Relationships Between Acrylamide Exposure and Cognitive Function in Older Adults: A Cross-Sectional Study
Abstract
Background: Cognitive decline is an important factor affecting the health and well-being of older adults. Previous studies have shown that acrylamide (AA) caused neurological damage among occupationally exposed workers. However, the effect of AA on cognitive function in general older adults is unclear yet. Therefore, this cross-sectional study is aimed at examining the relationships between blood markers of AA and cognitive function in the general elderly population.
Methods: Four hundred sixty-seven older adults (230 men and 237 women) aged 60 years and older from the National Health and Nutrition Examination Survey cycles (2013–2014) were included in this study. Logistic regression models were applied to explore the relationships between blood markers of AA and cognitive function.
Results: After adjusting for all confounders, at the animal fluency test (AFT) dimension, the odds ratio (OR) for individuals in the highest tertile of hemoglobin (Hb) adducts of acrylamide (HbAA) and its metabolite glycidamide (hemoglobin adducts of glycidamide (HbGA)) were 0.251 (95% confidence interval (CI): 0.090, 0.699) and 0.354 (95% CI: 0.164, 0.761), respectively, compared with individuals in the lowest tertile, indicating that both HbAA and HbGA were negatively associated with the decline in cognitive function in dimension AFT. No significant associations were seen on other dimensions. Moreover, HbGA/HbAA had no association with any dimensions of cognitive decline.
Conclusion: Collectively, our results suggest that HbAA and HbGA are not positively associated with cognitive decline in the general elderly population and are negatively related to the AFT dimension of cognitive impairment.
1. Introduction
In 2030, the World Health Organization predicts that the number of individuals over the age of 60 will increase to 1.4 billion, representing one in six of the world’s population [1]. With the acceleration of the aging process, age-related cognitive decline will pose a significant challenge to the health and well-being of older adults. People with cognitive decline display an array of symptoms encompassing impaired mental abilities to concentrate, learn, reason, remember, and make decisions, all of which can adversely affect their quality of life [2]. Therefore, a comprehensive understanding of the underlying risk factors contributing to cognitive decline in older adults is essential for improving their health and well-being.
Researchers have conducted numerous studies related to cognitive function in older adults. Past research has primarily focused on the relationship between lifestyle factors, including smoking, alcohol consumption, exercise, dietary factors, and cognitive function in this population [3–16]. Additionally, other studies have identified the influence of sociodemographic factors, such as age and education, on cognitive function among older adults [17–20]. Some researchers have also examined the link between chronic diseases and cognitive function in this demographic [21–28]. However, investigations into the effects of chemical exposure on cognitive function have been largely limited to substances such as lead, manganese, dichlorophenols, phthalates, phenols, ethylene oxide, polycyclic aromatic hydrocarbons, and parabens [29–36]. With the growth of the industrial sector and increased exposure to various chemicals, there is an urgent need for more comprehensive and in-depth research into chemical exposure in older adults.
Acrylamide (AA) is an important industrial chemical present in industrial wastewater, textiles, production of daily cosmetics, plastics, cigarettes, and baked and fried foods [37]. Studies have shown that humans can be exposed to AA through various routes such as the digestive tract, respiratory tract, skin, and mucous membranes [38–40]. It has been demonstrated that AA may be produced by the merged reaction in foods containing asparagine and the reduction of sugars. The strength of AA formation depends on the initial concentration or proportions of its precursors, as well as temperature, duration of heat treatment, and humidity. Once ingested, AA can rapidly diffuse into all body tissues and is metabolized by the cytochrome P450 2E1 (CYP2E1) enzyme to produce the more active epoxide glycidamide (GA) [41, 42]. Furthermore, AA and GA can bind hemoglobin (Hb) to form blood adducts, which can be used as biomarkers to reflect human AA exposure in the last 4 months [43].
This study investigated the relationship between blood AA biomarkers and cognitive function in a nationally representative group of older participants from the 2013–2014 National Health and Nutrition Examination Survey (NHANES). The results of this study will help to elucidate the effects of exposure to AA on cognitive function in older adults.
2. Materials and Methods
2.1. Study Population
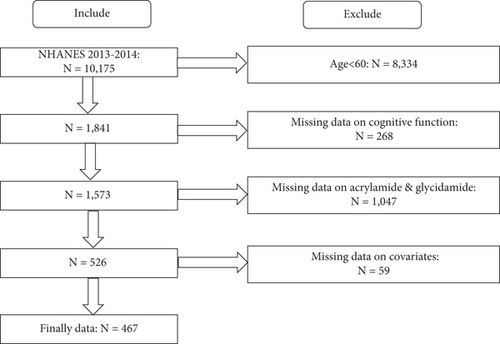
The inclusion criteria for this study were individuals aged 60 years and older with available information about hemoglobin adducts of acrylamide (HbAA), hemoglobin adducts of glycidamide (HbGA), cognitive function, and all covariates. The NHANES 2013–2014 survey included a total of 10,175 participants from the United States. After excluding 8334 participants who were under 60 years old, 268 who lacked cognitive function information, 1047 who had no HbAA and HbGA data, and an additional 59 who had no covariates, the final population included in this study was 467 (Figure 1).
2.2. Ethical Considerations
The NHANES study was granted approval by the Research Ethics Review Board of the National Center for Health Statistics. Informed written consent was obtained from all participants prior to their enrollment in the study. The ethical approval reference is Protocol #2011-17.
2.3. Measurement of HbAA and HbGA
The modified Edman reaction (which uses the effect of N-alkylated amino acids being able to form Edman products in neutral or alkaline conditions without changing the pH to acidic conditions required in conventional Edman reaction procedures) to measure HbAA and HbGA in human whole blood or erythrocytes. Specifically, the reaction products with the N-terminal valine of the Hb protein chains (N-[2-carbamoyl ethyl] valine and N-[2-hydroxycarbamoyl-ethyl] valine for AA and GA, respectively) are measured. The detailed method information can be referred to the Acrylamide and Glycidamide Lab Procedure Manual [44]. The detection thresholds for HbAA and HbGA were 3.9 and 4.9 pmol/g Hb, respectively. Based on the NHANES guidelines, the values were replaced with the value of the lower limit of detection (LLOD) divided by the square root of 2 if they were below the LLOD.
2.4. Cognitive Test
There were four parts of cognitive function definition: (1) the Consortium to Establish a Registry for Alzheimer’s Disease Immediate Recall Test (CERAD-IR), (2) the Consortium to Establish a Registry for Alzheimer’s Disease Delayed Recall Test (CERAD-DR), (3) the animal fluency test (AFT), and (4) the Digit Symbol Substitution Test (DSST). Please refer to the NHANES website for a detailed description of the cognitive functions [45]. Until now, there were no standard cut-off points in the CERAD-IR, CERAD-DR, AFT, and DSST. According to previous papers, we classified the scores into quartiles and defined the minimum quartile for each test as the reference group [6, 46]. Regarding the CERAD-IR, CERAD-DR, AFT, and DSST scores, the cut-off values were 16, 5, 12, and 33, respectively. Participants whose scores were lower than the corresponding cut-off values in tests were assigned to the low cognitive function group, and other participants were assigned to the normal cognitive function groups.
2.5. Covariate Assessment
Age, gender (male/female), race, education level (less than 9th grade, 9–11th grade, and above 11th grade), poverty income ratio, medical history (stroke, depression, diabetes, hypertension, and arteriosclerotic cardiovascular disease (ASCVD) diagnosis), physical activity, smoking status, and drinking status were used as covariates. All covariates were potential confounders in the relationship between HbAA, HbGA, and cognitive performance. (Classification criteria for covariates are presented in more detail in Table S1).
2.6. Statistical Analyses
This study’s continuous variable data are expressed as mean ± SD, and categorical variables are expressed as percentages. Independent variables HbAA and HbGA are continuous variables, which were divided into the following three groups based on trichotomies: low, middle, and high. The dependent variable, cognitive function, is a dichotomous variable that was divided into normal cognition and cognitive dysfunction. Three logistic regression models were developed to explore the relationship between HbAA, HbGA, HbGA/HbAA, and cognitive function. The unadjusted model was a univariate logistic regression with only independent and dependent variables, and Models 1 and 2 were multivariate logistic regressions with the addition of covariates. Model 1 was adjusted for the demographic variables of age, gender, race, educational qualifications, marriage, and poverty ratio. Model 2 was adjusted for all potential confounders, including age, gender, race, educational qualifications, marriage, poverty ratio, smoking, alcohol consumption, exercise, depression, diabetes, cardiovascular disease, stroke, and hypertension. We further conducted subgroup analyses for age, gender, and smoking status to explore group differences. Since the distributions of HbAA and HbGA were right-skewed, the ln-transformed values were used to improve the normality. R Version 4.2.2 and IBM SPSS 26.0 were used for analyses, and p value < 0.05 was considered significant.
3. Result
3.1. Characteristics of Study Participants
Table 1 describes the basic characteristics of the participants in this study. A total of 467 participants aged 60 years and older were included in this study, weighted to be representative of the 450,88,127 older American population. The average age is 68, most are non-Hispanic whites, and more than half have an education beyond high school. Additional relevant information is also described in Tables 1 and 2.
| CERAD-IR | CERAD-DR | AFT | DSST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal cognition | Low cognition | p value | Normal cognition | Low cognition | p value | Normal cognition | Low cognition | p value | Normal cognition | Low cognition | p value | |
| Number of participants | 363 | 104 | 293 | 174 | 363 | 104 | 351 | 116 | ||||
| Age | 68.13 ± 6.02 | 71.14 ± 6.67 | < 0.001 | 67.93 ± 5.92 | 70.28 ± 6.63 | < 0.001 | 68.02 ± 5.98 | 71.52 ± 6.61 | < 0.001 | 68.08 ± 6.22 | 70.98 ± 6.02 | < 0.001 |
| Gender | < 0.001 | < 0.001 | 0.621 | 0.001 | ||||||||
| Male | 162 (44.63%) | 68 (65.38%) | 125 (42.66%) | 105 (60.34%) | 181 (49.86%) | 49 (47.12%) | 158 (45.01%) | 72 (62.07%) | ||||
| Female | 201 (55.37%) | 36 (34.62%) | 168 (57.34%) | 69 (39.66%) | 182 (50.14%) | 55 (52.88%) | 193 (54.99%) | 44 (37.93%) | ||||
| Race | 0.039 | 0.067 | 0.192 | < 0.001 | ||||||||
| Mexican American | 23 (6.34%) | 16 (15.38%) | 19 (6.48%) | 20 (11.49%) | 30 (8.26%) | 9 (8.65%) | 18 (5.13%) | 21 (18.10%) | ||||
| Non-Hispanic Black | 79 (21.76%) | 26 (25.00%) | 58 (19.80%) | 47 (27.01%) | 73 (20.11%) | 32 (30.77%) | 68 (19.37%) | 37 (31.90%) | ||||
| Non-Hispanic White | 202 (55.65%) | 48 (46.15%) | 169 (57.68%) | 81 (46.55%) | 201 (55.37%) | 49 (47.12%) | 209 (59.54%) | 41 (35.34%) | ||||
| Other Hispanic | 27 (7.44%) | 6 (5.77%) | 20 (6.83%) | 13 (7.47%) | 28 (7.71%) | 5 (4.81%) | 23 (6.55%) | 10 (8.62%) | ||||
| Other race | 32 (8.82%) | 8 (7.69%) | 27 (9.22%) | 13 (7.47%) | 31 (8.54%) | 9 (8.65%) | 33 (9.40%) | 7 (6.03%) | ||||
| Education | < 0.001 | 0.013 | < 0.001 | < 0.001 | ||||||||
| Under high school | 24 (6.61%) | 20 (19.23%) | 19 (6.48%) | 25 (14.37%) | 25 (6.89%) | 19 (18.27%) | 9 (2.56%) | 35 (30.17%) | ||||
| High school | 133 (36.64%) | 40 (38.46%) | 108 (36.86%) | 65 (37.36%) | 129 (35.54%) | 44 (42.31%) | 118 (33.62%) | 55 (47.41%) | ||||
| More than high school | 206 (56.75%) | 44 (42.31%) | 166 (56.66%) | 84 (48.28%) | 209 (57.58%) | 41 (39.42%) | 224 (63.82%) | 26 (22.41%) | ||||
| Marital status | 0.615 | 0.323 | 0.021 | 0.029 | ||||||||
| Nonsingle | 209 (57.58%) | 57 (54.81%) | 172 (58.70%) | 94 (54.02%) | 217 (59.78%) | 49 (47.12%) | 210 (59.83%) | 56 (48.28%) | ||||
| Single | 154 (42.42%) | 47 (45.19%) | 121 (41.30%) | 80 (45.98%) | 146 (40.22%) | 55 (52.88%) | 141 (40.17%) | 60 (51.72%) | ||||
| Poverty income ratio | 2.56 ± 1.58 | 2.15 ± 1.46 | 0.018 | 2.62 ± 1.59 | 2.21 ± 1.48 | 0.006 | 2.61 ± 1.58 | 1.99 ± 1.42 | < 0.001 | 2.73 ± 1.56 | 1.67 ± 1.27 | < 0.001 |
| Smoking status | 0.325 | 0.197 | 0.669 | 0.042 | ||||||||
| Never | 153 (42.15%) | 41 (39.42%) | 131 (44.71%) | 63 (36.21%) | 147 (40.50%) | 47 (45.19%) | 156 (44.44%) | 38 (32.76%) | ||||
| Former | 109 (30.03%) | 39 (37.50%) | 88 (30.03%) | 60 (34.48%) | 118 (32.51%) | 30 (28.85%) | 110 (31.34%) | 38 (32.76%) | ||||
| Now | 101 (27.82%) | 24 (23.08%) | 74 (25.26%) | 51 (29.31%) | 98 (27.00%) | 27 (25.96%) | 85 (24.22%) | 40 (34.48%) | ||||
| Alcohol drinking status | 0.12 | 0.064 | 0.002 | 0.008 | ||||||||
| Never | 52 (14.33%) | 16 (15.38%) | 45 (15.36%) | 23 (13.22%) | 44 (12.12%) | 24 (23.08%) | 49 (13.96%) | 19 (16.38%) | ||||
| Former | 85 (23.42%) | 34 (32.69%) | 64 (21.84%) | 55 (31.61%) | 87 (23.97%) | 32 (30.77%) | 78 (22.22%) | 41 (35.34%) | ||||
| Now | 226 (62.26%) | 54 (51.92%) | 184 (62.80%) | 96 (55.17%) | 232 (63.91%) | 48 (46.15%) | 224 (63.82%) | 56 (48.28%) | ||||
| Physical activity | 0.042 | 0.005 | 0.023 | 0.241 | ||||||||
| No | 245 (67.49%) | 81 (77.88%) | 191 (65.19%) | 135 (77.59%) | 244 (67.22%) | 82 (78.85%) | 240 (68.38%) | 86 (74.14%) | ||||
| Yes | 118 (32.51%) | 23 (22.12%) | 102 (34.81%) | 39 (22.41%) | 119 (32.78%) | 22 (21.15%) | 111 (31.62%) | 30 (25.86%) | ||||
| Diabetes | 0.512 | 0.383 | 0.082 | 0.206 | ||||||||
| No | 246 (67.77%) | 74 (71.15%) | 205 (69.97%) | 115 (66.09%) | 256 (70.52%) | 64 (61.54%) | 246 (70.09%) | 74 (63.79%) | ||||
| Yes | 117 (32.23%) | 30 (28.85%) | 88 (30.03%) | 59 (33.91%) | 107 (29.48%) | 40 (38.46%) | 105 (29.91%) | 42 (36.21%) | ||||
| Hypertension | 0.121 | 0.607 | 0.003 | 0.01 | ||||||||
| No | 116 (31.96%) | 25 (24.04%) | 86 (29.35%) | 55 (31.61%) | 122 (33.61%) | 19 (18.27%) | 117 (33.33%) | 24 (20.69%) | ||||
| Yes | 247 (68.04%) | 79 (75.96%) | 207 (70.65%) | 119 (68.39%) | 241 (66.39%) | 85 (81.73%) | 234 (66.67%) | 92 (79.31%) | ||||
| ASCVD | 0.816 | 0.392 | 0.015 | 0.002 | ||||||||
| No | 290 (79.89%) | 82 (78.85%) | 237 (80.89%) | 135 (77.59%) | 298 (82.09%) | 74 (71.15%) | 291 (82.91%) | 81 (69.83%) | ||||
| Yes | 73 (20.11%) | 22 (21.15%) | 56 (19.11%) | 39 (22.41%) | 65 (17.91%) | 30 (28.85%) | 60 (17.09%) | 35 (30.17%) | ||||
| Depression | 0.133 | 0.024 | < 0.001 | 0.001 | ||||||||
| No | 268 (73.83%) | 69 (66.35%) | 222 (75.77%) | 115 (66.09%) | 279 (76.86%) | 58 (55.77%) | 267 (76.07%) | 70 (60.34%) | ||||
| Yes | 95 (26.17%) | 35 (33.65%) | 71 (24.23%) | 59 (33.91%) | 84 (23.14%) | 46 (44.23%) | 84 (23.93%) | 46 (39.66%) | ||||
| Stroke | 0.195 | 0.301 | 0.026 | 0.006 | ||||||||
| No | 344 (94.77%) | 95 (91.35%) | 278 (94.88%) | 161 (92.53%) | 346 (95.32%) | 93 (89.42%) | 336 (95.73%) | 103 (88.79%) | ||||
| Yes | 19 (5.23%) | 9 (8.65%) | 15 (5.12%) | 13 (7.47%) | 17 (4.68%) | 11 (10.58%) | 15 (4.27%) | 13 (11.21%) | ||||
- Note: Continuous variables—mean ± SD. Categorical variables—N (percent).
- Abbreviations: AFT, animal fluency test; ASCVD, arteriosclerotic cardiovascular disease; CERAD-DR, Consortium to Establish a Registry for Alzheimer’s Disease Delayed Recall; CERAD-IR, Consortium to Establish a Registry for Alzheimer’s Disease Immediate Recall; DSST, Digit Symbol Substitution Test.
| HbAA (pmol/g Hb) | HbGA (pmol/g Hb) | |||||||
|---|---|---|---|---|---|---|---|---|
| Low (16.40–35.90) | Middle (36.00–55.80) | High (55.90–416.00) | p value | Low (7.69–29.10) | Middle (29.30–44.70) | High (45.00–302.00) | p value | |
| Number of participants | 156 | 155 | 156 | 153 | 158 | 156 | ||
| Age | 69.98 ± 6.55 | 69.90 ± 6.38 | 66.53 ± 5.27 | < 0.001 | 70.18 ± 6.54 | 69.44 ± 6.36 | 66.81 ± 5.45 | < 0.001 |
| Gender | 0.014 | 0.359 | ||||||
| Male | 62 (39.74%) | 85 (54.84%) | 83 (53.21%) | 73 (47.71%) | 73 (46.20%) | 84 (53.85%) | ||
| Female | 94 (60.26%) | 70 (45.16%) | 73 (46.79%) | 80 (52.29%) | 85 (53.80%) | 72 (46.15%) | ||
| Race | < 0.001 | < 0.001 | ||||||
| Mexican American | 8 (5.13%) | 19 (12.26%) | 12 (7.69%) | 5 (3.27%) | 14 (8.86%) | 20 (12.82%) | ||
| Non-Hispanic Black | 31 (19.87%) | 25 (16.13%) | 49 (31.41%) | 35 (22.88%) | 31 (19.62%) | 39 (25.00%) | ||
| Non-Hispanic White | 80 (51.28%) | 94 (60.65%) | 76 (48.72%) | 74 (48.37%) | 95 (60.13%) | 81 (51.92%) | ||
| Other Hispanic | 14 (8.97%) | 9 (5.81%) | 10 (6.41%) | 16 (10.46%) | 10 (6.33%) | 7 (4.49%) | ||
| Other race | 23 (14.74%) | 8 (5.16%) | 9 (5.77%) | 23 (15.03%) | 8 (5.06%) | 9 (5.77%) | ||
| Education | 0.044 | < 0.001 | ||||||
| Under high school | 13 (8.33%) | 16 (10.32%) | 15 (9.62%) | 8 (5.23%) | 16 (10.13%) | 20 (12.82%) | ||
| High school | 57 (36.54%) | 45 (29.03%) | 71 (45.51%) | 63 (41.18%) | 40 (25.32%) | 70 (44.87%) | ||
| More than high school | 86 (55.13%) | 94 (60.65%) | 70 (44.87%) | 82 (53.59%) | 102 (64.56%) | 66 (42.31%) | ||
| Marital status | 0.023 | 0.29 | ||||||
| Nonsingle | 95 (60.90%) | 96 (61.94%) | 75 (48.08%) | 92 (60.13%) | 93 (58.86%) | 81 (51.92%) | ||
| Single | 61 (39.10%) | 59 (38.06%) | 81 (51.92%) | 61 (39.87%) | 65 (41.14%) | 75 (48.08%) | ||
| Poverty income ratio | 2.58 ± 1.56 | 2.70 ± 1.59 | 2.14 ± 1.50 | 0.004 | 2.57 ± 1.63 | 2.71 ± 1.55 | 2.13 ± 1.46 | 0.003 |
| Smoking status | < 0.001 | < 0.001 | ||||||
| Never | 98 (62.82%) | 77 (49.68%) | 19 (12.18%) | 86 (56.21%) | 77 (48.73%) | 31 (19.87%) | ||
| Former | 57 (36.54%) | 68 (43.87%) | 23 (14.74%) | 60 (39.22%) | 61 (38.61%) | 27 (17.31%) | ||
| Now | 1 (0.64%) | 10 (6.45%) | 114 (73.08%) | 7 (4.58%) | 20 (12.66%) | 98 (62.82%) | ||
| Alcohol drinking status | < 0.001 | 0.032 | ||||||
| Never | 37 (23.72%) | 25 (16.13%) | 6 (3.85%) | 33 (21.57%) | 20 (12.66%) | 15 (9.62%) | ||
| Former | 44 (28.21%) | 30 (19.35%) | 45 (28.85%) | 37 (24.18%) | 37 (23.42%) | 45 (28.85%) | ||
| Now | 75 (48.08%) | 100 (64.52%) | 105 (67.31%) | 83 (54.25%) | 101 (63.92%) | 96 (61.54%) | ||
| Physical activity | 0.614 | 0.202 | ||||||
| No | 113 (72.44%) | 108 (69.68%) | 105 (67.31%) | 114 (74.51%) | 103 (65.19%) | 109 (69.87%) | ||
| Yes | 43 (27.56%) | 47 (30.32%) | 51 (32.69%) | 39 (25.49%) | 55 (34.81%) | 47 (30.13%) | ||
| Diabetes | 0.97 | 0.153 | ||||||
| No | 106 (67.95%) | 106 (68.39%) | 108 (69.23%) | 103 (67.32%) | 117 (74.05%) | 100 (64.10%) | ||
| Yes | 50 (32.05%) | 49 (31.61%) | 48 (30.77%) | 50 (32.68%) | 41 (25.95%) | 56 (35.90%) | ||
| Hypertension | 0.048 | 0.356 | ||||||
| No | 36 (23.08%) | 55 (35.48%) | 50 (32.05%) | 41 (26.80%) | 54 (34.18%) | 46 (29.49%) | ||
| Yes | 120 (76.92%) | 100 (64.52%) | 106 (67.95%) | 112 (73.20%) | 104 (65.82%) | 110 (70.51%) | ||
| ASCVD | 0.004 | 0.01 | ||||||
| No | 134 (85.90%) | 127 (81.94%) | 111 (71.15%) | 126 (82.35%) | 134 (84.81%) | 112 (71.79%) | ||
| Yes | 22 (14.10%) | 28 (18.06%) | 45 (28.85%) | 27 (17.65%) | 24 (15.19%) | 44 (28.21%) | ||
| Depression | 0.006 | 0.023 | ||||||
| No | 121 (77.56%) | 118 (76.13%) | 98 (62.82%) | 117 (76.47%) | 120 (75.95%) | 100 (64.10%) | ||
| Yes | 35 (22.44%) | 37 (23.87%) | 58 (37.18%) | 36 (23.53%) | 38 (24.05%) | 56 (35.90%) | ||
| Stroke | 0.383 | 0.642 | ||||||
| No | 150 (96.15%) | 144 (92.90%) | 145 (92.95%) | 146 (95.42%) | 148 (93.67%) | 145 (92.95%) | ||
| Yes | 6 (3.85%) | 11 (7.10%) | 11 (7.05%) | 7 (4.58%) | 10 (6.33%) | 11 (7.05%) | ||
- Note: Continuous variables—mean ± SD. Categorical variables—N (percent).
- Abbreviations: ASCVD, arteriosclerotic cardiovascular disease; HbAA, hemoglobin adducts of acrylamide; HbGA, hemoglobin adducts of glycidamide.
3.2. AA Biomarkers and Cognitive Function
Table 3 presents the relationships between HbAA, HbGA, HbGA/HbAA, and different dimensions of cognitive function. The logistic regression showed that in the unadjusted model for the AFT dimension, the odds ratio (OR) for the highest tertile of HbAA compared to the lowest tertile was 0.516 (95% confidence interval (CI): 0.301, 0.886). This relationship remained significant in Model 1, adjusted for demographic variables, and Model 2, adjusted for all covariates, with results of 0.513 (95% CI: 0.277, 0.952) and 0.251 (95% CI: 0.090, 0.699), respectively. In the unadjusted model, the OR for the top and middle tertiles of HbGA compared to the lowest tertile were 0.509 (95% CI: 0.298, 0.869) and 0.545 (95% CI: 0.322, 0.923), respectively. In Model 1, adjusting for demographic variables, and Model 2, adjusting for all covariates, this relationship remained significant, with results of 0.503 (95% CI: 0.272, 0.931), 0.551 (95% CI: 0.307, 0.988), 0.354 (95% CI: 0.164, 0.761), and 0.566 (95% CI: 0.304, 1.054), respectively. However, in the unadjusted models for the CERAD-IR, CERAD-DR, and DSST dimensions, the p values for the highest tertile of HbAA compared to the lowest tertile were 0.118, 0.554, and 0.052, respectively. These p values were greater than 0.05, indicating that the correlation between the variables was not statistically significant. This relationship remained nonsignificant in both Model 1 and Model 2. Similarly, HbGA did not correlate with these cognitive dimensions. HbGA/HbAA did not correlate with any dimension of cognitive dysfunction.
| CERAD-IR | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | ||||
| HbAA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.280 (0.767, 2.136) | 0.345 | 1.126 (0.636, 1.994) | 0.684 | 1.105 (0.606, 2.014) | 0.745 |
| High | 0.636 (0.361, 1.122) | 0.118 | 0.576 (0.304, 1.094) | 0.092 | 0.412 (0.162, 1.047) | 0.063 |
| p for trend | 0.135 | 0.109 | 0.162 | |||
| HbGA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.892 (0.527, 1.511) | 0.671 | 0.931 (0.521, 1.663) | 0.810 | 0.925 (0.507, 1.688) | 0.799 |
| High | 0.809 (0.473, 1.384) | 0.439 | 0.786 (0.424, 1.458) | 0.445 | 0.810 (0.389, 1.687) | 0.573 |
| p for trend | 0.439 | 0.448 | 0.578 | |||
| HbGA/HbAA | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.938 (0.554, 1.589) | 0.812 | 1.177 (0.659, 2.101) | 0.582 | 1.101 (0.604, 2.009) | 0.753 |
| High | 0.830 (0.486, 1.418) | 0.496 | 0.958 (0.519, 1.766) | 0.890 | 0.844 (0.438, 1.625) | 0.611 |
| p for trend | 0.497 | 0.898 | 0.623 | |||
| CERAD-DR | ||||||
| Unadjusted | Model 1 | Model 2 | ||||
| HbAA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.331 (0.839, 2.110) | 0.224 | 1.255 (0.759, 2.075) | 0.377 | 1.157 (0.685, 1.957) | 0.586 |
| High | 1.150 (0.723, 1.829) | 0.554 | 1.189 (0.704, 2.010) | 0.517 | 0.814 (0.387, 1.714) | 0.589 |
| p for trend | 0.558 | 0.505 | 0.781 | |||
| HbGA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.005 (0.633, 1.594) | 0.984 | 1.106 (0.669, 1.827) | 0.695 | 1.047 (0.623, 1.761) | 0.861 |
| High | 1.083 (0.683, 1.716) | 0.736 | 1.199 (0.709, 2.029) | 0.498 | 0.963 (0.513, 1.809) | 0.907 |
| p for trend | 0.735 | 0.623 | 0.936 | |||
| HbGA/HbAA | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.038 (0.659, 1.636) | 0.871 | 1.255 (0.766, 2.057) | 0.367 | 1.239 (0.743, 2.067) | 0.411 |
| High | 0.756 (0.476, 1.203) | 0.238 | 0.916 (0.544, 1.541) | 0.740 | 0.940 (0.539, 1.640) | 0.827 |
| p for trend | 0.242 | 0.747 | 0.840 | |||
| AFT | ||||||
| Unadjusted | Model 1 | Model 2 | ||||
| HbAA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.642 (0.381, 1.080) | 0.095 | 0.694 (0.387, 1.244) | 0.220 | 0.678 (0.364, 1.263) | 0.220 |
| High | 0.516 (0.301, 0.886) | 0.017 | 0.513 (0.277, 0.952) | 0.034 | 0.251 (0.090, 0.699) | 0.008 |
| p for trend | 0.015 | 0.032 | 0.010 | |||
| HbGA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.545 (0.322, 0.923) | 0.024 | 0.551 (0.307, 0.988) | 0.046 | 0.566 (0.304, 1.054) | 0.073 |
| High | 0.509 (0.298, 0.869) | 0.013 | 0.503 (0.272, 0.931) | 0.029 | 0.354 (0.164, 0.761) | 0.008 |
| p for trend | 0.011 | 0.024 | 0.006 | |||
| HbGA/HbAA | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.378 (0.821, 2.312) | 0.225 | 1.848 (1.034, 3.302) | 0.038 | 1.654 (0.899, 3.043) | 0.106 |
| High | 0.751 (0.428, 1.318) | 0.319 | 0.839 (0.442, 1.592) | 0.590 | 0.616 (0.301, 1.261) | 0.185 |
| p for trend | 0.341 | 0.653 | 0.226 | |||
| DSST | ||||||
| Unadjusted | Model 1 | Model 2 | ||||
| HbAA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 1.215 (0.711, 2.077) | 0.476 | 1.299 (0.649, 2.600) | 0.460 | 1.326 (0.635, 2.766) | 0.452 |
| High | 1.671 (0.996, 2.804) | 0.052 | 2.009 (1.017, 3.968) | 0.045 | 1.221 (0.453, 3.290) | 0.693 |
| p for trend | 0.050 | 0.045 | 0.580 | |||
| HbGA (pmol/g Hb) | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.994 (0.588, 1.679) | 0.982 | 1.149 (0.587, 2.253) | 0.684 | 1.036 (0.502, 2.136) | 0.924 |
| High | 1.237 (0.741, 2.065) | 0.417 | 1.106 (0.567, 2.155) | 0.768 | 0.548 (0.236, 1.273) | 0.162 |
| p for trend | 0.411 | 0.770 | 0.185 | |||
| HbGA/HbAA | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Low | Reference | Reference | Reference | |||
| Middle | 0.676 (0.407, 1.125) | 0.132 | 0.764 (0.403, 1.450) | 0.411 | 0.715 (0.361, 1.414) | 0.335 |
| High | 0.646 (0.388, 1.078) | 0.094 | 0.538 (0.268, 1.081) | 0.082 | 0.528 (0.244, 1.139) | 0.104 |
| p for trend | 0.089 | 0.082 | 0.102 | |||
- Note: Model 1: adjust for age, gender, race, education, marital status, and poverty-income ratio. Model 2: adjust for age, gender, race, education, marital status, poverty-income ratio, smoking, alcohol drinking, physical activity, arteriosclerotic cardiovascular disease, diabetes, hypertension, stroke, and depression.
3.3. Subgroup Analysis
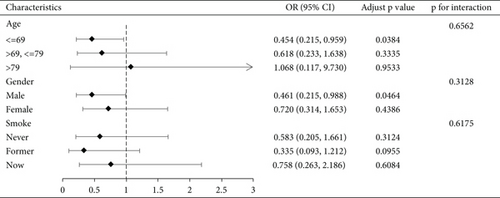
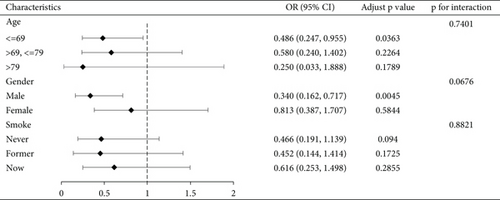
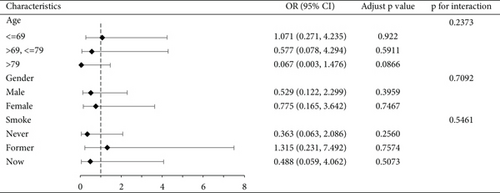
We conducted a subgroup analysis of the cognitive functioning profile of the AFT dimensions by age, gender, and smoking status, with the results shown in Figure 2, demonstrating the stability of our results. See Figure S1 for a subgroup analysis of CERAD-IR, CERAD-DR, and DSST dimensions.
4. Discussion
To the best of our knowledge, this is the first study to use NHANES data to explore the relationship between serum AA biomarkers and cognitive function in older adults. Overall, our results suggest a significant negative association between HbAA, HbGA, and cognitive impairment, mainly in the AFT dimension, which remained significant after adjusting for various factors, including sociodemographic factors such as age, gender, race, education, marriage, and poverty ratio; lifestyle factors such as alcohol consumption, smoking, and exercise; and a number of disorders highly associated with cognitive impairment, such as hypertension, diabetes, cardiovascular disease, and the severity of depressive symptoms. No significant associations were observed for the CERAD-IR, CERAD-DR, and DSST dimensions. Additionally, HbGA/HbAA did not correlate with any dimension of cognitive dysfunction.
AA has long been recognized as a neurotoxic substance. Researchers have conducted several basic experiments, one of which showed that AA causes damage to microglia through a mechanism of NLRP3-activated cytokine release [47]. In addition, a rodent experiment showed that subchronic exposure to AA exacerbated neuronal lesions in the cerebellum [48]. Subsequently, the research team conducted a follow-up study that confirmed the neurological damage caused by AA in rats and showed AA-induced synaptic damage in the rat hippocampus [49]. Multiple studies have reported evidence of damage to the nervous system in workers exposed to AA, including symptoms affecting both the central and peripheral nervous systems [50–52]. GA is a metabolite of AA. The researchers confirmed that GA can reduce cell viability. One study found that GA, at doses exceeding 500 μM for 24 h in human mammary epithelial MCF10A cells, significantly decreased cell viability [53]. Another investigation into mouse germ cells also showed the toxic effects of GA on rodent germ cells [54]. Beyond its cytotoxic effects, it has also been demonstrated that GA also has a promotive effect on the development of tumors [55–59].
However, our study did not find negative effects of HbAA and HbGA on cognitive function in the general older adult population. We believe the main reason is that our study involved the general older adult population, in whom the mean HbAA content is only 49.13 pmol/g Hb, which is well below occupational exposure levels [50, 51]. Additionally, previous researchers have concluded that low doses of AA are not associated with the incidence of a wide range of tumors [60–66]. Other studies have concluded that low doses of AA have a negative association with osteoarthritis, diabetes, and cardiovascular disease [67–69]. The effects of AA on the human body mainly depend on the concentration, and the mechanism of different effects according to AA dosage needs to be studied in the future.
The data for this study came from NHANES, a nationally representative health and nutrition survey in the United States. NHANES uses a stratified, multistage, probability cluster sampling design. This study has the following advantages. First of all, NHANES is a study design, data collection, and outcome evaluation conducted by professionals from the US Centers for Disease Control and Prevention, and the data obtained are very reliable. Secondly, previous research on AA, GA, and cognitive function mainly focused on basic research in animal experiments and cell experiments, and only a few population cohort studies were also aimed at occupational exposure populations, and our research focused on the relationship between the content of AA and its metabolite GA in the blood of the general population and cognitive impairment. Thirdly, we determine the concentration of AA and GA in the blood through laboratory tests, which is an objective and accurate value. Finally, we use four dimensions to assess cognitive function, which can lead to more comprehensive cognitive assessment results.
Admittedly, our study has certain limitations. First, this is a cross-sectional study; we can only derive associations between variables but cannot determine causation. In addition, the population in this study is the general population of the United States, so the results may not apply to other countries. Finally, due to the nature of cross-sectional studies, there were some unavoidable biases in this study, so prospective cohort studies or randomized controlled trials were needed to validate our results.
5. Conclusions
In this study, we found that blood metabolites of AA are not positively associated with cognitive decline in the general older adult population. Furthermore, these metabolites are negatively associated with the AFT dimension of cognitive impairment. Further prospective cohort studies or randomized controlled trials are needed in the future to confirm these results.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Jilin Provincial Science and Technology Department Project (YDZJ202301ZYTS078, 20220303003SF, 20220505034ZP, and 20230505040ZP).
Acknowledgments
We thank the participants and staff of the National Health and Nutrition Examination Survey 2013–2014 for their valuable contributions.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




