Clinical Insights Into Anti-Sulfatide Antibodies in Peripheral Neuropathies: A Retrospective Study
Abstract
Background: Sulfatide, synthesized from glycosphingolipids via sulfation of the hydroxyl group, is a prominent lipid antigen in both the peripheral and central nervous systems. Anti-sulfatide antibodies are detected in axonal and demyelinating neuropathies and are generally regarded as concomitant antibodies. However, their pathogenic role and precise diagnostic relevance remain unclear.
Methods: This double-center retrospective observational study tested anti-sulfatide and antiganglioside antibodies using immunoblot assays in serum and/or cerebrospinal fluid (CSF) from patients suspected of having peripheral neuropathy. Clinical symptoms, laboratory findings, and electrophysiological results were reviewed for patients with anti-sulfatide antibodies.
Results: The most common symptoms in adult-onset patients with anti-sulfatide antibody included motor weakness (81.25%) and superficial sensory disturbances (68.75%). Patients with anti-sulfatide antibodies in the CSF exhibited a higher frequency of CSF albuminocytological dissociation. Positive blood rheumatic antibodies and factors were more prevalent in seropositive patients. Electrophysiological findings revealed both axonal and demyelinating changes in these patients. Intravenous corticosteroids, immunoglobulins, and plasmapheresis proved effective treatments.
Conclusions: The clinical manifestations of patients with anti-sulfatide antibodies are highly heterogeneous. Anti-sulfatide antibodies cause axonal and demyelinating damage in autoimmune peripheral neuropathy, presenting distinct clinical and electrophysiological features.
1. Background
Gangliosides, glycosphingolipids containing one or more sialic acid residues, and paranodal/nodal molecules are critical targets of immune attacks in immune-mediated neuropathies. Autoantibodies targeting these structures cause nerve injury in nodo-paranodal regions, characterized by distinctive pathophysiological mechanisms, as well as electrophysiological and pathological findings, collectively termed nodo-paranodopathy [1]. Sulfatide, a glycolipid distinct from gangliosides due to its lack of sialic acid residues, is synthesized from galactocerebroside, a simple glycosphingolipid, via sulfation of the hydroxyl group on the third carbon of galactose [2]. Sulfatide is predominantly localized in the Golgi apparatus, lysosomes, and cell membranes and is abundant in the nervous system, kidneys, gastrointestinal tract, pancreatic islets, trachea, and various cancers and tumors. It plays roles in physiological and pathological processes involving the nervous system, immune response, hemostasis/thrombosis, diabetes, and bacterial/viral infections [3]. In the nervous system, sulfatide is primarily found in oligodendrocytes and Schwann cells, enriched in the noncompact myelin sheath [4]. In peripheral nerves, it is distributed along the myelin internode and concentrated at the paranodal loops of terminal heminodes [5]. Sulfatide contributes to the differentiation of myelinating cells and the maintenance of paranodal glia–axon junctions and signaling [3, 4]. In sulfatide-deficient mice, the glia–axon junction at the paranode, along with the clustering of Na + channels at nodes and K+ channels at juxtaparanodes, becomes disorganized [6], although the underlying mechanism remains unclear. Anti-sulfatide antibodies have been detected in autoimmune peripheral neuropathies such as Guillain–Barré syndrome (GBS), chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and multifocal motor neuropathy, as well as in IgM monoclonal gammopathy [7–9]. In one study, 85% of anti-sulfatide IgM-positive patients also exhibited elevated IgM antibody titers against myelin-associated glycoprotein (MAG) [7]. Since both sulfatide and MAG are predominantly localized in the myelin paranodal region [5, 10], this finding supports the hypothesis that anti-sulfatide /MAG antibodies contribute to nodo-paranodopathy. Nevertheless, the clinical, electrophysiological, and pathological features of anti-sulfatide antibody-positive autoimmune neuropathy remain poorly defined.
In this study, we reviewed the clinical and electrophysiological features as well as the treatment response of the anti-sulfatide positive patients with or without antiganglioside antibodies in two hospitals. Furthermore, the electrophysiological findings of paranodopathy mediated by anti-sulfatide and anti-NF155 antibodies were compared.
2. Methods
2.1. Patients and Samples
This double-center retrospective observational study analyzed clinical data from patients with anti-sulfatide antibody-positive neuropathy treated at Qilu Hospital of Shandong University and the First Affiliated Hospital of Shandong First Medical University between January 2019 and October 2022. Patients with suspected peripheral neuropathy who underwent testing for anti-sulfatide antibodies in serum or cerebrospinal fluid (CSF) were included. Patients with incomplete clinical data or unclear antibody results were excluded. CSF protein levels exceeding 0.45 g/L were considered clinically relevant.
Antiganglioside antibodies were assessed using a semiquantitative immunoblot method, following the manufacturer’s protocol. Briefly, patient serum and CSF samples were incubated with nitrocellulose membranes precoated with various ganglioside antigens. Following incubation and washing steps, bound antibodies were visualized via enzyme-conjugated secondary antibodies and a colorimetric substrate. The intensity of the resulting bands was analyzed by densitometry. Semiquantitative results were expressed in arbitrary units (AUs), calculated as the ratio of the grayscale intensity of the patient’s positive band to that of the cut-off control band provided with the kit. AU values represent relative units normalized to the cut-off control. A value of 0 AU was interpreted as negative, while increasing AU values indicated higher antibody levels.
During physical examinations, muscle strength was assessed using the manual muscle testing (MMT) scale. Muscle weakness was defined as an MMT score between 0 and 4. Treatment response was classified by the patients’ primary neurologists based on a review of neurological examination results recorded in medical charts.
This study was conducted in accordance with protocols approved by the Ethics Committees of Qilu Hospital of Shandong University and the First Affiliated Hospital of Shandong First Medical University (KYLL-2024(ZM)-096).
2.2. Clinical and Electrophysiological Studies
Physical examinations were performed by neurologists. Electrophysiological data from 32 of the 34 patients with anti-sulfatide antibodies were directly reviewed by the authors. Motor and sensory nerve conduction characteristics in the upper and lower extremities were analyzed, including distal motor latency, conduction velocity, and amplitude. For patients undergoing bilateral limb testing, data from both sides were included in the analysis.
2.3. Genetic Analysis
Genetic analysis was conducted for Patients 17 and 29 based on family history and early onset. DNA was extracted, and whole exome sequencing (WES) was performed for both patients. Multiplex ligation-dependent probe amplification (MLPA) was used in Patient 17 to evaluate repeat and depletion variations in SMN1 and PMP22. For Patient 29, WES and copy number variation sequencing were performed. Written informed consent was obtained from each patient or their legal representative.
2.4. Statistical Analysis
Qualitative variables were reported as percentages and absolute frequencies, while quantitative data were expressed as mean and standard deviation. The Kruskal–Wallis test was applied to multiple comparisons of onset age among groups, and the Mann–Whitney U test was used to analyze electrophysiological results. The χ2 test or Fisher’s exact test was employed to analyze qualitative variables, including clinical and laboratory findings. Statistical analyses were conducted using SPSS Version 25 (IBM Corp., Armonk, New York), with a significance level of p < 0.05.
3. Results
3.1. Accompanied Antiganglioside Antibodies in Anti-Sulfatide Positive Neuropathy
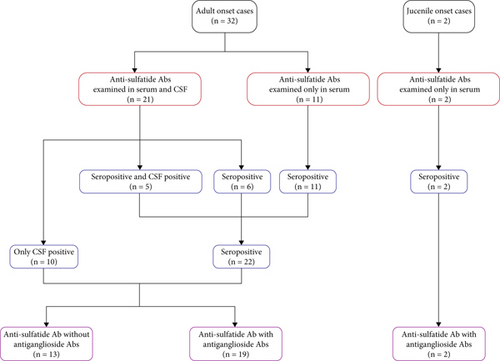
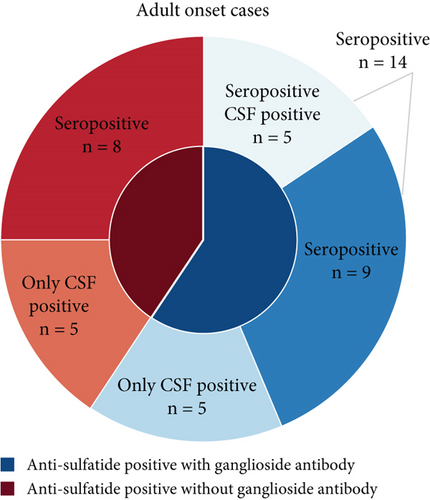
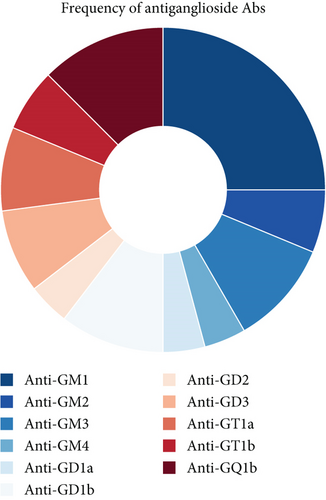
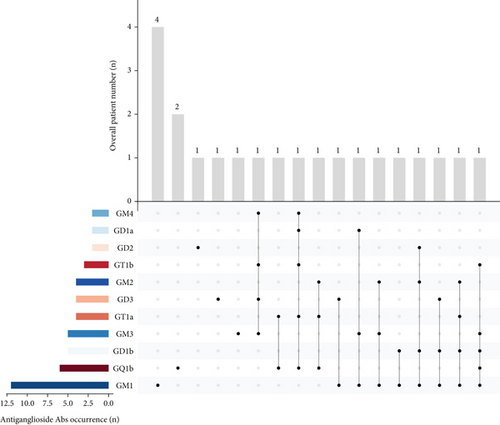
Among the 34 patients with antisulfatide-positive neuropathy, 32 were adult-onset cases, while 2 patients presented symptoms during adolescence (Figure 1a). The specific antibodies detected in each patient and their clinical diagnoses are summarized in Table 1. In adult-onset cases, 13 patients had only anti-sulfatide antibodies in serum or CSF, whereas 19 patients also had accompanying antiganglioside antibodies (Figure 1b). The most frequently co-occurring antiganglioside antibody was anti-GM1 (Figure 1c,d). Other commonly observed antibodies included anti-GQ1b, anti-GM3, and anti-GD1b. Additional antiganglioside antibodies detected in anti-sulfatide -positive patients included anti-GM2, anti-GM4, anti-GD1a, anti-GD2, anti-GD3, anti-GT1a, and anti-GT1b. Thirteen patients tested positive exclusively for anti-sulfatide antibodies.
| Patient no. | Serum | CSF | Diagnosis | ||||
|---|---|---|---|---|---|---|---|
| Anti-sulfatide | Antiganglioside abs | Anti-sulfatide | Antiganglioside abs | ||||
| IgG | IgM | IgG | IgM | ||||
| 1 | + | AMSAN | |||||
| 2 | + | Unclassified peripheral neuropathy | |||||
| 3 | + | AMAN | |||||
| 4 | + | Uncertain diagnosis | |||||
| 5 | + | Anti-GQ1b syndrome | |||||
| 6 | + | CIDP | |||||
| 7 | + | CIDP | |||||
| 8 | + | Unclassified peripheral neuropathy | |||||
| 9 | + | CIDP | |||||
| 10 | + | Unclassified peripheral neuropathy | |||||
| 11 | + | AMSAN | |||||
| 12 | + | AMSAN | |||||
| 13 | + | Unclassified peripheral neuropathy | |||||
| 14 | + | GM1 | AIDP | ||||
| 15 | + | GM1 | Unclassified peripheral neuropathy | ||||
| 16 | + | GM1 | Unclassified peripheral neuropathy | ||||
| 17 | + | GM1 | HNPP | ||||
| 18 | + | GM3 | Uncertain diagnosis | ||||
| 19 | + | GD2 | + | Unclassified peripheral neuropathy | |||
| 20 | GD3 | + | AMAN | ||||
| 21 | + | GM1, GM2, and GM3 | Uncertain diagnosis | ||||
| 22 | + | GM1 and GD1b | AMSAN | ||||
| 23 | GM1 and GD3 | + | AMAN | ||||
| 24 | + | GM1 and GM2, GD1b and GD2 | CIDP | ||||
| 25 | + | + | GM1, GM3, and GD1a | + | Uncertain diagnosis | ||
| 26 | GM1, GD1b, and GD3 | + | GD3 | AMAN | |||
| 27 | + | GM3, GM4, and GD3 | + | GT1b, GD3, GM3, and GM4 | CIDP | ||
| 28 | GQ1b | + | Anti-GQ1b syndrome | ||||
| 29 | + | GQ1b | CMT suspected | ||||
| 30 | GT1a and GQ1b | + | GT1a and GQ1b | Anti-GQ1b syndrome | |||
| 31 | GM1 and GD1b | + | GM2, GD1b, and GT1a | AMAN | |||
| 32 | + | GM2, GQ1b, and GT1a | Anti-GQ1b syndrome | ||||
| 33 | + | GM1, GM3, GD1b, GT1b, and GQ1b | CIDP | ||||
| 34 | + | GM4, GD1a, GT1a, GT1b, and GQ1b | + | GT1a and GQ1b | Anti-GQ1b syndrome | ||
- Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor sensory axonal neuropathy; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; CMT, Charcot–Marie–Tooth disease; HNPP, hereditary motor neuropathy with liability to pressure palsies.
3.2. Clinical and Laboratory Findings in Adult-Onset Patients
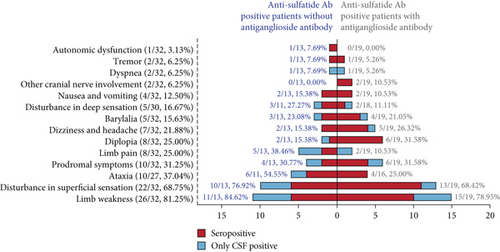
Patients with anti-sulfatide antibodies were categorized into seropositive (antibodies detected in serum and/or CSF) and CSF-positive (antibodies detected only in CSF). For clarity, the latter is referred to as the CSF-positive group. The demographic characteristics and clinical findings of adult-onset patients (n = 32) are summarized in Table 2. The average onset age was 55.22 ± 14.66 years among all patients, with no significant differences between groups. The male-to-female ratio was 18:14. Prodromal symptoms, including fever, respiratory, and gastrointestinal symptoms, were observed in approximately 30% of patients. Limb weakness was the most common symptom, affecting 26 patients (81.25%). Disturbance in superficial sensation (68.75%) was frequently observed, while only 16.67% of patients experienced deep sensory disturbances. Ataxia (37.04%), diplopia (25.00%), limb pain (25.00%), dizziness, and headache (21.88%) occurred occasionally. Less frequent symptoms included barylalia (15.63%), nausea and vomiting (12.50%), dyspnea, tremor, and cranial nerve involvement (other than ocular motor nerves) (6.25%). Only one patient (3.13%) reported autonomic dysfunction.
| Demographics | Seropositive (n = 22) | Only CSF-positive (n = 10) | Total (n = 32) |
|---|---|---|---|
| Age of onset (years, mean ± SD) | 55.41 ± 15.71 | 54.80 ± 14.33 | 55.22 ± 14.66 |
| Sex ratio (male/female) | 13:9 | 5:5 | 18:14 |
| Symptoms | |||
| Prodromal symptoms | 6/22 (27.27%) | 4/10 (40.00%) | 10/32 (31.25%) |
| Disturbance in superficial sensation | 16/22 (72.73%) | 6/10 (60.00%) | 22/32 (68.75%) |
| Disturbance in deep sensation | 3/21 (14.29) | 2/8 (25.00%) | 5/30 (16.67%) |
| Limb weakness | 16/22 (72.73%) | 10/10 (100%) | 26/32 (81.25%) |
| Ataxia | 8/20 (40.00%) | 2/7 (28.57%) | 10/27 (37.04%) |
| Diplopia | 7/22 (31.82%) | 1/10 (10.00%) | 8/32 (25.00%) |
| Other cranial nerve involvement | 2/22 (9.09%) | 0/10 (0.00%) | 2/32 (6.25%) |
| Barylalia | 4/22 (18.18%) | 1/10 (10.00%) | 5/32 (15.63%) |
| Autonomic dysfunction | 1/22 (4.55%) | 0/10 (0.00%) | 1/32 (3.13%) |
| Limb pain | 3/22 (13.64%) | 5/10 (50.00%) | 8/32 (25.00%) |
| Nausea and vomiting | 4/22 (18.18%) | 0/10 (0.00%) | 4/32 (12.50%) |
| Dyspnea | 0/22 (0.00%) | 2/10 (20.00%) | 2/32 (6.25%) |
| Dizziness and headache | 6/22 (27.27%) | 1/10 (10.00%) | 7/32 (21.88%) |
| Tremor | 2/22 (9.09%) | 0/10 (0.00%) | 2/32 (6.25%) |
| Diagnosis | |||
| AIDP | 1/22 (4.55%) | 0/10 (0.00%) | 1/32 (3.13%) |
| AMAN ∗ | 0/22 (0.00%) | 5/10 (50.00%) | 5/32 (15.63%) |
| AMSAN | 3/22 (13.64%) | 1/10 (10.00%) | 4/32 (12.50%) |
| Anti-GQ1b syndrome | 3/22 (13.64%) | 2/10 (20.00%) | 5/32 (15.63%) |
| CIDP | 5/22 (22.73%) | 1/10 (10.00%) | 6/32 (18.75%) |
| Unclassified peripheral neuropathies | 6/22 (27.27%) | 1/10 (10.00%) | 7/32 (21.88%) |
| Uncertain diagnosis | 4/22 (18.18%) | 0/10 (0.00%) | 4/32 (12.50%) |
| Comorbidity | |||
| DM | 5/22 (22.73%) | 2/10 (20.00%) | 7/32 (21.88%) |
| Tumor | 1/22 (4.55%) | 1/10 (10.00%) | 2/32 (6.25%) |
| MND | 2/22 (9.09%) | 0/10 (0.00%) | 2/32 (6.25%) |
| MSA | 1/22 (4.55%) | 0/10 (0.00%) | 1/32 (3.13%) |
- Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor sensory axonal neuropathy; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; DM, diabetes mellitus; MND, motor neuron disease; MSA, multiple systemic atrophy.
- ∗p < 0.05 between seropositive and only CSF-positive group.
To clarify the role of anti-sulfatide antibodies while minimizing the potential confounding effects of antiganglioside antibodies, we compared clinical characteristics between patients with and without antiganglioside antibodies. No significant differences were observed (Figure 2a, Table S1). Diagnoses varied among the patients, including CIDP (18.75%), anti-GQ1b syndrome (15.63%), and acute motor axonal neuropathy (AMAN) (15.63%). Four patients were diagnosed with acute motor-sensory axonal neuropathy (AMSAN), and one had acute inflammatory demyelinating polyneuropathy (AIDP). Seven patients presented with peripheral neuropathy symptoms that could not be classified due to unknown etiology. Four patients with uncertain diagnoses exhibited diplopia, but central nervous system infections could not be excluded. Notably, the frequency of AMAN was significantly higher in the CSF-positive group (p = 0.001). Furthermore, diagnoses appeared related to the isotype of anti-sulfatide antibody, as AMAN and anti-GQ1b syndrome were found only in patients with anti-sulfatide IgG, while AIDP and CIDP occurred exclusively in patients with anti-sulfatide IgM (Table S2).
Among the 32 adult-onset anti-sulfatide -positive patients, comorbidities included diabetes mellitus (21.88%), motor neuron disease (MND) (6.25%), thyroid or lung tumors (6.25%), and multiple system atrophy (MSA) (3.13%). Lumbar puncture and CSF routine examinations were conducted in 29 patients, with albumin-cytological dissociation observed in 18. This dissociation was present in 80% of CSF-positive patients but only about half of seropositive patients (Table 3). Serum immunofixation electrophoresis was performed on 18 patients, but none exhibited monoclonal gammopathy. Rheumatic antibodies and factors were screened in 28 patients, with 10 testing positive for rheumatoid factors and antibodies (including antinuclear antibodies (ANAs) and anticardiolipin antibodies (ACAs)). Among these 10 patients, eight patients had serum ANA, while two exhibited elevated rheumatoid factors, one of whom also had glucose-6-phosphate isomerase and ACA. In the CSF-positive group, only one patient (12.50%) tested positive for rheumatic antibodies, which was lower than in the seropositive group (Table 3).
| Laboratory findings | Seropositive, n/N (%) | Only CSF-positive, n/N (%) | Total, n/N (%) | p value |
|---|---|---|---|---|
| CSF albumincytological dissociation | 10/19 (52.63%) | 8/10 (80.00%) | 18/29 (62.07%) | 0.234 |
| Blood monoclonal gamma globulin | 0/13 (0.00%) | 0/5 (0.00%) | 0/18 (0.00%) | — |
| Blood rheumatic antibodies and factors | 9/20 (55.00%) | 1/8 (12.50%) | 10/28 (35.71%) | 0.194 |
- Note: N: number of cases received electrophysiology examination; n: number of efficacious cases.
- Abbreviation: CSF, cerebrospinal fluid.
3.3. Electrophysiology Changes in Adult-Onset Patients
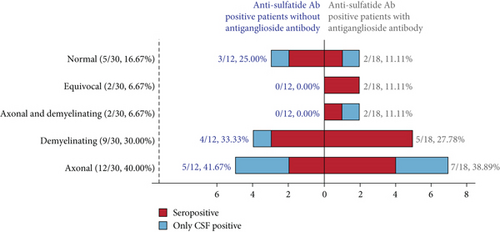
Of the 32 adult-onset patients, 30 underwent electrophysiological evaluation. Twelve patients (40.00%) showed axonal damage, nine had demyelinating changes, and two exhibited both axonal and demyelinating injuries (Figure 2b and Table 4). Five patients had normal motor and sensory nerve conduction. To elucidate the electrophysiological changes associated with anti-sulfatide antibodies, we focused exclusively on patients with anti-sulfatide positivity in the absence of antiganglioside antibodies (Table S3). Since sulfatide is predominantly localized in Schwann cell loops, we compared peripheral neuropathy caused by anti-sulfatide antibodies with that associated with anti-NF155 IgG4 in this study. Anti-sulfatide positive patients had shorter distal motor latency and faster motor nerve conduction velocity compared with anti-NF155 IgG4-seropositive patients and seronegative CIDP patients. In sensory nerves, anti-sulfatide positive patients had lower sensory nerve action potential (SNAP) amplitudes than patients with antiganglioside antibodies. Furthermore, sensory conduction velocity in anti-sulfatide positive patients was faster than that observed in anti-NF155 IgG4-seropositive patients and seronegative CIDP patients.
| Electrophysiology classification | Anti-sulfatide ab positive without antiganglioside abs, n/N (%) | Anti-sulfatide ab positive with antiganglioside abs, n/N (%) | Total | ||
|---|---|---|---|---|---|
| Seropositive | Only CSF-positive | Seropositive | Only CSF-positive | ||
| Axonal | 2/7 (28.57%) | 3/5 (60.00%) | 4/13 (30.77%) | 3/5 (60.00%) | 12/30 (40.00%) |
| Demyelinating | 3/7 (42.86%) | 1/5 (20.00%) | 5/13 (38.46%) | 0/5 (0.00%) | 9/30 (30.00%) |
| Axonal and demyelinating | 0/7 (0.00%) | 0/5 (0.00%) | 1/13 (7.69%) | 1/5 (20.00%) | 2/30 (6.67%) |
| Equivocal | 0/7 (0.00%) | 0/5 (0.00%) | 2/13 (15.38%) | 0/5 (0.00%) | 2/30 (6.67%) |
| Normal | 2/7 (28.57%) | 1/5 (20.00%) | 1/13 (7.69%) | 1/5 (20.00%) | 5/30 (16.67%) |
- Note: N: number of cases received electrophysiology examination; n: number of efficacious cases.
3.4. Juvenile Onset Patients With Anti-Sulfatide Antibody
Patient 29 is an adult male who has experienced symmetrical ankle sprains caused by distal muscle weakness over the past 10 years. His symptoms began in his teenage years with episodes of paroxysmal foot weakness, accompanied by numbness and pain in both legs. Over time, his limb weakness progressively worsened, leading to muscle atrophy in the lower limbs and an unsteady gait.
Physical examination revealed decreased muscle strength in the lower limbs. While superficial sensation was intact, deep touch perception (topesthesia) was impaired in both legs. Vibration and proprioceptive sensations were normal. Laboratory tests showed elevated homocysteine levels (20.8 μmol/L) and normal immunofixation electrophoresis. CSF analysis revealed normal cell count and protein levels but elevated IgG, IgA, and IgM.
Nerve conduction studies demonstrated axonal degeneration in both motor and sensory nerves, with more pronounced damage in the motor nerves of the lower limbs. Electromyography showed extensive neurogenic damage. Magnetic resonance imaging (MRI) of the lumbosacral plexus revealed no abnormalities.
He was diagnosed with peripheral neuropathy and hyperhomocysteinemia, with a suspicion of Charcot–Marie–Tooth (CMT) disease; however, no known genetic variation for CMT was identified. Treatment with methylprednisolone and neurotrophic drugs resulted in minimal symptom relief.
Patient 17 is a teenager who presented with weakness in the right foot for 10 days and was diagnosed with hereditary motor neuropathy with liability to pressure palsies (HNPP). Detailed information about this case is described elsewhere.
3.5. Treatment Response of Patients With Anti-Sulfatide Antibodies
Among patients with anti-sulfatide antibodies, five patients received intravenous immunoglobulins, which were reported to be effective in all cases (Table 5). In addition, nine patients received intravenous steroids combined with intravenous immunoglobulins, with an effective rate of 77.78% (7/9). By contrast, steroids were used in six patients, with symptom alleviation reported in only three. Plasmapheresis was performed in three patients and was reported to be effective. One patient was treated with antiviral drugs and intravenous steroids, but the treatment was ineffective. Another patient, treated with antiviral drugs, intravenous steroids, and intravenous immunoglobulins, was reported to have an effective outcome. Neurotrophic drugs alone demonstrated limited efficacy (14.29%, 1/7).
| Treatment | Efficacy, n/N (%) |
|---|---|
| Steroids | 3/6 (50.00%) |
| IVIg | 5/5 (100%) |
| Neurotrophic drugs | 1/7 (14.29%) |
| Plasmapheresis | 3/3 (100%) |
| Steroids + IVIg | 7/9 (77.78%) |
| Antiviral + steroids | 0/1 (0.00%) |
| Antiviral + steroids + IVIg | 1/1 (100%) |
- Note: N: number of cases collated; n: number of efficacious cases.
- Abbreviation: IVIg: intravenous immunoglobulin.
4. Discussion
This study highlights the underrecognized clinical importance of anti-sulfatide antibodies across various neuropathies. We find that anti-sulfatide antibody may exist in varied peripheral neuropathies, including AMAN, AMSAN, Anti-GQ1b syndrome, CIDP, and unclassified peripheral neuropathy. The patients with anti-sulfatide antibody mainly complained of limb weakness (81.25%), superficial sensation disturbance (68.75%), and ataxia (37.04%) no matter with antiganglioside antibodies or without. Electrophysiological examination shows that anti-sulfatide antibody may result in both axonal and demyelinating damage. Compared with anti-NF155 IgG4-seropositive and seronegative CIDP patients, anti-sulfatide antibody positive patients have shorter distal motor latency and faster motor and sensory nerve conduction velocities. Most patients responded to the IVIG and corticosteroid treatment. A recent study focused on pediatric GBS revealed that GBS spectrum disorders with positive anti-sulfatide antibodies present a distinct clinical phenotype, with shorter time to peak serving as an independent predictor of poor prognosis [11].
In our cohort, there were 10 patients with anti-sulfatide antibody only in CSF-positive. This prompted us to consider whether CSF anti-sulfatide antibodies entered the CNS through blood-nerve barrier (BNB) leakage or were synthesized intrathecally. Previous studies on anti-sulfatide IgG in acquired immunodeficiency syndrome (AIDS) suggested that CSF anti-sulfatide antibodies are probably synthesized intrathecally by infiltrating peripheral blood cells and are associated with peripheral neurological disorders in AIDs patients [12]. In fact, the blood-CSF barrier in the spinal nerve roots consists of meningeal cells in the pia mater, endothelial cells, and the basement membrane in capillary walls [13]. It is suggested that, in GBS, swelling nerve roots may cause venular congestion and result in disturbance of microcirculation. The anatomic changes of BNB in pathological conditions facilitated the entrance of mononuclear cells into nerves, as well as the leak of plasma protein. Here, CSF anti-sulfatide positive patients are prone to have CSF albumin-cytological dissociation. In addition, 3 of the 10 patients were accompanied by antiganglioside antibodies in their CSF samples, while in the other 7 patients, anti-sulfatide antibodies were the only autoantibody detected in CSF. Thus, CSF anti-sulfatide antibodies detected in patients with peripheral neuropathy are more likely to be synthesized intrathecally.
Previous results showed that anti-sulfatide antibodies are elevated in CSF from multiple sclerosis (MS) patients, and that both IgM and IgG antibodies are present [14]. A sulfatide-reactive monoclonal antibody obtained from an MS patient selectively binds to the surface of rat oligodendrocytes, indicating a potential role of anti-sulfatide autoantibodies in CNS demyelination [15]. However, whether CSF anti-sulfatide antibodies cause CNS demyelination remains unclear. Recent studies indicated that anti-sulfatide antibodies are rarely found in CSF [16] and that pediatric anti-sulfatide antibody-positive GBS patients exhibited inflammatory demyelinating lesions shown by brain MRI [11]. So far, the clinical symptoms between seropositive and CSF-positive patients show no significant difference.
In 1992, Aotsuka et al. revealed that serum anti-sulfatide antibodies were present in autoimmune rheumatic disease patients with a frequency between 10% and 81%, varied among diseases and disease stages [17]. Serum anti-sulfatide antibody levels were associated with the levels of anti-double-strand DNA antibodies. Antiganglioside antibodies were also found to be associated with peripheral neuropathy in rheumatoid arthritis [18]. Further investigations carried out by Alpa et al. showed elevated anti-sulfatide IgM/IgG and anti-GM1 IgM/IgG in patients with small vessel ANCA-associated vasculitis and other multiorgan immune-mediated diseases [19]. However, anti-sulfatide IgM/IgG showed a weak correlation with anti-dsDNA antibodies and were not correlated to other serologic rheumatic markers including ANA, RF, ANCA, anti-SSA, and SSB antibodies. Interestingly, there is a small portion of patients who had anti-GM1 or anti-sulfatide antibodies but were absent of neuropathy. The above studies all demonstrated the co-occurrence of serologic anti-sulfatide antibodies and rheumatic markers in autoimmune rheumatic diseases, but none focused on neuropathy solely. Herein, we discovered that the anti-sulfatide antibody seropositive patients tend to have elevated serologic rheumatic antibodies (ANA and ACA) and rheumatic factors even though not previously diagnosed as connective tissue diseases. We suspect that the coexistence of anti-sulfatide and rheumatic antibodies is due to cross-reactivity between these antibodies. It is reported that anti-sulfatide antibodies possess cross-reactivity with various anionic molecules (dsDNA, sulfatide, etc.) [17]. In addition, sulfatides are targets for ACAs [20]. We thus hypothesize that the exposure of sulfatide at paranodal loops in neuropathy patients boosted the production of some rheumatic antibodies.
Diabetes is the most common cause of peripheral neuropathy. Our study shows that there were seven DM patients among anti-sulfatide positive patients. All of them were diagnosed as Type 2 diabetes mellitus (T2DM) preceding the onset of neuropathy. Among them, three patients had only anti-sulfatide antibodies, and the other four patients were accompanied with antiganglioside antibodies. A previous study showed that nearly a quarter of Type 1 diabetes mellitus (T1DM) patients had sera antiganglioside antibodies, most of which were IgG isotype [21]. Anti-sulfatide IgG was found positive in 88% of patients diagnosed as T1DM [22]. The presence of antiganglioside and anti-sulfatide antibodies in T1DM may result from nonspecific immune hyperactivation and cross-reactivity between peripheral nerve and pancreatic cells. So far, anti-sulfatide antibodies were not found to correlate with T2DM; however, sulfatide might be involved in T2DM [23], and autoimmune reactions may also contribute to the pathogenesis of T2DM [24]. Herein, another reason may be the disruptive role of T2DM which is in consistency with Appeltshauser’s finding [25] suggesting that DM may be a potential risk factor for predisposing to developing nodo-paranodopathy. In their study, the frequency of DM is significantly elevated in CIDP patients with antiparanodal (including neurofascin-155, pan-neurofascin, contactin-1–associated protein 1, and contactin-1) antibodies, especially in anti–contactin-1-seropositive patients.
Sulfatide is a major component of the uncompacted myelin sheath in the peripheral nervous system [5]. It localizes at the paranodal loops and is involved in glial–axon interactions due to its essential role in the maintenance of the axo–glial junction and sodium/potassium ion channel clusters [4]. Other autoantigens at the paranodal loops include NF-155, Caspr1, and contactin 1, all of which mediate axo-glial attachment [26]. Though NF-155 and sulfatide both anchor on Schwann cells in paranodal loops, the clinical symptoms and electrophysiological changes caused by elevated anti-sulfatide and anti-NF-155 antibodies were not quite the same. The onset age of patients with anti-NF-155 antibody [27] was significantly younger (p = 0.0029) compared to anti-sulfatide antibody-positive patients. Most anti-NF155 antibody-positive patients were diagnosed as CIDP, while CIDP accounts for no more than 20% of anti-sulfatide antibody-positive patients. Recently, Martin-Aguilar et al. reported that anti-NF155 positive patients presented with a progressive (75%), sensory motor (87.5%), and symmetric distal predominant weakness in upper (97.2%) and lower extremities (94.5%), with tremor and ataxia (75%) [27]. Disturbances in deep sensation, ataxia, cranial nerve involvement, and tremor were more frequent in anti-NF155 antibody-positive patients [27–29]. In our anti-sulfatide positive cohort, the numbers of symptoms with tremor (6.25%) and ataxia (37.04%) are less than those of the anti-NF-155 positive patients. anti-sulfatide antibodies cause unique electrophysiological patterns when compared directly with those of anti-NF155 antibodies. Demyelination in motor and sensory nerves was more prominent in anti-NF-155 antibody-positive patients, as shown by electrophysiological data. Hence, though both NF-155 and sulfatide localize at paranodal loops, the clinical characteristics of patients with anti-sulfatide antibody differed from those with anti-NF-155 antibody-positive neuropathy.
The main limitations of our study arise from the small number of patients and its retrospective nature. First, due to the clinical significance of serum antiganglioside antibody in GBS, anti-sulfatide antibody and antiganglioside antibody detection in CSF were underestimated and therefore autoantibodies were not tested in all CSF samples. Also, the potential bias in data collection and lack of uniform testing may affect the validity and reliability of the results. Second, no patient was diagnosed with M-proteinemia in the current study, which is inconsistent with previous studies. This might be due to immunofixation electrophoresis not being commonly screened among patients with peripheral neuropathy. In addition, a previous study revealed that sulfatide-binding is a common property of antiganglioside antibodies [30]. Another limitation is that although autonomic dysfunction can be a clinical manifestation in peripheral neuropathies such as GBS, this study did not include assessments of autonomic electrophysiological parameters. Thus, valid methodology is crucial for the detection of anti-sulfatide and antiganglioside antibodies in peripheral neuropathy patients. A future prospective multicenter study with uniform testing for anti-sulfatide antibodies could be helpful to further reveal the role of anti-sulfatide antibody.
5. Conclusions
In conclusion, anti-sulfatide antibodies are associated with autoimmune peripheral neuropathy by causing axonal and demyelinating damage in adult patients. Though both sulfatide and NF155 localize at the paranodal loops, anti-sulfatide antibodies–related neuropathy causes a specific clinical spectrum and unique electrophysiological patterns.
Nomenclature
-
- AIDP
-
- acute inflammatory demyelinating polyneuropathy
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- AMAN
-
- acute motor axonal neuropathy
-
- AMSAN
-
- acute motor-sensory axonal neuropathy
-
- BNB
-
- blood-nerve barrier
-
- CIDP
-
- chronic inflammatory demyelinating polyradiculoneuropathy
-
- CMT
-
- Charcot–Marie–Tooth
-
- CSF
-
- cerebrospinal fluid
-
- GBS
-
- Guillain–Barre syndrome
-
- HNPP
-
- hereditary motor neuropathy with liability to pressure palsies
-
- MAG
-
- myelin associated glycoprotein
-
- MGT
-
- modified gomori trichrome
-
- MFS
-
- Miller–Fisher syndrome
-
- MND
-
- motor neuron disease
-
- MS
-
- multiple sclerosis
-
- MSA
-
- multiple system atrophy
-
- RF
-
- rheumatoid factor
-
- T1DM
-
- Type 1 diabetes mellitus
-
- T2DM
-
- Type 2 diabetes mellitus
Ethics Statement
This study was conducted according to a protocol approved by the Ethics Committee of the Qilu Hospital of Shandong University and the first affiliated hospital of Shandong First Medical University. All patients gave written informed consent to participate in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research is funded by the National Natural Science Foundation of China (Nos. 82101487 and 82171245), the Natural Science Foundation of Shandong Province, China (ZR2021QH161 and ZR2024MH051), and the Taishan Scholars Program of Shandong Province (202211318).
Acknowledgments
The authors thank the patients for their participation in this study.
Open Research
Data Availability Statement
The data that supports the findings of this study is available from the corresponding authors upon reasonable request.




