Impact of Depression on Cognitive Function and Phenoconversion to Neurodegenerative Diseases in Patients With Isolated REM Sleep Behavior Disorder
Abstract
Background: This study was aimed at analyzing cognitive function and quantitative electroencephalogram (qEEG) in patients with isolated REM sleep behavior disorder (iRBD) based on the presence of depression and at evaluating the impact of depression on phenoconversion to neurodegenerative diseases.
Methods: Individuals diagnosed with iRBD via polysomnography were included. Based on the presence of depression, patients were categorized into two groups. Neuropsychological tests and qEEG were conducted following the diagnosis of iRBD, and outcomes were compared between the two groups. Patients were regularly followed to monitor their phenoconversion status. Cox regression analysis was performed to assess the hazard ratio associated with depression.
Results: Ninety iRBD patients (70% males) were included, with a median age of 66.3 years. Depression was identified in 26 (28.9%) of these patients. The depressed group showed significantly poorer performance only in color reading subtest of Stroop (p = 0.029) compared to the nondepressed group, showing reduced processing speed. In qEEG, relative gamma power (p = 0.034) and high gamma power (p = 0.020) in the parietal region were significantly higher in the depressed group than in the nondepressed group. Depression was associated with a hazard ratio of 3.32 for the risk of phenoconversion to neurodegenerative diseases in iRBD patients (p = 0.011).
Conclusion: Depressive symptoms in iRBD patients should be closely monitored as they could aggravate cognitive dysfunction and increase the risk of phenoconversion to neurodegenerative diseases.
1. Introduction
REM sleep behavior disorder (RBD) is a sleep disorder defined by atypical behaviors that occur during REM sleep. Individuals with RBD experience vivid dreams and lack typical muscle atonia during REM sleep [1]. Isolated REM sleep behavior disorder (iRBD) refers to instances of RBD that manifest without any associated neurodegenerative disorders or identified etiologies [2]. The majority of individuals with iRBD have a high likelihood of developing neurodegenerative diseases associated with the accumulation of α-synuclein, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and Alzheimer’s disease (AD) [3–6]. Thus, RBD has been suggested as a prodromal phase of α-synucleinopathies. Clinicians diagnosing and treating it are paying special attention to the presence of neurodegenerative diseases [3, 7, 8].
Depression frequently accompanies neurodegenerative disorders and can accelerate their progression. Notably, depression is a common nonmotor symptom in PD patients, affecting approximately 35% of them [9, 10]. It is also prevalent among patients with iRBD, with an estimated rate of 28.8% to 44.7% [11–14], often preceding the onset of PD [11]. Although research is limited, studies have explored whether depression contributes to phenoconversion in iRBD patients. For instance, Wing et al. found that the presence of psychiatric disorders in iRBD is significantly associated with an increased risk of phenoconversion to PD [15]. Conversely, a multicenter iRBD study reported that depression was not a significant risk factor for phenoconversion to neurodegenerative diseases [8]. These conflicting findings leave it unresolved whether depression contributes to phenoconversion in iRBD patients.
In addition, patients with iRBD often exhibit cognitive impairments, especially in domains of working memory, executive function, and visuospatial abilities [16–22]. The presence of mild cognitive impairment (MCI) and decline in executive function are associated with an increased risk of phenoconversion [21–24]. Additionally, abnormalities in electroencephalogram (EEG) during sleep and wakefulness have been reported in iRBD compared to normal controls [25, 26]. EEG slowing is the most prominent finding, characterized by higher theta and lower beta power. The dominant occipital frequency is also reduced in iRBD. Furthermore, for iRBD patients who have concomitant MCI, waking EEG slowing in posterior cortical regions can predict future phenoconversion to PD or DLB [27]. While depressive symptoms are known to impact neurodegenerative progression, particularly cognitive decline [28–31], the specific impact of depression on cognitive function in iRBD remains inadequately elucidated, warranting further investigation.
Given the fact that depression and iRBD are both prodromal signs of α-synucleinopathies, we aimed to unravel interplays between depression and iRBD, by exploring cognitive change, EEG activity, and phenoconversion risk. We hypothesized that (1) depressed iRBD patients would exhibit decreased cognitive function and distinct EEG patterns compared to nondepressed iRBD patients and (2) depression would be associated with an increased risk of phenoconversion to neurodegenerative diseases.
2. Materials and Methods
2.1. Participants
Patients diagnosed with iRBD at the sleep clinic of Seoul National University Bundang Hospital between February 2014 and December 2015 were recruited and followed up. All the participants had undergone video polysomnography (PSG) and structured interview for the diagnosis of iRBD.
The diagnosis of RBD was determined based on the standard criteria provided in the International Classification of Sleep Disorders—third edition (ICSD-3) [32]: (1) the presence of REM sleep without atonia (RSWA) observed during PSG; (2) documented occurrence of sleep-related injurious, potentially injurious, or disruptive behaviors through patient history or PSG monitoring; (3) lack of epileptiform activity on EEG during REM sleep; and (4) absence of any alternative explanation for the behavior. Given the concerns surrounding the perceived arbitrariness and leniency of the RSWA criteria in the ICSD-3, we decided to adopt a stricter definition of RSWA by referring to the guidelines outlined in the American Academy of Sleep Medicine (AASM) manual [33]. Once a patient was diagnosed as having RBD, we arranged the patient a neuropsychological test and referred the patient to a neurologist for neurological examination. Patients with RBD without neurological signs or cognitive impairment were confirmed as having iRBD. Individuals with a history of head trauma, cerebrovascular diseases, psychiatric disorders (including alcohol or substance use disorders), or other sleep disorders such as sleepwalking or narcolepsy were excluded.
Patients with iRBD visited our sleep clinic regularly in a term ranged between 1 month and 1 year. The last follow-up was conducted between September 2019 and July 2023 to determine the final status of the patients, whether phenoconversion occurred or not. Participants provided written informed consents. This study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-1907-553-301).
2.2. Diagnosis of Depression
Initial assessments were conducted through a comprehensive initial interview to discern the presence of depressive symptoms and to rule out alternative explanations. The diagnosis of depression was based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [34], including major depressive disorder (MDD) and other specified depressive disorder (OSDD). OSDD is diagnosed when a person shows depressive symptoms but does not fully fit the criteria for any specific depressive disorder. It may include situations where there is insufficient information to make a more specific diagnosis or when symptoms do not match the criteria for any single depressive disorder. Additionally, individuals who were taking antidepressants were also categorized as having depression.
2.3. Neuropsychological Assessments
A comprehensive neuropsychological battery including Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet Neuropsychological Assessment Battery (CERAD-K-N) was given to individuals diagnosed with iRBD [35, 36]. The evaluation encompassed following cognitive domains [37]: (1) digit span, Stroop color word test, and Trail Making Test for attention and working memory; (2) word list memory, word list recall, word list recognition, and recall of constructional praxis for memory function; (3) categorical fluency, executive clock drawing Task I (CLOX I), and frontal assessment battery for executive function; (4) constructional praxis and executive clock drawing Task II for visual–spatial function; and (5) the 15-Item Boston Naming Test for language. Raw scores were converted into demographically adjusted z-scores based on factors including age, gender, and education [38]. Z-scores that were less than −1.5 were classified as clinically meaningful impairment [36].
2.4. Questionnaires and qEEG
At baseline, right after diagnosis as iRBD, patients were led to conduct questionnaires and qEEG. Assessments included the Geriatric Depression Scale (GDS) for depression [39], the Pittsburgh Sleep Quality Index (PSQI) for sleep quality [40], and the Epworth Sleepiness Scale (ESS) for daytime sleepiness [41].
For resting-state qEEG, EEG is recorded for 15 mins while a patient remains awake. Examinees were instructed to close their eyes in a sitting position but remain awake and relaxed. A 64-channel Neuroscan SynAmp (Compumedics, Charlotte, NC, United States) was used to amplify and digitize EEG signals at a sample rate of 1 kHz. The expanded international 10–20 method was used to determine the placement of the electrodes. An artifact-free 90-s EEG recording was visually examined and selected for spectral analysis. Through the fast Fourier transform, the absolute and relative power for each frequency band was calculated: delta (1.0–4.0 Hz), theta (4.0–8.0 Hz), alpha (8.0–12.0 Hz), beta (12.0–25.0 Hz), high beta (25.0-30.0 Hz), gamma (30.0-80.0 Hz), and high gamma (> 80.0 Hz). In this study, we analyzed relative power values to reduce interindividual variation. Relative power indicates the proportion of power in each band in relation to the overall power. The mean values of relative power detected in electrodes were used to represent the five brain areas: frontal (FP1, FP2, F3, F4, F7, and F8), temporal (T3, T4, T5, and T6), central (C3 and C4), parietal (P3 and P4), and occipital (O1 and O2).
2.5. Phenoconversion
Phenoconversion was determined when an experienced neurologist diagnosed parkinsonism-related disorders [42] or geriatric psychiatrists confirmed dementia based on established clinical criteria [43]. For parkinsonism-related disorders, the UK Parkinson’s Disease Society Brain Bank criteria were used to confirm the diagnosis [44]; for dementia, the diagnosis was based on the DSM-5 criteria [34], requiring significant cognitive decline in one or more cognitive domains, interfering with independence in everyday activities. For these patients, neuroimaging (i.e., F-18 fluorodeoxyglucose positron emission tomography, dopamine transporter imaging, and magnetic resonance imaging) was conducted for objective diagnosis of parkinsonian disorders and for differential diagnosis of dementia.
Regular follow-up assessments were conducted to monitor any phenoconversion event over the study period. The date of phenoconversion was defined as the first observation of objective cognitive impairment that led to a dementia diagnosis or the date of the development of overt parkinsonism symptoms.
2.6. Statistical Analysis
iRBD patients were divided into two groups, iRBD with depression and iRBD without depression. Normality was examined by the Kolmogorov–Smirnov test. Comparison of clinical manifestation and neurocognitive function at baseline between the two groups was conducted by Student’s t-test for parametric data and the Mann–Whitney U-test for nonparametric data. Results are presented as mean ± standard deviation (SD) for parametric variables and median (interquartile range [IQR]) for nonparametric variables. For categorical variables, χ2 test was used. Generalized estimating equations (GEEs) were used to compare qEEG at baseline between depression and nondepressed groups among patients with iRBD, adjusting for covariates (age, sex). The Kaplan–Meier survival analysis method was utilized to compute cumulative incidence of phenoconversion to neurodegenerative disorders. Log rank test and Cox regression analysis were performed to assess the impact of depression on the risk of phenoconversion in iRBD. Statistical significance was set as p values < 0.05. Data analysis was conducted using SPSS Statistics 27.0 for Windows (SPSS, Chicago, Illinois, United States).
3. Results
A summary of the study flow is presented in Figure 1. A total of 90 patients diagnosed with iRBD between 2014 and 2015 were included for cross-sectional evaluation. Of these 90 patients with iRBD, 26 had depression at baseline. At baseline, 89 patients completed neurocognitive function tests and 82 patients had qEEG data available.
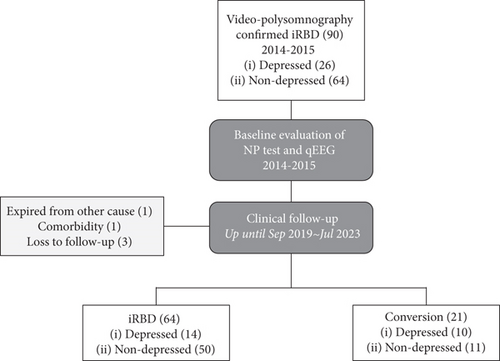
3.1. Cross-Sectional Comparison Between the Two Groups
Table 1 shows demographic and clinical characteristics of the 90 patients diagnosed with iRBD in depression (n = 26) and nondepression (n = 64) groups. There was a higher rate of females in the depressed group, showing a significant gender difference (53.8% vs. 20.3%, p = 0.002). As expected, the depression scores were significantly higher in the depressed group than in the nondepressed group (GDS: 13.5 [6.5, 17.8] vs. 5.0 [3.0, 10.0], p = 0.002). While depression is commonly associated with sleep disturbances that can also affect cognitive function and EEG activity, this study found no significant differences in self-reported sleep quality or polysomnographic metrics (e.g., total sleep time, sleep structure, and sleep apnea severity) between the two groups.
| Characteristic | Depression (n = 26) | Nondepression (n = 64) | p |
|---|---|---|---|
| Age (years) | 69.1 (62.3–74.8) | 66.0 (62.5–71.7) | 0.325 |
| Female, n | 14 (53.8%) | 13 (20.3%) | 0.002 ∗∗ |
| Body mass index (kg/m2) | 24.5 ± 2.4 | 24.7 ± 2.9 | 0.757 |
| Questionnaires | |||
| GDS | 13.5 (6.5–17.8) | 5.0 (3.0–10.0) | 0.002 ∗∗ |
| PSQI | 9.0 (3.5–12.5) | 6.0 (4.0–9.0) | 0.161 |
| ESS | 7.0 ± 3.8 | 8.4 ± 4.1 | 0.534 |
| Polysomnographic characteristics | |||
| Total sleep time, min | 346.6 ± 47.4 | 354.7 ± 45.7 | 0.459 |
| Sleep efficiency (%) | 73.9 ± 11.6 | 75.0 ± 9.6 | 0.646 |
| Stage N1 (%) | 6.7 (5.6–9.2) | 9.3 (5.6–12.9) | 0.062 |
| Stage N2 (%) | 42.5 ± 10.4 | 44.8 ± 9.1 | 0.303 |
| Stage N3 (%) | 8.8 (2.8–17.2) | 7.0 (2.8–12.2) | 0.325 |
| Stage REM (%) | 18.3 ± 8.3 | 17.5 ± 5.4 | 0.617 |
| Sleep latency (min) | 15.8 (6.6–24.1) | 12.8 (4.3–35.4) | 0.950 |
| REM latency (min) | 104.8 (65.4–173.6) | 89.5 (69.8–158.6) | 0.873 |
| Apnea–hypopnea index | 3.9 (0.4–12.9) | 7.1 (1.7–16.6) | 0.087 |
- Note: Data are presented as mean ± standard deviation for parametric continuous variables and median (quarter) for nonparametric continuous variables.
- Abbreviations: ESS, Epworth’s Sleepiness Scale; GDS, Geriatric Depression Scale; PSQI, Pittsburgh Sleep Quality Index.
- ∗∗p < 0.01.
Table 2 shows the comparison of standardized z-scores of neuropsychological tests between the two groups. The depressed group showed poorer performance in color naming of the Stroop test (−0.01 ± 1.04 vs. 0.54 ± 1.02, p = 0.024) compared to the nondepressed group. In addition, the depressed group tended to have lower z-scores than the nondepressed group in word reading of the Stroop test (0.09 ± 1.05 vs. 0.47 ± 0.91, p = 0.096), although such differences were not statistically significant.
| Neuropsychological test | Depression (n = 25) | Nondepression (n = 64) | p |
|---|---|---|---|
| Attention and working memory | |||
| Digit span forward | 0.09 (−0.12, 0.44) | −0.12 (−0.82, 0.62) | 0.496 |
| Digit span backward | −0.04 (−1.06, 0.09) | −0.04 (−0.64, 1.00) | 0.130 |
| Stroop test, word reading | 0.09 ± 1.05 | 0.47 ± 0.91 | 0.096 |
| Stroop test, color naming | −0.01 ± 1.04 | 0.54 ± 1.02 | 0.029 ∗ |
| Stroop test, color–word interference | −0.05 (−0.84, 0.24) | 0.00 (−0.51, 0.63) | 0.117 |
| Trail Making Test, part A | 0.87 (0.29, 1.20) | 1.03 (0.58, 1.29) | 0.141 |
| Memory | |||
| Word list memory | 0.08 ± 1.10 | 0.39 ± 0.94 | 0.182 |
| Word list recall | −0.78 (−1.13, 0.48) | 0.00 (−0.53, 0.58) | 0.163 |
| Word list recognition | −0.25 (−1.20, 0.67) | 0.13 (−0.39, 0.71) | 0.143 |
| Constructional recall | −0.08 (−0.79, 0.64) | −0.02 (−0.65, 0.81) | 0.434 |
| Executive function | |||
| Categorical fluency | 0.35 ± 1.02 | 0.35 ± 1.30 | 0.985 |
| CLOX 1 | 0.66 (−0.36, 0.79) | 0.47 (0.18, 0.78) | 0.993 |
| Frontal assessment battery | 0.15 (−0.38, 0.64) | 0.37 (−0.28, 0.67) | 0.222 |
| Visuospatial function | |||
| Constructional praxis | 0.53 (−0.34, 0.62) | 0.60 (−0.40, 0.60) | 0.598 |
| CLOX 2 | 0.38 (0.33, 0.46) | 0.38 (0.33, 0.38) | 0.845 |
| Language | |||
| 15-Item Boston Naming Test | 0.68 (−0.07, 1.38) | 0.79 (0.16, 1.21) | 0.996 |
- Note: Data are presented as mean ± standard deviation for parametric continuous variables and median (quarter) for nonparametric continuous variables.
- ∗p < 0.05.
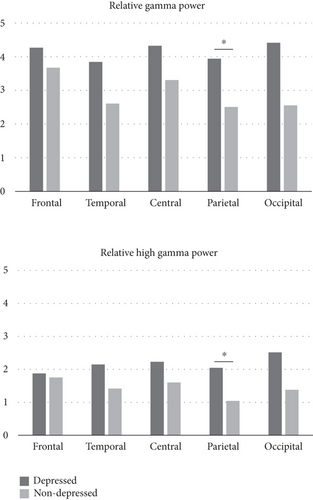
Significant difference in EEG activity between the two groups was also presented (Figure 2). The depressed group showed higher relative gamma (p = 0.034) and high gamma (p = 0.020) power in the parietal region compared to the nondepressed group. No differences were observed in other EEG band activities between the two groups.
3.2. Longitudinal Study for Phenoconversion Risk
During follow-up, one patient expired, one patient had primary central nervous system lymphoma, and three patients refused follow-up evaluation (Figure 1). Twenty-one patients phenoconverted to neurodegenerative disease, including PD (n = 15), DLB (n = 1), AD (n = 4), and MSA (n = 1). Sixty-four patients remained as having iRBD at follow-up.
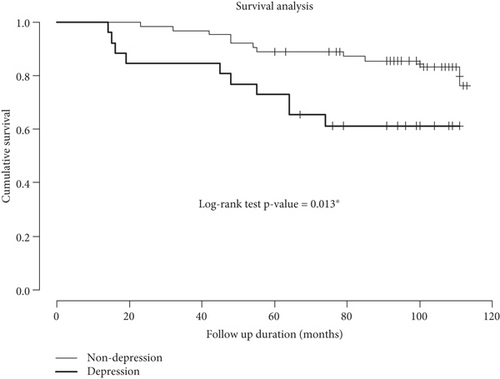
The median observation period was 98.0 (76.8, 108.3) months between vPSG diagnosis and last follow-up, ranging from 14 to 113 months. Estimated 5-year and 8-year risks of neurodegenerative disease were 15.6% and 21.5%, respectively. Survival curves for the phenoconversion in iRBD patients with and without depression are shown in Figure 3. The risk of phenoconversion significantly increased in iRBD patients with depression than in iRBD patients without depression (p = 0.013). After adjusting for age, gender, and apnea–hypopnea index, depression was associated with a hazard ratio of 3.32 (p = 0.011) for phenoconversion to neurodegenerative diseases in iRBD (Table 3).
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | Exp(β) (95% CI) | p value | β | Exp(β) (95% CI) | p value | |
| Age | 0.099 | 1.105 (1.025–1.190) | 0.009 ∗ | 0.102 | 1.107 (1.027–1.194) | 0.008 ∗ |
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | −0.177 | 0.838 (0.338–2.079) | 0.703 | 0.345 | 1.412 (0.533–3.742) | 0.488 |
| Depression | ||||||
| Nondepressed | 1 | 1 | ||||
| Depressed | 1.038 | 2.825 (1.194–6.682) | 0.018 ∗ | 1.2 | 3.319 (1.310–8.412) | 0.011 ∗ |
| Apnea–hypopnea index | −0.003 | 0.997 (0.963–1.032) | 0.851 | 0.002 | 1.002 (0.963–1.043) | 0.909 |
- Note: likelihood ratio test: χ2 = 14.498, p = 0.006.
- ∗p < 0.05.
4. Discussion
In this study, depression was identified in over one-quarter of iRBD patients. The depressed group showed poorer performance in the color naming subtest of Stoop compared to the nondepressed group. qEEG analysis revealed increased gamma and higher gamma power in the parietal region in the depressed group. Finally, survival analysis indicated that depression significantly increased the risk of phenoconversion in iRBD.
The prevalence of depression among iRBD in this study was 28.9%, aligning with the 28.8% reported in a recent meta-analysis [12–14], which is significantly higher than the estimated 5% in general population [45]. Notably, this study showed that the prevalence of depression was higher in women with iRBD (51.85%) compared to men (19.05%), reflecting the general trend where depression is up to 50% more common in women than in men [46, 47]. A study that used tract-specific statistical analysis to assess tract-specific microstructural alterations associated with iRBD discovered that microstructural changes in the right inferior fronto-occipital fasciculus are linked to depressive symptoms in iRBD [48]. This finding may help to explain why depression has become prevalent in iRBD. Small white matter structural alterations have been seen in the right inferior fronto-occipital fasciculus and bilateral anterior thalamic radiation of individuals with iRBD, indicating that inferior fronto-occipital fasciculus alterations may play a role in the development of depressive symptoms [48].
Depression often occurs as a frequent complication of conditions related to α-synucleinopathies. Additionally, a previous study [9] has reported that 35% of people with PD are depressed, which is 3.02 times higher than the rate in the general population [14, 49]. This confirms that the presence of depression increases the risk of developing PD [50]. Both depression and RBD are recognized as early warning signs of PD [51]. In this regard, while antecedent mood disorders have been identified as important antecedent clinical markers of PD, the impact of depression in patients with iRBD remains unclear. Thus, in the present study, we sought to determine the extent to which comorbid depression in patients with iRBD was a risk factor for phenoconversion to a neurodegenerative disorder such as PD. Our study was in line with previous studies [15, 52] showing that depression was associated with an increased risk of phenoconversion to PD in patients with iRBD. There seems to be a connection between depression and PD, suggesting that they may share a common cause [53, 54]. This suggests that there might be a biological interaction between depressive disorder and PD within iRBD, consistent with our findings. There may be a connection between PD and the loss of dopaminergic and noradrenergic innervation in certain areas of the brain. This reduction in monoamine activity could indicate a shared biological mechanism between PD and psychiatric disorders [15, 55].
According to a meta-analysis, all cognitive domains are affected in iRBD compared to normal controls, including general cognition, memory, executive function, processing speed, attention, language, and visuospatial abilities [22]. While depression can adversely affect neurocognitive functions—such as psychomotor speed, attention, visual learning, memory, and executive functioning [56]—it is reasonable to hypothesize that comorbid depression in iRBD may have a more pronounced impact on these cognitive abilities. In this study, the depressed group showed significantly poorer performance only in the color naming subtest of the Stroop test compared to the nondepressed group. The addition of depression does not appear to significantly affect performance on other neuropsychological tests, at least at the time of iRBD diagnosis, but processing speed. The color naming subtest requires participants to simply recognize and name the color of each box in a list, mainly assessing processing speed and attention. Other attention-related tasks, such as digit span forward and backward, showed no significant differences between the groups. However, the word reading subtest of the Stroop test, which similarly reflects processing speed, also indicated a trend toward reduced performance in the depressed group (p < 0.1). Given that cognitive slowing and psychomotor retardation are common symptoms of depression, this finding is not surprising. It can be inferred that processing speed is the cognitive domain most impacted by depression in iRBD, despite the pre-existing cognitive deficits associated with iRBD.
In this study, high-frequency activities such as relative gamma power and high gamma power were increased in the parietal region in the depressed group of iRBD compared to the nondepressed group. In the context of depression, typically, alpha and theta band rhythms have been the primary focus of EEG investigations pertaining to depression since they have demonstrated correlations with both depressed states and recovery [57–60]. Nevertheless, the gamma band, despite its growing significance in neural processing in both humans and rodents, has not been extensively studied in this context [61]. Research by Fitzgerald and Watson found that individuals with unipolar depression displayed higher gamma power compared to healthy controls, suggesting a possible link to mood alterations [62]. Experimental variations, such as assessing gamma power during cognitive tasks [63, 64] versus baseline states [65–67], have led to conflicting findings. Nevertheless, gamma rhythms are known to relate to sensory and attentional systems [68] and may reflect mood changes in certain contexts [62]. Moreover, the parietal lobe, crucial for sensory perception, integration, and spatial attention [69], shows increased gamma activity linked to cognitive coordination during tasks involving visual and spatial information [70]. Previous research highlighted reduced posterior brain connectivity in iRBD, with specific changes in the parietal lobule associated with processing speed [71]. In this study, the elevated gamma activity in the parietal area of depressed iRBD patients might compensate for their impaired processing speed and attention, suggesting a complex interplay between depression and neurocognitive function in iRBD.
Our study had several limitations. First, the inclusion of mildly depressed patients in our study might have introduced variability in depression severity and potentially limited the generalizability of our findings to a broader population. Future investigations employing a more stringent categorization of depression severity would contribute to a more nuanced understanding of the relationship between different levels of depressive symptoms and the phenoconversion of iRBD to neurodegenerative diseases. Second, the potential confounding factors such as medication use were not adjusted, which could impact both depressive symptoms and EEG findings. Third, the relatively small sample size, particularly within the depressed iRBD group, might have impacted the statistical power of our study, potentially influencing the detection of statistically significant differences in certain cognitive measures and EEG parameters. Lastly, the absence of a normal control group limits our ability to compare cognitive and EEG findings of iRBD patients with and without depression to healthy controls.
Despite these limitations, this study used multiple assessment tools to capture changes in depression in iRBD and performed survival analysis with 7 years of follow-up to explore the relationship of depression with phenoconversion to neurodegenerative diseases. In conclusion, when depressive symptoms, even mild, are identified in patients with iRBD, regular monitoring and appropriate treatment should be considered as they may exacerbate cognitive dysfunction and serve as a risk factor for phenoconversion to neurodegenerative diseases. Future research should explore the predictive value of different types of depression on iRBD phenoconversion; investigate if depression specifically predicts conversion to diseases like PD, MSA, or DLB; and examine whether treating depression can mitigate the risk of phenoconversion.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.H.M.: statistical analysis, visualization, and writing–original draft; J.K.H.: investigation, projection administration, statistical analysis, writing–revised draft, and editing; M.L.: methodology, data curation, and review; H.H.K.: conceptualization, data curation, statistical analysis, and review I.-Y.Y.: conceptualization, funding acquisition, investigation, projection administration, supervision, and review. All authors read and approved the manuscript. S.H.M. and J.K.H. contributed equally (co–first author).
Funding
This work was supported by a grant (No. 02-2019-0039) from the Seoul National University Bundang Hospital (SNUBH) Research Fund.
Acknowledgments
This work was supported by a grant (No. 02-2019-0039) from the Seoul National University Bundang Hospital (SNUBH) Research Fund.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




