshwater omnivore, it thrives on a variety of organisms in the wild, ranging from aquatic invertebrates to small seeds, including macrophytes, plant remains, and aquatic insects [6, 39, 40].
The objectives of the study were to establish the best weaning age of H. niloticus fry and to examine if LC-PUFAs in the form of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) and the supplementation of (PL) (soy lecithin) had an effect on fry survival and growth and enzymatic activity at the tested weaning ages.
2. Materials and Methods
The study was conducted at the wet laboratory of the Department of Fisheries and Watershed Management, Kwame Nkrumah University of Science and Technology (KNUST), Ghana. The experimental set-up consisted of 21 rectangular plastic tanks of 65 L volume (L-53 cm × W-38 cm × H-33 cm) each filled with 30 L of freshwater and provided with gentle aeration to maintain DO above 70% saturation, that is, 5 mg l−1 throughout the experiment. The system was flow-through and each tank was continuously supplied with well water from a reservoir with a flow rate of 0.5 L min−1 for 2 h daily (daily water exchange rate of 200%) at 26°C. The experiment was performed in a ventilated room with fluorescent roof light. Larvae/fry were subject to a constant photoperiod of 12:12 h of light/dark (lights turned on and off at 6:00 and 18:00), respectively, throughout the experiment.
2.1. Experimental Diets
Two extruded diets that differed in PL content and LC-PUFA content of EPA and DHA were evaluated as weaning diets using live Artemia as a control (AF strain, Artemia systems, Ghent, Belgium). The two experimental dry diets were formulated to be iso-nitrogenous and iso-lipidic (48% CP and 23% CL) (Table 1). The diets were prepared and extruded as 0.5 mm granulates (SPAROS Olhao, Portugal), using high-quality fish meal, fish protein hydrolysate, squid meal, fish gelatine, wheat gluten, and wheat starch, as protein sources, and soy lecithin, salmon oil, krill PL oil, and olive oil as lipid sources. Two inclusion levels of Algatrium DHA70 (a glyceride product high in DHA and EPA) and soy lecithin provided different content of DHA, EPA, and PL, respectively. Diets were labeled, LPP (low phospholipid, low LC-PUFA) and HPP (high phospholipid; high LC-PUFA) (Table 2).
| Ingredient | Diets | ||||
|---|---|---|---|---|---|
| LPP | HPP | AF Artemia | |||
| Fishmeala | 35.0 | 35.0 | — | ||
| Fish protein hydrolysateb | 7.5 | 7.5 | — | ||
| Squid mealc | 19.0 | 19.0 | — | ||
| Fish gelatind | 2.0 | 2.0 | — | ||
| Wheat glutene | 2.0 | 2.0 | — | ||
| Wheat starchf | 14.4 | 10.6 | — | ||
| Vitamin and mineral premixg | 1.0 | 1.0 | — | ||
| Choline chlorideh | 0.2 | 0.2 | — | ||
| Antioxidanti | 0.4 | 0.4 | — | ||
| Monoammonium phosphate (MAP)j | 0.2 | 0.2 | — | ||
| Soy lecithink | 4.1 | 13.2 | — | ||
| Salmon oill | 0.0 | 2.4 | — | ||
| Arachidonic acid 40%m | 0.0 | 1.0 | — | ||
| Fish oil (DHA70%)n | 0.0 | 3.5 | — | ||
| Krill phospholipid oilo | 0.0 | 2.0 | — | ||
| Olive oilp | 14.2 | 0.0 | — | ||
| Proximate composition | |||||
| Dry matter (%) | 91.7 | 91.1 | 93.3 | ||
| Crude protein (%) | 48.0 | 48.3 | 47.1 | ||
| Crude lipid (%) | 23.2 | 24.9 | 1.7 | ||
| Ash (%) | 8.0 | 8.8 | 0.8 | ||
| NFE (%)q | 12.5 | 9.1 | 44.5 | ||
| DHA (%) (calculated) | 0.66 | 3.04 | — | ||
| EPA (%) (calculated) | 0.10 | 0.75 | — | ||
| Phospholipids (%) (calculated) | 3.16 | 12.96 | — | ||
| Gross energy (MJ/kg)r | 22.7 | 22.8 | 19.5 | ||
- aFishmeal LT 71.9 CP%, 6.8% CF, Scandinavian Sopropêche, France.
- bFish protein hydrolysate 82.6% CP, 9.6% CF, Sopropêche, France.
- cSquid meal 83% CP, 4% CF, Sopropêche, France.
- dFish gelatin 94% CP, % CF, 160 Bloom 60 mesh, WEISHARDT International, Slovakia.
- eWheat gluten 80.4% CP, 5.8% CF, Roquette, France.
- fWheat starch 90% starch, Roquette, France.
- gVitamins (IU or mg/kg diet): DL, alphatocopherol acetate; 100 mg; sodium menadione bisulfate, 25 mg; retinyl acetate, 20,000 IU; DL, cholecalciferol; 2000 IU; thiamine, 30 mg; riboflavin, 30 mg; pyridoxine, 20 mg; cyanocobalamin, 0.1 mg; nicotidin acid, 200 mg; folic acid, 15 mg; ascorbic acid, 1000 mg; inositol, 500 mg; biotin, 3 mg; calcium panthotenate, 100 mg; choline chloride, 1000 mg, betaine, 500 mg. Minerals (g or mg/kg diet): cobalt carbonate, 0.65 mg; copper sulfate, 9 mg; ferric sulfate, 6 mg; potassium iodide, 0.5 mg; manganese oxide, 9.6 mg; sodium selenite, 0.01 mg; zinc sulfate. 7.5 mg; sodium chloride, 400 mg; calcium carbonate, 1.86 g; excipient wheat middling’s Premix Lda., Portugal.
- hCholine chloride, ORFFA, The Netherlands.
- iAntioxidant, Kemin Europe NV, Belgium.
- j(Monoammonium phosphate, MAP), 26% P, Phosphea, Serbia.
- kSoy lecithin, Acetone insoluble >95%, Berg & Schmidt GmbH & Co. KG, Germany.
- lSalmon oil 98.3% CF, 4.6% EPA; 5.2% DHA Sopropêche, France.
- mArachidonic acid 40%, 96.9% CF, 42% ARA, Huatai Biopharm Inc., China.
- nFish oil (DHA70%) 98.7% CF, 70% DHA, Brudy Technology Spain.
- oKrill phospholipid oil 98% CF, 37% PL, Aker Biomarine, Norway.
- pOlive oil 98.4% CF 60% Oleic acid Henry Lamotte Oils GmbH Germany.
- qNitrogen-free extract (NFE), calculated as: 100−(crude protein% + crude lipid% + ash% + moisture %).
- rGross energy (kJ/g), calculated as: crude protein% × 23.6 + crude lipid% × 39.5 + nitrogen-free extract (NFE) × 17.2 [41].
| Fatty acids | LPP | HPP | Artemia |
|---|---|---|---|
| 14:0 | 0.80 | 0.73 | 1.78 |
| 15:0 | — | 0.83 | 1.37 |
| 16:0 | 14.47 | 16.43 | 11.9 |
| 17:0 | 0.24 | 0.90 | 1.80 |
| 18:0 | 3.28 | 6.22 | 6.42 |
| 20:0 | 0.38 | 0.94 | 0.36 |
| Sum SFA | 19.2 | 26.1 | 21.1 |
| 16:1 (n-7) | 1.78 | 3.03 | 13.0 |
| 18:1 (n-9) | 54.15 | 11.8 | 16.1 |
| 18:1 (n-7) | 2.31 | 8.08 | 11.30 |
| 20:1 (n-7) | 1.78 | 1.31 | 0.45 |
| 22:1 (n-11) | 0.22 | 0.24 | 0.06 |
| 22:1 (n-9) | — | 0.52 | — |
| 24:1 (n-9) | 0.33 | 0.22 | — |
| Sum MUFA | 60.57 | 25.2 | 41.8 |
| 18 : 2 (n-6) | 10.66 | 0.74 | 3.85 |
| 18:3 (n-6) | 1.13 | 0.73 | 3.15 |
| 20:2 (n-6) | 0.08 | 1.77 | 0.07 |
| 20:3 (n-6) | — | — | 0.28 |
| 20:4 (n-6) | 0.2 | 4.01 | 3.13 |
| Sum n-6 | 12.07 | 7.25 | 10.48 |
| 16:2 (n-4) | — | 0.44 | 0.47 |
| 16:3 (n-4) | 0.05 | 0.89 | 4.40 |
| 16:4 (n-3) | — | 0.60 | 0.09 |
| 18:3 (n-4) | 0.32 | — | 0.13 |
| 18:3 (n-3) | — | — | 0.99 |
| 18:4 (n-3) | — | — | 0.04 |
| 20:3 (n-3) | 0.09 | 0.19 | — |
| 20:4 (n-3) | 0.11 | — | 0.49 |
| 20:5 (n-3) | 1.68 | 17.84 | 11.18 |
| 21:5 (n-3) | 0.06 | — | — |
| 22:5 (n-3) | 0.19 | 1.23 | — |
| 22:6 (n-3) | 2.75 | 14.12 | — |
| Sum n-3 PUFA | 4.88 | 33.98 | 12.71 |
| n-3/n-6 | 0.40 | 4.69 | 1.21 |
- Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
2.2. Fish and Experimental Design
A total of 8000 larvae of H. niloticus were obtained from two wild breeding nests from the Barekese Reservoir, Kumasi, and transported to the laboratory. The age of larvae was estimated to be 5 days old determined by comparing with a previous study of larvae size from hatching [5]. Until the start of experiment, all larvae were reared in four fiberglass tanks (L—96 cm × W—46 cm × H—38 cm) in a flow through system with 200% water exchange and fed with Artemia nauplii three times a day at a density of 1 nauplii ml−1. A 2 × 6 factorial experiment was performed to examine the effect of diet (2) and weaning age (6) (i.e., 15, 25, 35, 45, 65, and 85 DAH, each treatment was performed in triplicate tanks. When larvae reached the required weaning age, larvae from the holding tanks were counted and stocked in triplicate in the experimental tanks. Table 3 illustrates the stocking density and average start weight (g/ind., mean ± SD [standard deviation]) for the various weaning ages.
| Weaning age | DAH | Initial number | Initial average weights (g) |
|---|---|---|---|
| WA1 | 15 | 180 | 0.04 ± 0.01 |
| WA2 | 25 | 180 | 0.11 ± 0.02 |
| WA3 | 35 | 120 | 0.34 ± 0.06 |
| WA4 | 45 | 90 | 0.94 ± 0.05 |
| WA5 | 65 | 60 | 1.16 ± 0.27 |
| WA6 | 85 | 60 | 3.78 ± 0.07 |
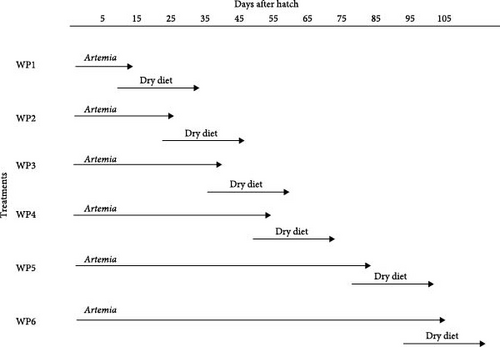
Weaning to experimental diets was done as follows; all weaning groups were cofed with Artemia nauplii and experimental extruded feeds for 7 days, the quantity of Artemia fed was gradually reduced daily until they were completely substituted with the experimental feeds followed by 10 days solely on dry feeds (Figure 1). Four times daily, from 9:00 to 17:30 h, similar preweighed quantities of the weaning diets were hand fed to the fish. The fish were fed the experimental diets at a daily rate of approximately 5% of the fish biomass, whereas the control group was kept on live Artemia nauplii. The tanks were cleaned daily by siphoning residual feed and faeces.
2.3. Dietary Proximate Analysis and FA Analysis
Briefly, the diets and Artemia were finely ground using a Krups Speedy Pro homogenizer and analyzed for crude protein, [42], crude fat [43], and dry matter and ash [44].
Prior to FA determination, total lipids were extracted by incubating samples in chloroform–methanol (2:1) for 24 h [45]. Subsequently, FAs were determined by gas chromatography (HP-5890A, Agilent Technologies, Santa Clara, USA) and separated on a column (Agilent DB wax 127-7012, 10 m × 100 µm × 0.1 µm, Agilent Technologies). A standard mixture of FA methyl esters (Nu Check Prep 68D, USA) was used for FA identification. FAs were reported as the area percentage of total identified FAs.
2.4. Water Quality
Temperature (T, °C), dissolved oxygen (DO, mgl−1), total dissolved solids (TDSs, mgl−1), and pH were recorded daily with a multiparameter probe (YSI professional plus 18H105806, USA) at 8 am ammonia nitrogen (mgl−1) was determined spectrophotometrically [46] weekly in all rearing tanks.
2.5. Sampling and Processing
For each of the six weaning ages, a sample of 20 fry was randomly taken at the beginning and end for growth and analytical purposes. The total length (TL) of each fry was taken to the nearest 0.01 mm using a Vernier caliper (Mitutoyo 500-196-20, Foshan, China), and body weight (BW) was taken to the nearest 0.01 g using an analytical balance (UW1020H, Kyoto, Japan). Ten fry from the different weaning ages were used for analysis of digestive enzyme activity, these individuals were rinsed with distilled water and frozen at −80°C until analysis.
2.6. Determination of Digestive Enzyme Activity
Digestive enzyme activity was determined by dissecting to remove the guts and mechanically homogenizing the guts of individual fry in ice-cold Milli-Q water, the homogenate was centrifuged (10 min at 15,800 g), and the supernatant was used to assay enzymatic activity. Amylase activity was determined with a commercial kit (Ultra Amylase Assay kit E33651, Thermo Scientific, USA). The kit contains a starch derivate labeled with a fluorophore dye as a substrate. This substrate was diluted in 3- (N-morpholino) propane sulfonic acid (MOPS; pH 6.9) and substrate solvent (sodium acetate; pH 4.0), to a final concentration of 200 µg ml−1. For analysis, 50 µl of the substrate solution, and 15 µl of the enzyme extract were added to the microplate. Fluorescence was measured at 485 nm (excitation) and 538 nm (emission). For trypsin, the fluorogenic substrate Boc-Gln-Ala-Arg-7-methylcoumarin hydrochloride (BOC-SIGMA B4153) was diluted in dimethyl sulfoxide (DMSO), to a final concentration of 20 µM. For analysis, 5 µl of this substrate, 190 µl of 50 mM Tris + 10 mM CaCl2 buffer (pH 8.5), and 15 µl of the enzyme extract were added to the microplate and measured at 355 nm (excitation) and 460 nm (emission). Lipase activity was assayed using 4-methylumbelliferyl heptanoate (M2514, Sigma–Aldrich). The substrate was dissolved in phosphate buffer pH 7.0 to a final concentration of 0.4 mM, following a modified method from Rotllant et al. [47]. Exactly, 15 µl of the extract was added to the microplate and mixed with 250 µl of 0.4 mM substrate for the analysis. Fluorescence was measured at 355 nm (excitation) and 460 nm (emission). All enzyme activities were assayed using the methods of Rotllant et al. [47] modified as described in Goncalves et al. [48]. All enzyme activities were expressed as RFU (relative fluorescence units) per milligram of individual gut weight. The results were expressed as the specific enzyme activity per individual.
2.7. Statistical Analysis
Results of length and weight measurements and the enzyme activity were presented as means ± SD. Statistical analysis was conducted using Graph Pad Prism 8. All data passed the normality test (Shapiro–Wilk). Data on digestive enzymes for each weaning age were also analyzed using a one-way analysis of variance. Data for growth and survival for each weaning age were analyzed using a two-way analysis of variance. Dunn’s multiple comparisons test was used to determine the significant difference between the mean values of the treatments. All statistical analyses were performed at p < 0.05.
3. Results
3.1. Diets Composition
Proximate composition was similar between the two diets, while FA content differed due to different inclusion levels of algatrium oil and soy lecithin. As such the DHA content of the HPP diet was some 4.6 times higher than in the LPP diet while EPA was some 1.85 times higher. For the relative content of FA main differences were related to oleic acid (18:1n-9) content much higher in the LPP diet than the HPP diet due to the higher inclusion of olive oil, which contains some 55% oleic acids of total fatty acids (TFAs). Similarly, LA content was relatively higher in the LPP diet, while EPA and DHA content was much higher in the HPP diet. Consequently, n/3:n/6 ratio was 0.4 for the LPP diet and 4.7 for the HPP diet.
3.2. Growth and Survival
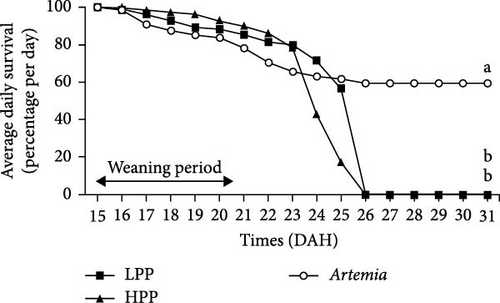
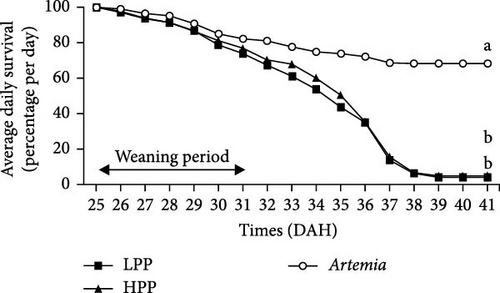
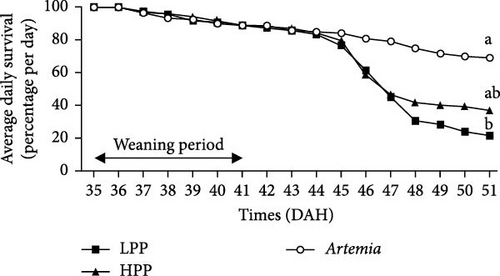
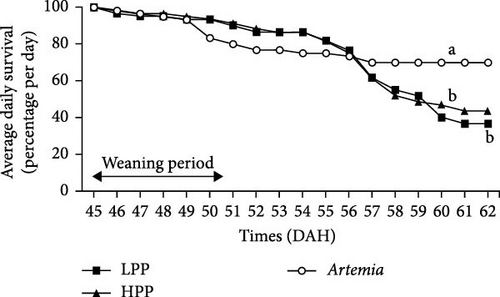
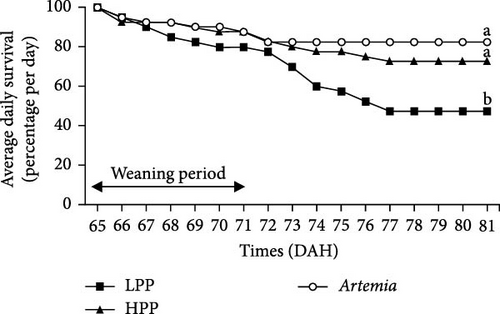
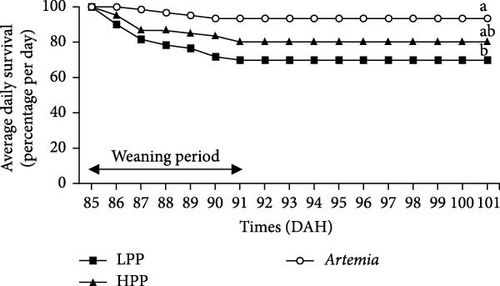
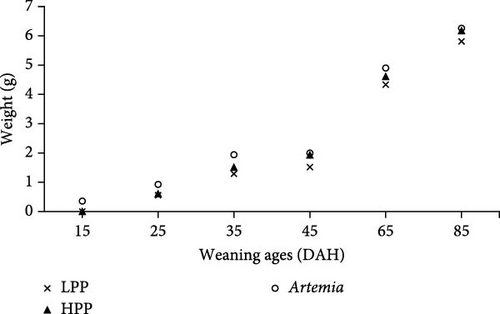
Comparison of the formulated diets with Artemia diet is complicated by the low lipid content in Artemia, but in relative terms (% TFA), Artemia had a very high content of EPA, while DHA was almost 0, thus very different from both formulated diets. The survival rates of H. niloticus fry improved with age. The control diet (Artemia) had the highest survival, which was significantly higher than that of fry fed the experimental diets (Table 4) (p < 0.05). Of the two experimental diets, the HPP diet obtained the highest survival (p < 0.05) (Figure 2). The survival rate of fry fed the HPP diet increased from 0% at the start weaning from 15 DAH to 80% ± 6.67% when weaning was conducted at 85 DAH, while survival rate of fry fed the LPP diet increased from 0% at 15 DAH to 70% ± 3.33% at 85 DAH. The HPP diet had a significantly higher survival rate than the LPP diet from weaning age 65 DAH (p = 0.045); however, at the earlier weaning ages (25, 35, and 45 DAH), there was no significant difference (p = 0.271) and similarly at 85 DAH there was no significant difference in survival between two diets (p = 0.638). Growth performance calculated as increase in mean weight and specific growth rate (SGR) was higher in fry fed the HPP diet compared to fry fed the LPP diet (Figure 3). However, there were no differences in weight gain until 45 DAH (p < 0.001), while significant differences were observed at 65 DAH (p < 0.01) and at 85 DAH (p < 0.001). It was observed that mortality during the experiment was caused by starvation, as evidenced by empty guts of dying fry causing shrinkage in size and weight loss. The slow-sinking granulates used to feed the fry did not really attract the fry to feed on them in the water column compared to fry fed the live prey and most feeds were observed to be eaten from the bottom of the tank instead.
| Weaning diets | Weaning age DAH | IBW (g) | FBW (g) | SGR (% day−1) | Weight gain (%) | Survival (%) | p-Value |
|---|---|---|---|---|---|---|---|
| LPP | 15 | 0.04 ± 0.01 | — | — | — | — | — |
| 25 | 0.11 ± 0.02 | 0.56 ± 0.17 | 9.57 | 405.31 | 4.4 ± 1.92a | — | |
| 35 | 0.34 ± 0.06 | 1.29 ± 0.05 | 7.84 | 278.15 | 21.7 ± 1.81a | — | |
| 45 | 0.94 ± 0.05 | 1.52 ± 0.09 | 2.83 | 61.92 | 36.6 ± 8.86a | — | |
| 65 | 1.16 ± 0.27 | 4.33 ± 0.05 | 7.75 | 138.09 | 41.6 ± 3.92a | — | |
| 85 | 3.78 ± 0.07 | 5.81 ± 1.21 | 2.53 | 66.83 | 70.0 ± 3.33ab | 0.034 | |
| HPP | 15 | 0.04 ± 0.01 | — | — | — | — | — |
| 25 | 0.11 ± 0.02 | 0.61 ± 0.16 | 10.08 | 450.43 | 5.0 ± 3.33a | — | |
| 35 | 0.34 ± 0.06 | 1.51 ± 0.09 | 8.77 | 345.00 | 35.8 ± 7.65a | — | |
| 45 | 0.94 ± 0.05 | 1.93 ± 0.12 | 4.23 | 105.98 | 43.3 ± 4.14a | — | |
| 65 | 1.16 ± 0.27 | 4.61 ± 0.14 | 8.12 | 156.24 | 69.4 ± 3.93a | — | |
| 85 | 3.78 ± 0.07 | 6.17 ± 1.25 | 2.88 | 81.41 | 80.0 ± 6.67ab | 0.032 | |
| Artemia | 15 | 0.04 ± 0.01 | 0.36 ± 0.04 | 12.92 | 788.09 | 59.4 ± 5.39a | — |
| 25 | 0.11 ± 0.02 | 0.93 ± 0.04 | 12.56 | 744.23 | 68.3 ± 1.73a | — | |
| 35 | 0.34 ± 0.06 | 1.94 ± 0.05 | 10.24 | 469.93 | 69.2 ± 7.64a | — | |
| 45 | 0.94 ± 0.05 | 2.00 ± 0.14 | 4.44 | 112.89 | 70.0 ± 4.71a | — | |
| 65 | 1.16 ± 0.27 | 4.90 ± 0.16 | 10.49 | 174.71 | 80.0 ± 9.42a | — | |
| 85 | 3.78 ± 0.07 | 6.26 ± 0.91 | 2.97 | 82.67 | 93.0 ± 3.33a | >0.05 | |
- Note: A different superscript letters for each diet indicate a significant effect of weaning age significantly different. Values are means ± SD for each parameter for each weaning age.
3.3. Enzyme Activity
Figure 4 shows the specific enzyme activity of H. niloticus fry at the different weaning ages. Trypsin, amylase, and lipase activity were all relatively higher at the earlier weaning ages (15, 25, and 35 DAH) compared to the later weaning ages (45, 65, and 85 DAH). Overall, a noticeable decline in the specific enzyme activity with age was observed for the three enzymes. The maximum specific trypsin activity was 99.2 ± 35.7 RFU mg−1 BW at 15 DAH, which gradually decreased to 82.4 ± 31.9 RFU mg−1 BW at 25 DAH, and then further decreased to 23.3 ± 18.7 RFU mg−1 BW at 35 DAH and to 5.6 ± 2.1 RFU mg−1 BW at 45 DAH. From 65 to 85 DAH, it decreased further to 2.6 ± 2.0 RFU mg−1 BW at 65 DAH and to 1.5 ± 0.7 RFU mg−1 BW at 85 DAH.
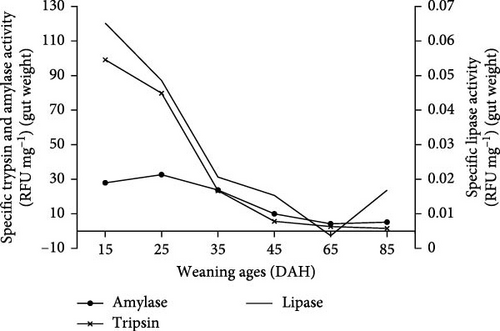
Amylase activity was 27.9 ± 4.1 RFU mg−1 BW at 15 DAH, and increased to 32.6 ± 9.6 RFU mg−1 BW at 25 DAH after which it declined gradually with a value of 23.7 ± 8.70 RFU mg−1 BW at 35 DAH; 9.9 ± 5.1 RFU mg−1 BW at 45 DAH, and 4.20 ± 1.4 RFU mg−1 BW at 65 DAH. Following that, it appeared to increase slightly to 5.17 ± 3.62 RFU mg−1 BW at 85 DAH.
Lipase activity was highest at 0.065 ± 0.008 RFU mg−1 BW at 15 DAH. From 25 to 65 DAH, it decreased to 0.049 ± 0.018 RFU mg−1 BW at 25 DAH and to 0.021 ± 0.013 RFU mg−1 BW at 35 DAH, then to 0.015 ± 0.005 RFU mg−1 BW at 45 DAH, and 0.004 ± 0.001 RFU mg−1 BW at 65 DAH. Following that, the lipase activity increased slightly to 0.017 ± 0.008 RFU mg−1 BW at 85 DAH.
4. Discussion
Fry survival is an important indicator to evaluate if a given weaning diet or weaning age has a positive effect on the fry. Fry adaptation to microdiets requires a period of significant morphological, physiological, and behavioral changes [49]. According to Soligo, Garcia, and Cerqueira [50], weaning age is regarded as an essential phase in larval rearing because it occurs at the same time that many species transform from larvae to early juveniles. In this study, 5 DAH H. niloticus fry readily ingested live Artemia; however, the dry feed weaning attempt was unsuccessful and survival to dry diets was not observed until 25 DAH. Although the H. niloticus fry were fed high-quality, nutritionally balanced diets, the weaning diets elicited lower survival rates at ages 15, 25, 35, and 45 DAH, with survival rates between 0% and 36% (LPP) and between 0 and 43% (HPP) compared to a significantly higher survival rate for fry fed live Artemia (59.5%–70%). Reports of no survival to very low survival during early weaning have previously been reported [3], which is similar to observations for a range of other species including common perch (Perca fluviatilis) when weaned at 13 and 15 DAH; and summer flounder (Paralichthys dentatus) when weaned between 14 and 21 DAH [51–53]. In the sand-spotted bass (Paralabrax maculatofasciatus), low survival was also reported (1.7%−2.7%), during early weaning when fed a microparticulate diet at 25 and 30 DAH.
To ascertain the earliest possible weaning time without compromising survival is not only of interest from a biological point but also of economic importance in hatcheries. In this study, however, early weaning was preceded by a higher fry mortality. Total mortality was observed in fry weaned at 15 DAH and the cause is most likely due to rejection (no intake) of the dry diet (authors’ observation). H. niloticus appears to be conservative in its feeding behavior during the fry stages, according to Ofori-Darkwah et al. [5]. Therefore, it seems very difficult to switch from live prey to the granulate feeds as observed in the present experiment, even with the relatively long cofeeding of Artemia and the gradual withdrawal, which for many other fish species, would likely have been successful. Larvae seemed more likely to starve to death than consume the dry diets offered at the tested fry stages. Fish starvation occurs regularly during the weaning stage of many species, as exemplified by observations of Senegalese sole (Solea senegalensis) [54] and sand bass (Paralabrax maculatofato fasciatus) [55]. It requires delicate feeding procedures with a slow removal of live preys, as performed here, that typically may trigger the starved larvae and fry to pick on new feed items. The slow-sinking granulates used were aimed at ensuring that fish had a better chance to consume the feed while it was in the water column. However, the Heterotis fry seemed not adequately attracted to the feed in the water column until the granulates landed at the bottom of the tank, when some fry were observed to be feeding on them. The results of the proximate analysis of the feeds indicated that the protein contents of the dry meals and Artemia were comparable; however, the live Artemia produced the best results in terms of SGR and weight gain compared to the weaning diets. The high quality of ingredients used in the dry feeds suggests that the lack of feed intake was caused by the nonmobility of the feed offered more than the quality, smell, or palatability and that the better growth and survival observed with Artemia nauplii was more related to feed intake than nutritional composition of the diets.
No information exists on the smell and chemosensory attraction in Heterotis fry, so at present it is unknown if feed intake could be stimulated by certain substances or if feed intake of Heterotis fry is merely triggered by feed movement, size, color, etc., or a combination of these. The relatively higher survival and growth rates of the fry introduced to formulated diets at later ages (65 and 85 DAH) prove a higher intake and also suggest that guts were more developed at this stage for better digestion and utilization of formulated feed thus capable of handling the particulate diets.
Research has shown that the growth and development of especially marine fish larvae depend on highly unsaturated FAs, including EPA, DHA, and arachidonic acid [56]. Majority of freshwater fish can to some extent produce n-3 LC-PUFA and n-6 series LC-PUFA from alpha-linolenic acid (LNA) (18:3n-3) and linoleic acid (LA) (18:2n-6), respectively [23, 57]. For H. niloticus, little is known about this species‘ capacity to synthesize LC-PUFAs or desaturase FA precursors. Several feeding trials have demonstrated the negative effects of diets deficient in LC-PUFA on growth and survival rates of fish fry [22, 58]. Between the two diets, the HPP fed fry had better survival, final weight, weight gain, and SGR with increasing age of the fry. The relatively higher survival and growth rate of H. niloticus fry fed the HPP diet compared to the LPP diet maybe due to high inclusion of DHA. The DHA and EPA are particularly crucial for fry growth and development, with DHA being essential for neural growth and development, improving stress tolerance, and even reducing mortality [28, 59, 60].
In addition, it is possible that the fry’s survival and growth were enhanced by the HPP diets’ higher PL supplementation, which may increase its uptake and utilization from the gut [31, 34]. Research conducted on percid larvae indicates that feeding farmed pikeperch with PLs and/or particular vitamins can improve their health status and strengthen their ability to withstand osmotic stress [35, 37, 61, 62]. These observations suggest that improving the levels of dietary LC-PUFA, EPA, and DHA, and PLs in the diets can improve survival and promote the growth of H. niloticus fry and fingerlings.
It is generally known that differences in species, age and diets can affect how digestive enzymes act in an organism [63]. From the start of exogenous feeding, H. niloticus larvae/fry have a poor developed digestive system and rely mostly on live prey organisms (Artemia) for the delivery of exogenous enzymes [3, 5]. According to Mandal and Ghosh [64], the growth of a fish may be an indication of how effectively the digestive enzymes contribute to the digestion of nutritional components. The enzymes, amylase, lipase, and trypsin, were shown to be active at the beginning of exogenous feeding from 5 DAH onward, based on investigations on the digestive enzyme activity of H. niloticus [5].
Trypsin is a crucial nutritional indicator that is directly related to protein metabolism. According to Grendell and Rothman [65] and Tseng, Grendell, and Rothman [66], it is mostly regulated by the protein level and amino acid profile of the diet. Trypsin activity in H. niloticus fry was identified on the 15 DAH at high levels and, thereafter, there was decreased trypsin activity at the various weaning ages until the 85 DAH. Similar activity profiles have been reported for Sobaity sea bream (Sparidentex hasta) [67], Mayan cichlid (Cichlasom urophthalmus) [68], and Chinese perch (Siniperca chuatsi) [69] where trypsin activities measured were high at the initial stages, however, declined progressively with age. This might be associated with the metamorphosis and the development of gastric glands which has also been reported in other species such as common dentex (Dentex dentex) [70–72]. Furthermore, trypsin pattern variations may be genetically regulated, according to Cahu and Infante [63].
At 25 DAH, H. niloticus fry showed a peak in their amylase activity, which thereafter decrease until the end of the study. The reduced carbohydrate content of live prey may be the cause of the age-related decline in amylase activity [73]. Common carp have a similar trend, with significant amylase activity seen at the early life stages and a decline during fry growth [74]. Chakrabarti et al. [75] have documented the same in silver carp and bighead carp hybrids.
At 15 DAH, a specific lipase activity was detected, although it remained at low levels until 65 DAH. At the end of the trial, the specific lipase 85 DAH began to slightly increase. Similar patterns have been observed in Mystus nemurus and Clarias magur [76, 77].
5. Conclusion
The findings of this study showed that weaning H. niloticus fry between 65 and 85 DAH to a formulated high-quality granulated diets had a higher survival and better growth performance compared to weaning at early ages. Early weaning of fry (15–25 DAH) following live Artemia nauplii feeding was unsuccessful and results in complete starvation, even though the size of fry indicates that formulated diets should be a viable alternative to Artemia. Growth, body composition, and survival were enhanced for H. niloticus fry fed high dietary levels of PLs, DHA, and EPA. More research is needed on formulation and feeding of artificial diets to substitute live preys to reduce weaning age considerably, which is a considerable practical and economical bottleneck in the commercial rearing of this species.
Ethics Statement
The study was conducted according to the guidelines of the Animal Research Ethics Committee (AREC) of the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (https://www.knust.edu.gh/sites/default/files/201902/AREC%20Reviewed%202018.pdf).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conception and designed the research: Daniel Adjei-Boateng, Regina Esi Edziyie, Prince Ofori-Darkwah, Nelson Winston Agbo, and Ivar Lund. Data collection: Prince Ofori-Darkwah, Daniel Adjei-Boateng, Regina Esi Edziyie, and Nelson Winston Agbo. Data analysis, interpretation, and drafting the article: Prince Ofori-Darkwah, Daniel Adjei-Boateng, Regina Esi Edziyie, Nelson Winston Agbo, and Ivar Lund. Critical revision of the article: Prince Ofori-Darkwah, Ivar Lund, Daniel Adjei-Boateng, Regina Esi Edziyie, and Nelson Winston Agbo. All authors approved the final version of the manuscript to be published.
Funding
The Danish International Development Agency (DANIDA) under the “Improving the Productivity of Ghanaian Aquaculture project” (DFC no. 18-16-GHA) funded this research.
Acknowledgments
The authors wish to thank Javis Bediako Asare, Joyce Temah Baah, Barbara Baasubinin Campion, Eric Boakye, and Evans Kwabena Konadu all at Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, for assisting the fieldwork and analytical support, respectively.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




