Anatomical Dimensions of the Proximal Carpal Tunnel Entrance and Its Relationship With Carpal Tunnel Syndrome
Abstract
Background: Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy in humans. It is characterized by paresthesias in the median nerve (MN) area, distal to the carpal tunnel (CT). It is more common in middle-aged women. Its incidence increases with repetitive manual activity and obesity, although its ultimate etiology is not well known. Our aim was to determine the ultrasound anatomical dimensions of the proximal CT entrance and their relationship with sex, age, anthropometric data, MN, and the presence or absence of CTS and to assess their possible etiological role in this neuropathy.
Methods: We analyzed the anatomical measurements of the proximal entrance of 793 CTs using ultrasound—height, width, and ellipsoid area—in patients with CTS (578) and in healthy subjects (215). We also analyzed their relationships with age, sex, height, weight, dominant hand, and degree of nerve involvement.
Results: The three anatomical variables studied at the proximal entrance of the CT were height (12.63 ± 1.44 mm), width (22.06 ± 2.01 mm), and ellipsoid area (173 ± 22 mm2). All three measurements studied were higher in cases than in controls and in male than in female. Height and area were strongly associated with the degree of MN involvement.
Conclusions: These results suggest that the proximal CT entrance is a site of adaptability rather than the site of greatest biomechanical stress within the CT in the pathophysiology of CTS.
1. Introduction
The median nerve (MN) is one of the three main nerves that descend along the upper limb. After the elbow joint, it divides on its anterior surface into two branches: a deep and essentially motor branch, the anterior interosseous branch, which descends over the interosseous membrane, and another more superficial, mixed, and thicker branch, which descends between the superficial and deep muscle planes [1]. In the distal third of the forearm, it becomes more superficial as the muscles become tendons.
Before reaching the wrist, in most cases, it gives off a branch, the palmar cutaneous branch, which provides sensory innervation to the palm [2, 3]. It then crosses the carpus through a tunnel just inferior to the flexor retinaculum. In its distal emergence, it divides, giving its sensory branches to the first, second, third, and medial edge of the fourth digit, and the recurrent motor branch, which, although with variations, provides motor innervation to the dependent muscles of the thenar eminence [1]. Through the tunnel, the MN can show variations in its morphology, although minor, such as bifid or trifid, and accompanied by a persistent median artery (PMA) [2, 4, 5].
The carpal tunnel (CT) is an osteofibrous structure located at the anterior aspect of the wrist, with a trapezoidal to cylindrical longitudinal shape and an elliptical cross-sectional shape. The carpal bones form its concave base and lateral surfaces, but its roof is formed by a fibrous septum, the flexor retinaculum [6, 7], which moves apically or bulges in wrists with carpal tunnel syndrome (CTS) with marked intraneural edema. The CT is estimated to have a mean length of 12.7 ± 2.5 mm, a mean width of 19.2 ± 1.7 mm, and a mean height or depth of 8.3 ± 0.9 mm [8] and is not homogeneous along its course [7]. Its course is longitudinally descending distally.
Its proximal entry is bounded by the scaphoid (lateral) and the pisiform (medial) and is immediately followed by the radioulnar carpal joint (Figure 1). It exits distally through the trapezium and hamate. Most authors argue that it has a greater cross-sectional area (CSA) proximal than distal [6, 9, 10]. The tunnel is crossed by nine tendons and the MN in the upper external part. Of the tendons, four upper tendons correspond to the flexor digitorum superficialis and four lower tendons correspond to the flexor digitorum profundus. These eight tendons have a common synovial sheath [11]. The ninth tendon corresponds to the flexor pollicis longus and has its own synovial sheath, which passes laterally and inferiorly and is inserted into the base of the distal phalanx of the thumb [5]. In addition, according to Borekci et al. [12], accessory muscles or variations of the tendon connections are present within the CT in up to 50% of cases, most commonly unilateral.
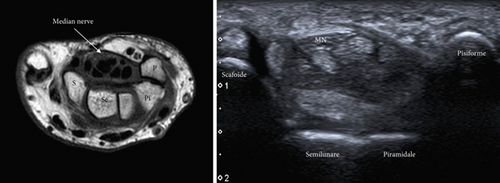
CTS is the most common entrapment neuropathy in humans. It is characterized by the presence of paresthesias, predominantly at night, in the MN-dependent digits [13–15]. It is more common in middle-aged females, with a 3:1 ratio to males, and its prevalence is estimated to be up to 10% of the population in western countries. It usually affects the dominant limb with repetitive use of the hand, wrist, and forearm, although it can be bilateral. In more advanced stages, paresthesias become more persistent, may involve the forearm, and may be accompanied by pain with neuropathic characteristics [16]. In advanced stages, there is a loss of pinch strength and amyotrophy of the thenar eminence. Tinel’s or Phalen’s signs may be present [15].
The diagnosis of CTS is clinical, supported by electrodiagnostic tests such as electroneurography (ENG), which has a level of Evidence A [17]. In recent decades, ultrasonography (US) has been widely used for this purpose [18] (Figure 2). Clinical guidelines suggest that US is an appropriate adjunct to ENG in diagnosing CTS [19]. Although US is an easily accessible and more comfortable technique for the patient, it has reasonable limitations in the diagnosis and staging of primary and idiopathic CTS [20]. However, it does provide relevant information about the morphology of the nerve and surrounding structures [11, 14, 21].
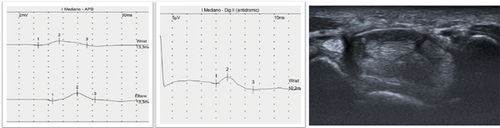
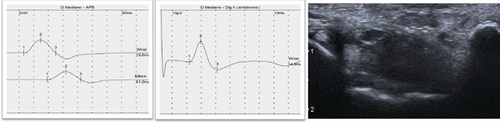
The primary cause of CTS is a chronic compression of the MN secondary to increased pressure in the CT. In recent decades, there have been several attempts to understand its pathophysiology and discover its etiology, especially its relationship with sex, age, obesity, and occupation [22–24]. Also, with the development of imaging techniques, there have been attempts to understand CT and its dimensions and to find out if they are the cause of the appearance of CTS or not, since its decrease has been intuited as a possible origin of MN entrapment. There is a consensus that CT has smaller dimensions in females from the fetal period to adulthood [25]. However, previous studies have had small sample sizes.
Our aim was to determine the ultrasound anatomical dimensions of the proximal CT entrance and their relationship with sex, age, anthropometric data, MN, and the presence or absence of CTS and to assess their possible etiological role in this neuropathy.
2. Material and Methods
This is a prospective case–control study of patients suspected of having CTS undergoing ENG and US. The numerical values of the US of the proximal entrance of the CT and the MN were studied. In all cases, their relationship with age, sex, height, weight, body mass index (BMI), dominant hand, and laterality was studied. Informed consent was obtained from each patient. The study was approved by the Ethics Committee of the HM Foundation.
Inclusion criteria included patients aged 18–85 years with suspected CTS who attended the Clinical Neurophysiology Service of HM La Esperanza to undergo ENG between April 2023 and November 2024 in Santiago de Compostela, northwest Spain.
Exclusion criteria included patients with previous CTS or surgery on the forearm, carpus, or hand; previous significant trauma or fracture of the forearm, carpus, or hand; and underlying neuromuscular diseases, diabetes mellitus, acromegaly, or rheumatoid arthritis.
2.1. Ultrasound Studies
The MN was explored using US in transverse and longitudinal sections from the middle third of the forearm to the distal exit of the CT. The presence of anatomical variations such as bifid or trifid nerves and PMA was evaluated. Images and measurements were taken in the axial plane of the proximal entrance of the CT, at the level of the pisiform bone. The width of the tunnel was measured from the medial edge of the pisiform bone to the medial edge of the scaphoid, the height from the bony floor to the flexor retinaculum at its midpoint, and the ellipse area of the sheath surrounding the nine tendons and the MN (Figure 3). The intraneural area of the MN was measured at the entrance of the CT, marking the inner edge of the epineurium and the longitudinal diameter (LD) [11, 19, 21]. A Toshiba Aplio 400 ultrasound system with a 12-MHz linear probe was used. The wrists were examined in the supine and neutral positions after the ENG examination.
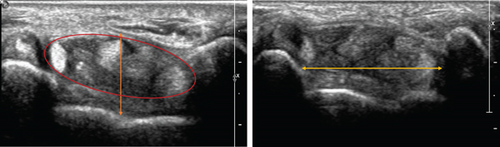
A clinical neurophysiologist performed the ENG and ultrasound examinations. Images of each nerve were then reviewed by a musculoskeletal radiologist in a blinded fashion to avoid confirmation bias.
2.2. ENG Studies
- •
Incipient: CV of the fourth digit less than or equal to 49 m/s, with no difference of 0.5 ms with the fourth ulnar digit.
- •
Mild: CV at the fourth digit < 49 m/s and difference equal to or greater than 0.5 ms with respect to the fourth ulnar digit. Distal motor latency (DML) < 4.2 ms.
- •
Moderate: CV in the third and fourth digits to 40 m/s or less and DML equal to or greater than 4.2 m/s.
- •
Severe: CV in third and fourth digits < 35 m/s, decrease in amplitude in third and fourth digits, and DML > 4.2 m/s.
2.3. Statistical Analysis
Frequencies, means, and standard deviations (SDs) were used for descriptive statistics. Student’s t-test was used to calculate the variance of means between two groups, and the ANOVA test was used to calculate the variance of means between three or more groups. The association between categorical variables was examined using the chi-squared test. Pearson’s correlation coefficients were calculated as the variables showed normal behavior. Statistical analysis was carried out using SPSS 29.0.
3. Results
The sample studied consisted of 793 carpals from 585 individuals, 181 males and 404 females. Of the carpals, 244 were from males and 549 were from females. The mean age of the sample was 51.95 ± 12.35 years (20–85 years). On the other hand, 578 carpals corresponded to STC cases and 215 to controls.
The three anatomical variables studied at the proximal entrance of the CT presented the following means (±SD) in the total sample: tunnel height (12.63 ± 1.44 mm), tunnel width (22.06 ± 2.01 mm), and ellipsoid area (173 ± 22 mm2) (Table 1).
| ANOVA/t-test | Tunnel height (mm) | Tunnel width (mm) | Tunnel area (mm2) | p | |
|---|---|---|---|---|---|
| Total sample | Media ± SD | 12.63 ± 1.44 | 22.06 ± 2.01 | 173 ± 22 | |
| Sex | Men (244) | 13.39 ± 1.59 | 23.51 ± 1.88 | 191 ± 21 |
|
| Women (549) | 12.30 ± 1.22 | 21.41 ± 1.71 | 169 ± 61 | ||
| Age groups | AG1 (20–40 years) (129) | 12.22 ± 1.56 | 21.96 ± 2.00 | 167 ± 20 |
|
| AG2 (41–60 years) (478) | 12.70 ± 1.40 | 22.07 ± 2.01 | 172 ± 23 | ||
| AG3 (61–85 years) (186) | 12.71 ± 1.50 | 22.10 ± 2.01 | 177 ± 22 | ||
| BMI | I (15–20) (28) | 11.91 ± 1.28 | 21.03 ± 1.73 | 157 ± 23 |
|
| II (20.1–25) (261) | 12.20 ± 1.38 | 21.41 ± 1.86 | 165 ± 20 | ||
| III (25.1–30) (314) | 12.78 ± 1.40 | 22.42 ± 1.99 | 178 ± 22 | ||
| IV (30.1–35) (135) | 13.01 ± 1.52 | 22.53 ± 1.93 | 177 ± 21 | ||
| V (35.1–40) (38) | 13.02 ± 1.37 | 22.59 ± 2.67 | 182 ± 22 | ||
| VI (40.1–45) (16) | 13.21 ± 1.03 | 22.18 ± 2.10 | 184 ± 27 | ||
| VII (45.1–50) (1) | 10.9 | 21.4 | 155 | ||
| Laterality | Right (442) | 12.66 ± 1.41 | 22.01 ± 1.95 | 175 ± 45 |
|
| Left (349) | 12.58 ± 1.47 | 22.12 ± 2.08 | 175 ± 62 | ||
| Dominance | Yes (458) | 12.65 ± 1.41 | 22.08 ± 2.00 | 176 ± 45 |
|
| No (335) | 12.61 ± 1.47 | 22.03 ± 2.03 | 175 ± 63 | ||
| CTS degree of severity | Control (215) | 11.91 ± 1.40 | 21.79 ± 2.01 | 163 ± 19 |
|
| Incipient (131) | 12.60 ± 1.21 | 22.01 ± 2.24 | 170 ± 19 | ||
| Mild (248) | 12.70 ± 1.27 | 22.02 ± 1.87 | 174 ± 21 | ||
| Moderate (105) | 13.46 ± 1.25 | 22.68 ± 1.80 | 183 ± 23 | ||
| Severe (94) | 13.11 ± 1.71 | 22.16 ± 2.15 | 186 ± 23 | ||
In relation to sex, the three variables showed larger sizes in males (p < 0.001), both in cases and controls. Also, the CSA-MN/CSA-CT index shows a proportional increase in cases compared to controls, both in males and females (Table 2).
| t-test | n | Tunnel height (mm) | Tunnel width (mm) | CSA-CT (mm2) | CSA-MN (mm2) | Index area (CSA-MN/CSA-CT) [26] (%) | p | |
|---|---|---|---|---|---|---|---|---|
| Males | Cases | 183 | 13.6 | 23.6 | 196 | 12.7 | 6.5 |
|
| Controls | 61 | 12.6 | 23.2 | 176 | 8.3 | 4.7 | ||
| Total | 244 | 13.4 | 23.5 | 191 | 11.6 | 6.1 | ||
| Females | Cases | 395 | 12.5 | 22.0 | 168 | 12.1 | 7.2 |
|
| Controls | 154 | 11.6 | 21.2 | 157 | 8.2 | 5.2 | ||
| Total | 549 | 12.1 | 21.6 | 164 | 11 | 6.7 | ||
| Sample | Cases | 578 | 12.9 | 22.2 | 1.77 | 12.3 | 7.0 |
|
| Controls | 215 | 11.9 | 21.8 | 1.62 | 8.2 | 5.1 | ||
| Total | 793 | 12.6 | 22.1 | 173 | 11.2 | 6.5 | ||
In relation to the stratified age groups, all three variables have increased with age; however, both height and area have done so in a statistically significant way (p < 0.001), although this phenomenon occurs only in cases due to the presence of CTS. On the other hand, the increase in stratified BMI showed significant relationships with the increase in the three anatomical variables studied (p < 0.001) (Table 1).
The three variables studied did not show variations between dominant or nondominant hands and left or right extremities (laterality). Regarding the degree of impairment of CTS with the mean values of the anatomical variables studied, we see a progressive increase in the three variables with increasing severity, mainly height and CSA-CT (p < 0.001) (Table 1), although in severe cases the average height and width decrease in relation to moderate cases.
In terms of Pearson’s CC, of the three variables, only CT width was weakly related to height (0.413) (Table 3). The other relationships with anthropometric data are not significant. Among them, however, the height of the CT was related to the CSA-CT (0.611). The width of the CT was also related to the CSA-CT (0.529), also in a weak way, while height and width were not related to each other (0.285). The CSA-MN has the same relationship with the height as with the CT area (0.355).
| Pearson’s CC | Age | Height | Weight | BMI | CSAf | CSAc | LD | Tunnel height | Tunnel width | Tunnel area |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | — | −0.158 | −0.003 | 0.103 | 0.016 | 0.072 | 0.055 | 0.137 | 0.072 | 0.187 |
| Height | −0.158 | — | 0.493 | 0.058 | 0.156 | −0.051 | −0.020 | 0.236 | 0.413 | 0.358 |
| Weight | −0.003 | 0.493 | — | 0.861 | 0.129 | 0.180 | 0.138 | 0.285 | 0.356 | 0.391 |
| BMI | 0.103 | 0.058 | 0.861 | — | 0.062 | 0.237 | 0.170 | 0.227 | 0.204 | 0.275 |
| CSA-MN forearm | 0.016 | 0.156 | 0.129 | 0.062 | — | 0.164 | 0.075 | 0.150 | 0.077 | 0.269 |
| CSA-MN wrist | 0.072 | −0.051 | 0.180 | 0.237 | 0.164 | — | 0.360 | 0.355 | 0.080 | 0.355 |
| LD | 0.055 | −0.020 | 0.138 | 0.170 | 0.075 | 0.360 | — | 0.124 | 0.080 | 0.138 |
| Tunnel height | 0.137 | 0.236 | 0.285 | 0.227 | 0.150 | 0.355 | 0.124 | — | 0.285 | 0.611 |
| Tunnel width | 0.072 | 0.413 | 0.356 | 0.204 | 0.077 | 0.080 | 0.080 | 0.285 | — | 0.529 |
| Tunnel area | 0.187 | 0.358 | 0.391 | 0.275 | 0.269 | 0.355 | 0.138 | 0.611 | 0.529 | — |
- Note: Bold data denote the highest correlations.
When the sample was divided into two groups, cases and controls, we observed that the mean values of the three variables studied were higher in the cases than in the controls (Table 4), with full statistical significance in height and CSA (p < 0.001). We also observed higher mean values in the ultrasound measurements of the MN in the carpus (CSA-MN, LD) in the cases (p < 0.001). Regarding anthropometric variables, weight and BMI were higher in cases, whereas height was higher in controls (cases: 164.54 ± 8.64 mm; controls: 166.83 ± 9.62 mm; total: 165.16 ± 8.97 mm). Dominance, laterality, and CT width were not significantly different between cases and controls. In Table 2, we observe the means by sex of the independent samples, as the tendency is that, for height, width, and CSA-CT, the means are higher in cases than in controls and in males than in females.
| t-test | Cases (578) | Controls (215) | Total (793) | p | |
|---|---|---|---|---|---|
| Tunnel height (mm) | 12.88 ± 1.38 | 11.88 ± 1.38 | 12.61 ± 1.45 | < 0.001 | |
| Tunnel width (mm) | 22.17 ± 2.01 | 21.76 ± 2.00 | 22.06 ± 2.01 | 0.062 | |
| Tunnel area (mm2) | 179 ± 50 | 163 ± 19 | 174 ± 44 | < 0.001 | |
| CSA-MN forearm (mm2) | 4.71 ± 0.82 | 4.69 ± 0.79 | 4.71 ± 0.81 | 0.326 | |
| CSA-MN wrist (mm2) | 12.26 ± 3.39 | 8.21 ± 1.33 | 11.17 ± 3.48 | < 0.001 | |
| LD-MN (mm) | 2.50 ± 0.94 | 2.07 ± 0.283 | 2.39 ± 0.84 | < 0.001 | |
| Age (years) | 53.77 ± 12.14 | 46.73 ± 11.03 | 51.87 ± 12.25 | < 0.001 | |
| Height (cm) | 164.54 ± 8.64 | 166.83 ± 9.62 | 165.16 ± 8.97 | < 0.001 | |
| Weight (kg) | 76.17 ± 16.70 | 70.88 ± 16.23 | 74.74 ± 16.73 | < 0.001 | |
| BMI | 27.88 ± 5.10 | 25.30 ± 4.33 | 27.17 ± 5.04 | 0.027 | |
| Side | Right | 328 | 114 | 442 | 0.323 |
| Left | 248 | 101 | 349 | ||
| Dominance | Yes | 339 | 119 | 458 | 0.403 |
| No | 239 | 96 | 335 | ||
Regarding the number of MN and PMA, 719 carpals presented a single MN, 71 were bifid, and three were trifid. Thirty-six carpals had one PMA, and one had two PMA. The presence of these anatomical variables was not associated with changes in the mean measured dimensions (height, width, and area) of the CT entrance.
4. Discussion
Over the past few decades, several studies have been carried out to determine the size of the CT, particularly its entrance and exit, and its possible relationship to MN entrapment in idiopathic and majority CTS. Studies have been carried out on cadavers, healthy volunteers, and patients with CTS using radiography, ultrasound, CT, and magnetic resonance imaging (MRI). Although US does not approximate cadaver anatomy as closely as MRI [27], very few have focused on the careful study of the proximal entrance of CT.
Dekel and Coates in 1979 [28], using radiographs, found that the proximal and distal CSA of CT were smaller in females. Merhar et al. [29], using tomography, added that the distal CSA was smaller than the proximal in CT. In 2008, Bower et al., who studied eight carpals in healthy subjects using MRI in various flexion and extension positions, showed that both the CT and its content were smaller in females and that the relationship between CT dimensions and content was proportional between the sexes (see details in Table 5). Pacek et al. [8] found that females had narrower CTs and smaller CSAs in 10 cadavers. Kim et al. [10] also demonstrated with US that the proximal CSA of the CT was larger than the distal CSA in both cases and controls.
| Author and year | Technique | Carpals | ENG | Cases | Proximal CSA (mm2) | Proximal width (mm) | Proximal height (mm) | Distal CSA (mm2) | Distal width (mm) | Distal height (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Winn and Habes, 1990 [30] | TC | 58 | Yes | 25 |
|
— | — |
|
— | — |
| Allmann et al., 1997 [9] | MRI 1T | 40 | — | 20 |
|
— | — |
|
— | — |
| Cobb et al., 1997 [31] | MRI 1, 5T; Rx | 14 | Yes | 7 | — | — | — |
|
|
|
| Pierre-Jerome et al., 1997 [32] | MRI 0, 5T | 114 | — | 0 |
|
— | — |
|
— | — |
| Monagle, 1999 [33] | MRI | 39 | Yes | 14 | 353 | — | — | 316 | — | — |
| Kamolz et al., 2001 [34] | US cadaver | 40 | — | — | — | — |
|
|
|
|
| Uchiyama et al., 2005 [35] | MRI | 141 | — | 105 |
|
— | — |
|
— | — |
| Bower et al., 2006 [36] | MRI | 8 | — | 0 | 173 | — | — | 182 | — | — |
| Mogk and Keir, 2008 [37] | MRI | 6 | — | 180.5 | 22.5 | 10.5 | 170 | 21.4 | 10.4 | |
| Pacek et al., 2010 [8] | Cadaver; silicone mold | 10 | — | 134.9 | 19.2 | 8.3 | 134.9 | 19.2 | 8.3 | |
| Kim et al., 2012 [26] | US | 60 | Yes | 0 |
|
|
||||
| Kim et al., 2013 [10] | US | 102 | Yes | 78 |
|
— | — |
|
— | — |
| Gabra, 2015 [38] | MRI cadaver | 8 | — | — | — | — | 183.5 | 21.3 | 10.9 | |
| Rodríguez et al., 2022 [39] | MRI cadaver | 20 | — | 188 | — | — | — | — | — | |
| Rani et al., 2024 [40] | US | 65 | — |
|
|
|
— | — | — | |
| González-Uriel and Suárez-Quintanilla, 2025 (current study) | US | 793 | Yes | 578 |
|
|
|
— | — | — |
Recently, Rodríguez et al. [39] found that both the CSA of CT and other parameters as muscle mass within the TC in cadavers were higher in males (Table 5). In 2023, Kondak et al. showed that CT CSAs were lower in females from the fetal period. Rani et al. [40] found in 65 carpals examined by ultrasound that the CT was narrower in females and that the CSA was lower (142 mm2 in total, 163 mm2 in men, and 137 mm2 in women). They also found that the higher the age, the higher the CSA-CT. They all agree with our research, in which the three variables studied were higher in men, both in cases and controls (Table 5).
With regard to the anthropometric data studied, while laterality, dominance, or height is not associated with CTS, there is ample evidence that obesity is a risk factor for its development [24, 41, 42], especially before the age of 63, where it is the second risk factor after repetitive manual work [22]. In our sample, both average weight and BMI were significantly higher in cases than in controls.
In relation to the presence or absence of CTS, Allman et al. [9] described a mean CSA of 178 mm2 at the level of the pisiform bone (179 mm2 in our cases) in the MRI of 20 wrists with CTS symptoms. Kim et al. [10] examined the mean areas of CT and MN at the entry and exit of the carpus. They found that at the proximal entrance, both the CSA of the CT and the MN increased in cases compared to controls, whereas at the distal exit, the CSA of the carpus did not increase in cases. This may indicate that the anatomical point of greatest biomechanical stress is the tunnel exit, as it is less extensible [6]. According to Rotman and Donovan [7], the narrowest part of the canal is between 2 and 2.5 cm from the wrist crease and corresponds to the hourglass deformity seen in patients with CTS. Also, Al-Qattan described that in 30 patients with severe CTS who underwent surgery, the site of greatest entrapment was 2.5 cm from the distal wrist crease, at the level of the hook of the hamate [43].
Regarding the higher incidence of CTS in females, some authors have postulated that the flexor retinaculum is less elastic in this sex, a factor that could increase intracarpal pressure and increase its incidence in response to the same environmental factors [44]. Lakshminarayanan et al. [45] measured the carpal arches of 10 healthy females and 10 healthy males and objectified that they were smaller in females, both proximally and distally.
Going deeper into the pathophysiology of CTS, when the pressure within the CT rises above 30–60 mmHg [46], it compresses the MN and its vasa nervorum, affecting its vascularization, causing intermittent ischemia, depolarization with alteration of axonal transport, and symptoms of paresthesia and hypoesthesia [47]. Intraneural venous return is also impaired, leading to fluid accumulation in the form of edema.
Nerve entrapment is the result of an imbalance between the internal pressures of the CT and the nerve itself, when the former exceeds the latter. Causes of increased pressure include repetitive flexion–extension movements of the wrist and digits, leading to hyperemia of the tendons, increased fatty tissue, and the presence of accessory muscles that can enter and exit both the proximal end of the CT (such as the palmaris longus) and the distal end (such as the lumbricals) [48, 49]. It can also be produced by hypertrophy of the flexor retinaculum and consequent loss of elasticity of its fibers [15, 50].
The main physiological adaptability mechanism to withstand this pressure is the apical displacement of the carpal annular ligament or flexor retinaculum [51, 52]. For this reason, when this compensatory mechanism fails and neuropathy develops, pressure will also increase due to increased intraneural edema. From moderate cases onward, the treatment is dissection of this ligament. Several studies have analyzed the biomechanics of the flexor retinaculum and have reached similar conclusions. The collagenous fibers of the flexor retinaculum are mostly arranged transversely and have greater elasticity at its proximal entry, at the radial edge [51], and less elasticity distally, at its ulnar edge (at the hook of the hamate) [53]. This allows us to better understand our findings and the role of the proximal CT inlet in CTS, as it is a site of adaptability to withstand the increased pressure within the tunnel and whose height and CSA will be higher in CTS cases, regardless of sex.
5. Conclusions
In this study, we observed that both the height and CSA of the proximal CT entrance were increased in patients with CTS compared to healthy subjects, largely due to the elasticity of the flexor retinaculum. These results suggest that the proximal CT entrance is a site of adaptability rather than the site of greatest biomechanical stress within the CT in the pathophysiology of CTS.
Nomenclature
-
- BMI
-
- body mass index
-
- CSA
-
- cross-sectional area
-
- CSA-CT
-
- cross-sectional area carpal tunnel
-
- CSA-MN
-
- cross-sectional area median nerve
-
- CT
-
- carpal tunnel
-
- CTS
-
- carpal tunnel syndrome
-
- CV
-
- conduction velocity
-
- ENG
-
- electroneurography
-
- LD
-
- longitudinal diameter
-
- MN
-
- median nerve
-
- MRI
-
- magnetic resonance imaging
-
- PMA
-
- persistent median artery
-
- SD
-
- standard deviation
-
- US
-
- ultrasound
Ethics Statement
The study has been approved by the Ethics Committee of the HM Foundation.
Consent
Informed consent was obtained from each patient.
Disclosure
This work was carried out in the clinical neurophysiology service of the HM La Esperanza Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.




