Insights Into Modifiable Risk Factors of Vertigo Using the Mendelian Randomization Approach
Abstract
Background: Vertigo is a highly prevalent symptom with wide-ranging causes and adverse consequences. While common risk factors for vertigo have been identified, their causal relationship with vertigo remains not fully known. Thus, identifying the modifiable factors causally related to vertigo is crucial for preventing vertigo.
Methods: A comprehensive Mendelian randomization study was employed to investigate the causal effects of vertigo among more than 40 genetically predicted modifiable risk factors, categorized into lifestyle traits, blood parameters, and metabolic comorbidities. This study used two different vertigo summary statistics from the deCODE and FinnGen consortia. Estimates were calculated using the inverse-variance weighted method and validated through alternative approaches.
Results: The results indicated that genetically predicted higher educational level was significantly associated with a decreased risk of vertigo (deCODE: odds ratio (OR) = 0.757, 95% CI = 0.697–0.822, pFDR[false discover rate] < 0.001; FinnGen: OR = 0.796, 95%CI = 0.703–0.901, pFDR = 0.007), while genetically predicted longer television watching was significantly associated with an increased risk of vertigo (deCODE: OR = 1.193, 95%CI = 1.076–1.323, pFDR = 0.011; FinnGen: OR = 1.269, 95%CI = 1.085–1.483, pFDR = 0.030). Additionally, genetically predicted elevated levels of alanine transaminase (ALT) were positively associated with the risk of vertigo. Genetically predicted increased physical activity was suggestively related to a reduced risk of vertigo, while higher triglyceride, body mass index (BMI), and diastolic blood pressure (DBP) were suggestively associated with an increased risk of vertigo (praw < 0.05).
Conclusions: Our findings indicate that genetically predicted increased educational levels and physical activity were associated with a decreased risk of vertigo, while higher levels of ALT and triglycerides, television watching time, BMI, and DBP were positively associated with the risk of vertigo. Thus, modifying these factors would decrease the risk of vertigo.
1. Introduction
Vertigo, a debilitating symptom characterized by an illusory sensation of spinning, is a prevalent and distressing condition affecting millions of people worldwide [1]. While the etiology underlying vertigo is multifactorial and complex, growing evidence suggests there are common risk factors associated with vertigo [2, 3], encompassing a diverse range of factors such as lifestyle-related behaviors, hypertension, metabolic disorders, and inflammation [4–6]. For instance, previous observational studies showed that lifestyle-related factors, including physical activity, dietary habits, tobacco and alcohol consumption, educational levels, and sleep disorders have been regarded as potentially modifiable determinants of vertigo [4, 7, 8]. However, the latest meta-analysis showed that smoking, alcohol consumption, and sleep disorders were not associated with the recurrence of benign paroxysmal positional vertigo (BPPV), the most common cause of recurrent vertigo [9]. The discrepancies observed in the above findings might be due to differences in the specific symptoms or pathologies examined across studies, such as BPPV, audio-vestibular dysfunction, vestibular vertigo, or general vertigo. Additionally, due to confounding factors, reverse causation, and selection bias, it is still challenging to establish the causal relationship between these factors and vertigo from conventional observational studies.
Mendelian randomization (MR), employing genetic variants as instrumental variables (IVs), has emerged as a promising approach to overcome these challenges [10]. MR analysis leverages the random assortment of alleles during meiosis to mimic the randomization seen in randomized controlled trials (RCT) and provides a powerful tool to investigate causal relationships between modifiable risk factors and health outcomes [11]. The fundamental principle of MR relies on the stable inheritance of genetic variants that serve as proxies for modifiable risk factors [12, 13]. As the genetic variant is independent of potential confounding factors and remains unaltered throughout an individual’s life, it can reduce the risk of confounding and bias in estimating causal effects. Thus, the application of MR can obtain robust evidence of causality, bridging the gap between observational associations and causal relationships.
In this study, we performed a comprehensive MR analysis to uncover causal relationships of 43 potential predominate factors associated with vertigo, which is essential for designing effective preventive strategies.
2. Material and Methods
2.1. Data Source
Genome-wide association studies (GWAS) summary-level data on 43 modifiable risk factors and vertigo of European descent were used in this MR study (Supporting Information 1: Table S1). The 43 modifiable risk factors can be divided into the following three categories: 1) lifestyle traits: age of initiation smoking, smoking initiation, cigarettes per day, smoking cessation, and drinks per week from the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) consortium [14]; dietary component (carbohydrate, sugar, fat, and protein), physical activity, educational attainment, and cognitive performance from the Social Science Genetic Association Consortium (SSGAC) [15–17]; insomnia and other sleep traits (snoring, sleep duration, napping, and morningness) from the Complex Trait Genetics lab (CTGlab) [18]; coffee and tea consumption [19]; and leisure sedentary behaviors (driving behavior, computer use, and television watching) [20]; 2) blood parameters: including fasting glucose (FG), 2 h-glucose post challenge, hemoglobin A1c (HbA1c), and fasting insulin from the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) [21]; triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), and total cholesterol from the Global Lipids Genetics Consortium (GLGC) [22]; gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), and alanine transaminase (ALT) for liver function [23]; interleukin-6 (IL-6), interleukin-8 (IL-8), and C-reactive protein (CRP) for inflammatory response [24, 25]; 3) metabolic comorbidities: waist circumference, hip circumference, body mass index (BMI), and waist-to-hip ratio from the Genetic Investigation of ANthropometric Traits (GIANT) consortium [26, 27]; systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure from the International Consortium for Blood Pressure (ICBP) consortium [28]. The summary statistics for vertigo were from the latest large-scale GWAS meta-analysis performed by the deCODE consortium, which included samples from the deCODE, UK Biobank, and FinnGen (release 6) with up to 942,613 participants [29]. As some of the modifiable risk factors contained partial samples from the UK Biobank, to avoid potential bias caused by sample overlapping, we also used the summary-level data on vertigo from the FinnGen (release 9) with 374,012 individuals to further confirm the results [30]. This study was based on publicly available summary statistics, and all data were de-identified and anonymized. Therefore, ethical approval was not separately required for this MR study.
2.2. Instrumental Variable Selection
All IVs used in this MR study were genetically predicted based on genetic variants robustly associated with each risk factor, allowing assessment of causal effects on vertigo without direct measurement [10]. For a valid two-sample MR study, IVs should meet the following criteria: (1) strongly relevant to the modifiable risk factor in GWAS, (2) independent of confounding factors, and (3) not directly associated with vertigo [11]. To identify relevant IVs for modifiable risk factors, genetic variants robustly (p < 5e − 8) associated with the modifiable risk factors were applicable as IVs, which were then clumped based on the 1000 genome project for independence (linkage disequilibrium (LD): r2 < 0.001 within 10,000 kb, European). In addition, to avoid bias introduced by weak IVs, the F-statistic of each genetic variant was calculated using (beta/se)2 as previously described [31], and those IVs with F-statistic values less than 10 were excluded (Figure 1). A detailed list of IVs for each exposure and outcome pair used in this MR study was presented in Supporting Information 1: Table S2.

2.3. Mendelian Randomization Analysis
In this MR, we used the inverse-variance weighted (IVW) method, a commonly used approach in MR, to estimate the causal effect of modifiable risk factors on vertigo. The IVW estimates the causal effect by taking a weighted average of individual genetic variant associations with the modifiable risk factor and vertigo. In sensitivity analysis, we also employed other complementary methods, including the weighted median, maximum likelihood, MR-Egger, simple median, and weighted mode, to further confirm the estimates. To evaluate the presence of horizontal pleiotropy, we examined the intercept from the MR-Egger regression [32], and we used the Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test to identify and correct for any outliers with heterogeneous causal estimates [33]. Additionally, Cochran’s Q test was applied to assess the heterogeneity in MR analysis. Finally, we performed leave-one-out analyses to assess the robustness of our findings by iteratively excluding individual genetic variants from the analysis. All statistical analyses were conducted using the TwoSampleMR package (version 0.5.7) in R software (version 4.2.2) [34]. All p values were two-sided, and the false discovery rate (FDR) correction (pFDR) was applied to adjust for multiple testing at a statistical significance level of 0.05, while a raw p value (praw) less than 0.05 indicated a suggestive significance.
3. Results
After harmonization, the number of valid IVs for each exposure ranged from 2 to 542, and the F-statistic values for all the IVs ranged from 12.29 to 10212.66 (Supporting Information 1: Table S2).
3.1. Lifestyle Traits on the Risk of Vertigo
Using summary-statistics of vertigo from the deCODE consortium, genetically predicted educational attainment, cognitive performance, and napping were significantly and negatively associated with the risk of vertigo (educational attainment: odds ratio (OR) = 0.757, 95%confidence interval (CI) = 0.697–0.822, pFDR < 0.001; cognitive performance: OR = 0.865, 95%CI = 0.797–0.939, pFDR = 0.009; and napping: OR = 0.868, 95%CI = 0.793–0.951, pFDR = 0.029, respectively), while genetically predicted television watching time was positively associated with the risk of vertigo (OR = 1.193, 95%CI = 1.076–1.323, pFDR = 0.011). Using summary statistics from the FinnGen consortium, we successfully replicated the MR estimates for educational attainment and television watching but not for cognitive performance and napping (Figure 2). Meanwhile, genetically predicted increased diet protein was significantly linked to an elevated risk of vertigo only in the FinnGen dataset (OR = 2.283, 95%CI = 1.466–3.556, pFDR = 0.007). Additionally, in the deCODE dataset, genetically determined increased physical activity and computer use were suggestively and negatively linked to the risk of vertigo (physical activity: OR = 0.712, 95%CI = 0.552–0.919, praw = 0.009; computer use: OR = 0.796, 95%CI = 0.643–0.987, praw = 0.038, respectively), but only the MR estimate for physical activity was successfully replicated in the FinnGen dataset (Figure 2). Meanwhile, in the FinnGen dataset, genetically predicted drinks per week (OR = 0.669, 95%CI = 0.484–0.924, praw = 0.015) and sleep duration (OR = 1.134, 95%CI = 1.066–1.685, pFDR = 0.012) were suggestively associated with a reduced and increased risk of vertigo, respectively. No potential pleiotropy was observed in the MR-Egger intercept test (Table 1 and Supporting Information 1: Table S3). There were no causal effects of genetically predicted insomnia, snoring, morningness, coffee and tea intake, smoking, driving behavior, and diet components (fat, carbohydrate, and sugar) on the risk of vertigo in both the deCODE and FinnGen datasets (Figure 2).
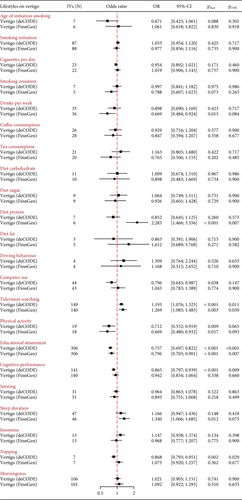
| Exposure | Outcome (vertigo) | IVs (N) | Cochran Q test | MR-Egger intercept (p) | MR-PRESSO RSSobs (p) | |
|---|---|---|---|---|---|---|
| IVW (p) | MR-Egger (p) | |||||
| Log-triglycerides | deCODE | 519 | 709.264 (< 0.001) | 688.604 (< 0.001) | 0.0029 (< 0.001) | 711.821 (< 0.001) |
| FinnGen-R9 | 514 | 620.562 (0.001) | 620.491 (0.001) | 0.0003 (0.808) | 622.926 (0.002) | |
| Alanine transaminase | deCODE | 225 | 301.656 (< 0.001) | 299.478 (< 0.001) | 0.0013 (0.204) | 304.718 (< 0.001) |
| FinnGen-R9 | 223 | 286.460 (0.002) | 285.664 (0.002) | 0.0010 (0.433) | 287.409 (0.004) | |
| Television watching | deCODE | 149 | 210.438 (0.001) | 209.710 (0.001) | −0.0026 (0.476) | 213.187 (< 0.001) |
| FinnGen-R9 | 140 | 171.714 (0.031) | 171.643 (0.027) | 0.0014 (0.811) | 174.207 (0.023) | |
| Physical activity | deCODE | 19 | 35.431 (0.008) | 34.875 (0.006) | 0.0111 (0.609) | 39.927 (0.006) |
| FinnGen-R9 | 18 | 22.445 (0.168) | 21.239 (0.170) | 0.0270 (0.355) | 25.761 (0.177) | |
| Educational attainment | deCODE | 306 | 412.082 (< 0.001) | 410.456 (< 0.001) | −0.0025 (0.273) | 414.815 (< 0.001) |
| FinnGen-R9 | 306 | 369.094 (0.007) | 367.947 (0.007) | -0.0033 (0.331) | 371.568 (0.009) | |
| Body mass index | deCODE | 501 | 688.561 (< 0.001) | 681.364 (< 0.001) | 0.0027 (0.022) | 692.301 (< 0.001) |
| FinnGen-R9 | 512 | 629.510 (< 0.001) | 624.603 (< 0.001) | 0.0036 (0.046) | 632.803 (< 0.001) | |
| Diastolic blood pressure | deCODE | 440 | 578.128 (<0.001) | 577.894 (< 0.001) | 0.0005 (0.674) | 580.988 (< 0.001) |
| FinnGen-R9 | 445 | 546.518 (0.001) | 546.089 (0.001) | 0.0011 (0.555) | 548.814 (0.002) | |
- Abbreviations: IVs, instrumental variables; IVW, inverse-variance weighted; MR-PRESSO, Mendelian Randomization Pleiotropy RESidual Sum and Outlier; N, number; NA, not applicable with less than 4 IVs; p, p value.
3.2. Blood Parameters on the Risk of Vertigo
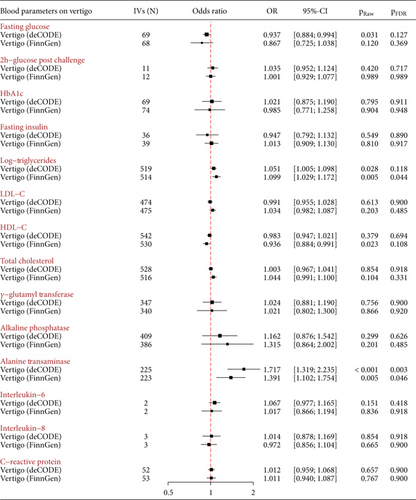
In the deCODE dataset, genetically determined increased blood ALT levels were positively associated with an increased risk of vertigo (OR = 1.717, 95%CI = 1.319–2.235, pFDR = 0.003), which was successfully replicated using the FinnGen dataset (OR = 1.391, 95%CI = 1.102–1.754, pFDR = 0.046). Additionally, in the deCODE dataset, genetically determined increased FG and TG were suggestively linked to a decreased and increased risk of vertigo (FG: OR = 0.937, 95%CI = 0.884–0.994, praw = 0.031 and TG: OR = 1.051, 95%CI = 1.005–1.098, praw = 0.028), respectively (Figure 3). There was possible pleiotropy for TG observed in the MR-Egger intercept test (p < 0.001) (Table 1 and Supporting Information 1: Table S3), but the MR estimate remained stable in the MR-PRESSO-corrected analysis after removing outliers (Supporting Information 1: Table S4). In the FinnGen dataset, we successfully replicated the MR estimates for TG (OR = 1.099, 95%CI = 1.029–1.172, praw = 0.005), but not for FG. Meanwhile, genetically predicted increased levels of HDL-C were suggestively associated with a reduced risk of vertigo in the FinnGen dataset (OR = 0.936, 95%CI = 0.884–0.991, pFDR = 0.023). There were no causal effects of genetically predicted 2 h-glucose post-challenge, HbA1c, fasting insulin, LDL-C, total cholesterol, GGT, ALP, and inflammatory cytokines (IL-6, IL-8, and CRP) on the risk of vertigo (Figure 3).
3.3. Metabolic Comorbidities on the Risk of Vertigo
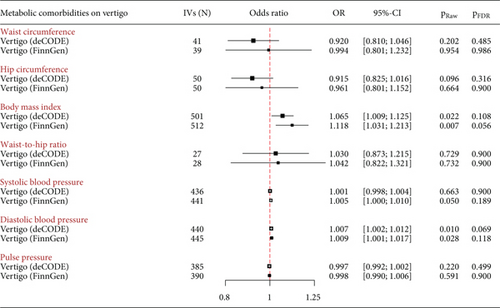
At the statistical significance level, there were no significant causal associations between genetically predicted metabolic comorbidities and the risk of vertigo (Figure 4). However, at suggestive significance level, genetically determined increased BMI and DBP were associated with an increased risk of vertigo in the deCODE dataset (BMI: OR = 1.065, 95%CI = 1.009–1.125, praw = 0.022; DBP: OR = 1.007, 95%CI = 1.002–1.012, praw = 0.010) and the FinnGen dataset (BMI: OR = 1.118, 95%CI = 1.031–1.213, praw = 0.007; DBP: OR = 1.009, 95%CI = 1.001–1.017, praw = 0.028). There was possible pleiotropy for BMI observed in the MR-Egger intercept test (deCODE: p = 0.022; FinnGen: p = 0.045) (Table 1), but the MR estimates remained stable in the MR-PRESSO-corrected test after removing outliers (Supporting Information 1: Table S4). There were no obvious causal effects of genetically predicted waist circumference, hip circumference, waist-to-hip ratio, and SBP on the risk of vertigo (Figure 4).
3.4. Sensitivity Analysis
Sensitivity analysis using other approaches showed consistent results with the same trend as IVW estimates, with a few exceptions such as for the MR-Egger method (Supporting Information 1: Table S5). The discrepancy in the direction of association using the MR-Egger approach might be due to a violation of the Instrument Strength Independent of Direct Effect (InSIDE) assumption [35]. There were some outliers found in the MR-PRESSO test (Table 1), but the estimates were consistent before and after removing potential outliers (Supporting Information 1: Table S4). Leave-one-out analysis plots showed there was no single genetic variant driving the bias of the positive estimates (Supporting Information 2: Figure S1–S7).
4. Discussion
The present study screened the causal relationships between 43 genetically predicted modifiable risk factors and vertigo. Using two different GWAS datasets on vertigo, our findings provided evidence that genetically predicted increased educational level and physical activity are linked to a reduced risk of vertigo, while genetically predicted increased television watching time, blood levels of ALT and TG, BMI, and DBP are positively associated with the risk of vertigo. By utilizing genetic variants as IVs, our MR study overcame the limitations of conventional observational studies and established the role of modifiable risk factors in the occurrence of vertigo.
Our results showed that individuals with higher educational attainment were prone to having a lower risk of vertigo. Indeed, a previous population-based study with 4869 participants showed that the prevalence of BPPV was lower in people with a middle-level and higher-level school education (≥ 10 years) [36]. There are some potential explanations for the inverse association between educational attainment and vertigo. First, people with higher educational attainment are often associated with better health awareness and access to healthcare, allowing for timely diagnosis, prevention, and treatment of vertigo-related disorders. Second, educational attainment has been proposed to be highly correlated with cognition [37], which was associated with vertigo [38, 39]. Consistently, our MR estimates also showed that better cognitive performance was associated with a reduced risk of vertigo using the deCODE dataset. Third, people with higher educational attainment usually have a balanced diet and regular physical activity, which might contribute to a lower risk of vertigo [4]. For example, our data suggested that diets rich in protein were linked to an increased risk of vertigo in the FinnGen dataset. This association may be mediated by psychological factors, as previous studies indicate that a protein-rich diet might induce anxiety and depressive symptoms [40], which are linked to a higher risk of vertigo [41]. However, this finding should be interpreted with caution, as it was observed only in the FinnGen dataset and not replicated in deCODE. Further research is needed to clarify this relationship.
Numerous observational studies have suggested that physical inactivity was associated with an increased risk of vertigo [3, 42]. Our MR results provided causal evidence supporting the beneficial effect of physical activity and the detrimental effect of television watching, a common leisure sedentary behavior, on the risk of vertigo. Physical inactivity is associated with various cardiovascular risk factors and metabolic impairment, such as hypertension, lipids disorders, and obesity [43, 44], which could affect the vestibular system and contribute to vertigo. For instance, previous studies indicated that patients with hypertension had a higher recurrence of BPPV [45], and people with increased TG levels were significantly linked to a history of positional vertigo [46]. A recent meta-analysis showed that hypertension and hyperlipidemia were associated with an increased risk of BPPV recurrence [3]. Similarly, our MR results showed that genetically predicted increased DBP, TG, and BMI were correlated with an elevated risk of vertigo. These findings highlight the importance of regular physical activity and exercise as potential preventive measures for vertigo, which might be mediated by regulating blood pressure and energy metabolism.
Sleep disorders have been previously reported to be associated with an increased risk of vertigo [47, 48], but our findings did not support a causal link between insomnia and vertigo. However, the MR estimates revealed a significantly negative association between napping and vertigo risk in the deCODE dataset, indicating napping might reduce the risk of vertigo. Previous studies have suggested that short napping (< 30 min) could alleviate sleepiness and fatigue [49], while extended napping (> 60 min) was linked to an increased risk of cardiovascular disease and mortality [50]. Thus, short napping might be a preferable strategy for reducing vertigo risk. Additionally, genetically predicted sleep duration was positively correlated with the risk of vertigo in the FinnGen dataset. Indeed, previous studies have reported that abnormal sleep duration was associated with an increased risk of vertigo [51]. Abnormal sleep duration is commonly defined as less than 7 h or more than 9 h per night [52]. Studies have shown that both shorter and longer sleep durations are associated with an increased risk of cardiovascular disease and mortality [53]. Thus, maintaining a normal sleep duration (7–9 h) might be optimal for reducing vertigo risk. It is important to note that since the above positive findings between sleep traits and vertigo were only observed in one dataset of vertigo, the results should be interpreted cautiously and need further investigation.
Although previous observational studies did not find an association between ALT and vertigo [54], our study showed that genetically predicted increased blood levels of ALT, but not GGT and ALP, were positively associated with the risk of vertigo. Given that few studies explored the association between hepatic function and vertigo-related diseases, further studies with larger sample sizes are warranted to confirm whether liver function impairment is implicated in the occurrence of vertigo. In this MR study, there were no significant causal relationships between genetically predicted consumption of coffee, tea, and tobacco and vertigo; however, we observed a suggestively negative association between drinking and vertigo in the FinnGen dataset. Indeed, previous studies have shown that alcohol consumption was more common in healthy participants than in BPPV patients and was associated with a delayed age at the onset of Meniere’s disease [55, 56], another common cause of vertigo. A possible explanation for the inverse association observed between alcohol consumption and vertigo might be that individuals who have previously experienced vestibular disorders might subsequently reduce their alcohol intake.
The present MR study provided valuable insights into causal relationships between genetically predicted modifiable risk factors and vertigo, but some limitations should be addressed here. First, there were potential pleiotropic effects observed for the IVs of BMI and TG, which might influence the reliability of these MR estimates. Although we have performed an MR-PRESSO-corrected test to assess the robustness of our results, residual pleiotropy cannot be completely ruled out. Second, the MR results from the deCODE dataset were not fully replicated in the FinnGen dataset and should be interpreted very cautiously. It is important to note that some modifiable risk factor datasets include samples from the UK Biobank, such as napping, DBP, SBP, and BMI, potentially leading to sample overlapping and overestimated results between these exposures and vertigo from the deCODE dataset. Additionally, the smaller sample size of the FinnGen dataset (N = 374,012) compared to the deCODE dataset (N = 942,613) might further explain differences between the datasets. Third, the MR study assumes a linear relationship between continuous exposures and vertigo, and whether there are potential nonlinear relationships between them needs to be determined based on individual-level data in future studies. Fourth, some of the estimates are positive only in either the deCODE or FinnGen dataset, which weakens the reliability of these MR results. Fifth, since our study was based on publicly available GWAS datasets of European descent, the findings in the present MR analysis might have limitations in their generalizability to other ethnic groups due to potential genetic and environmental differences. Finally, a key limitation of this study is that MR relies on genetic variants as proxies for modifiable risk factors rather than direct measurements. This indirect approach assumes that genetic predisposition accurately reflects actual exposure, an assumption that might be violated due to environmental influences, behavioral choices, and other confounding factors. Therefore, MR estimates might not fully reflect the true impact of interventions targeting these risk factors, and future studies combining MR findings with observational and experimental evidence are needed to validate causal inference.
5. Conclusions
In summary, our MR study has identified causal links between genetically predicted educational attainment, physical activity, television watching, ALT, BMI, TG, and DBP and the occurrence of vertigo. These findings highlight the importance of addressing lifestyle-related and metabolic risk factors to reduce the incidence and burden of vertigo and have significant clinical implications. Implementation of lifestyle interventions, such as managing body weight, improving lipid metabolism and blood pressure, and promoting physical activity, may serve as potential preventive measures for vertigo and reduce the burden of vertigo at the population level.
Nomenclature
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine transaminase
-
- BMI
-
- body mass index
-
- BPPV
-
- benign paroxysmal positional vertigo
-
- CI
-
- confidence interval
-
- CRP
-
- C-reactive protein
-
- CTGlab
-
- Complex Trait Genetics lab
-
- DBP
-
- diastolic blood pressure
-
- FDR
-
- false discovery rate
-
- FG
-
- fasting glucose
-
- GGT
-
- gamma-glutamyl transferase
-
- GIANT
-
- Genetic Investigation of ANthropometric Traits
-
- GLGC
-
- Global Lipids Genetics Consortium
-
- GWAS
-
- Genome-wide association studies
-
- GSCAN
-
- GWAS and Sequencing Consortium of Alcohol and Nicotine use
-
- HbA1c
-
- hemoglobin A1c
-
- HDL-C
-
- high-density lipoprotein-cholesterol
-
- ICBP
-
- International Consortium for Blood Pressure
-
- IL-6
-
- interleukin-6
-
- IL-8
-
- interleukin-8
-
- InSIDE
-
- Instrument Strength Independent of Direct Effect
-
- IVs
-
- instrumental variables
-
- LD
-
- linkage disequilibrium
-
- LDL-C
-
- low-density lipoprotein-cholesterol
-
- MAGIC
-
- Meta-Analyses of Glucose and Insulin-related traits Consortium
-
- MR
-
- Mendelian randomization
-
- MR-PRESSO
-
- Mendelian Randomization Pleiotropy RESidual Sum and Outlier
-
- OR
-
- odds ratio
-
- RCT
-
- randomized controlled trials
-
- SBP
-
- systolic blood pressure
-
- SSGAC
-
- Social Science Genetic Association Consortium
-
- TG
-
- triglycerides
Ethics Statement
As this study utilized publicly available data, no separate ethical approval was necessary.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
X.G., X.Z., N.G., P.L., and R.L. conceived and designed the project. X.G., C.H., and P.T. collected and analyzed the data. X.G., C.H., and P.T. drafted the manuscript. L.C., P.L., and R.L. critically revised the manuscript. All authors have reviewed and approved the final manuscript.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors thank the deCODE, FinnGen, MAGIC, GLGC, GIANT, GSCAN, SSGAC, ICBP consortium, and all the participants and investigators who contributed to this study.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The GWAS summary statistics used in this MR study were publicly available.




