Evaluation of the Clinical and Prognostic Characteristics of Myasthenia Gravis Patients Followed Up in a Tertiary Neuromuscular Disease Center in Thrace Region of Turkey
Abstract
Objective: This study is aimed at comparing the clinical, serological, and prognostic characteristics of myasthenia gravis (MG) subtypes based on data obtained from patients monitored at a tertiary neuromuscular disease center in Türkiye, within the framework of MGFA and MGFA-PIS classifications. The limited number of published studies from Türkiye in this field enhances the originality and potential contribution of this study to the regional literature on the local patient profile.
Methods: A total of 190 patients who were monitored between 2012 and 2023 and diagnosed with MG according to clinical, serological, or electrophysiological criteria were included in the study. Patients were classified according to antibody profile, involved muscle group, and age at disease onset. Clinical and demographic characteristics, treatment strategies, and prognosis were assessed.
Results: In the classification of MG based on age at onset, 78.3% of patients in the early-onset MG (EOMG) subgroup were female, while 57.9% of those in the late-onset MG (LOMG) subgroup were male (p < 0.001). Significant differences were found between the EOMG and LOMG groups in the use of azathioprine and corticosteroids (p = 0.006 and p = 0.002, respectively). LOMG was more frequently observed in both the ocular MG (OMG) and generalized MG (GMG) groups. Electrophysiological abnormalities were detected more frequently in the GMG group (p = 0.045). Among patients initially diagnosed with OMG, 41.2% developed generalization during a median follow-up period of 5 years.
Conclusion: This study revealed significant differences among MG subtypes in terms of clinical features, autoantibody profiles, and treatment requirements. The MGFA and MGFA-PIS classifications offer useful tools for individualized treatment planning. The findings provide valuable insights into the potential role of early immunosuppressive therapy in reducing the risk of generalization in patients with OMG.
1. Introduction
Myasthenia gravis (MG) is a heterogeneous disorder characterized by an autoimmune response against postsynaptic components at the neuromuscular junction, leading to fatigue and weakness in striated muscles [1]. In its pathogenesis, the most frequently implicated antibodies target the acetylcholine receptor (AChR); however, antibodies against muscle-specific kinase (MuSK), Low-Density Lipoprotein Receptor–Related Protein 4 (LRP4), agrin, titin, and ryanodine receptors have also been identified [2].
MG demonstrates a bimodal age distribution, peaking between the ages of 20 and 39 in women and after the age of 50 in men [1, 3]. With increasing clinical awareness and advances in diagnostic capabilities, a marked rise in the diagnosis of MG in older adults has been observed [1]. Clinically, MG is characterized by varying degrees of muscle weakness affecting the extraocular, bulbar, axial, respiratory, and limb muscles [2]. The most common initial symptoms are ptosis and diplopia, whereas bulbar manifestations such as dysphagia and dyspnea may lead to life-threatening conditions like myasthenic crisis [4–7]. Worsening of symptoms with exertion and diurnal fluctuation are distinguishing features of MG [5].
Antibody testing plays a critical role in the diagnosis of MG. However, in seronegative cases, electrophysiological methods such as single-fiber electromyography MG (SFEMG) and repetitive nerve stimulation (RNS) contribute to diagnostic confirmation. While SFEMG offers higher sensitivity, the specificity of the RNS test is greater [6, 8].
Therapeutic options for MG include acetylcholinesterase inhibitors, corticosteroids, immunosuppressive agents, and thymectomy in appropriate patients, while immunomodulatory approaches such as intravenous immunoglobulin (IVIG) and plasmapheresis are preferred for managing exacerbations (Es) [9]. The classification of MG into subtypes based on antibody profile, age at onset, and thymic pathology is of great importance in terms of treatment strategy and prognosis [7, 9].
The most common clinical subtypes of MG include ocular MG (OMG), characterized by involvement limited to the ocular muscles, and generalized MG (GMG), which involves widespread muscle groups. Based on antibody profile, MG is classified as AChR-positive or MuSK-positive. According to age at disease onset, it is divided into early-onset MG (EOMG) and late-onset MG (LOMG), while another subtype is associated with the presence of thymoma.
Approximately 12%–80% of patients with OMG eventually progress to a generalized form, while in 15–20% of cases, the disease remains limited to ocular symptoms [5, 7, 10, 11]. AChR antibodies constitute the most common serological subtype of MG and are typically associated with GMG [3, 12]. MuSK antibody positivity is predominantly observed in female patients and is characterized by prominent bulbar muscle involvement and a tendency toward treatment resistance. In this subgroup, plasmapheresis and rituximab (RTX) have been found to be more effective than IVIG [5, 13].
In seronegative MG patients, clustered AChR antibodies can be detected through cell-based assays, which may pose diagnostic challenges [7, 14]. Thymoma-associated MG typically follows a generalized course and is characterized by a more severe clinical presentation. Thymoma is observed in approximately 10%–15% of MG patients, while MG has been reported to develop in 30% of individuals with thymoma [2, 5, 6, 11, 15].
EOMG represents a female-predominant subgroup characterized by the onset of symptoms before the age of 50, with frequent thymic hyperplasia and autoimmune comorbidities. LOMG, on the other hand, is more common in men, with initial symptoms occurring after the age of 50 and thymic hyperplasia being rare. Most patients with LOMG exhibit a generalized course with more severe symptoms [1, 2, 7].
This retrospective study was aimed at comparing the clinical, serological, and prognostic characteristics of MG subtypes using data from patients followed at a tertiary neuromuscular disease center in Türkiye, based on the MG Foundation of America (MGFA) and MGFA Post-Intervention Status (MGFA-PIS) classifications. In addition to evaluating demographic features, antibody profiles, comorbidities, and treatment strategies, the study assessed their impact on disease prognosis. Given the limited number of multidimensional retrospective studies on MG subtypes in Türkiye, the present findings provide region-specific insights into the national patient profile and contribute original data to the international MG literature.
2. Materials and Methods
2.1. Study Design and Setting
This retrospective observational study was conducted at the Neuromuscular Disorders Unit of Tekirdağ Namık Kemal University Training and Research Hospital, Türkiye. Patients diagnosed with MG and monitored between January 1, 2012, and December 12, 2023, were included.
2.2. Inclusion Criteria
Patients were eligible for inclusion if they were aged 18 years or older at the time of diagnosis; had a confirmed diagnosis of MG based on clinical symptoms, autoantibody positivity (AChR or MuSK), and/or electrophysiological findings indicating a neuromuscular transmission disorder; received regular follow-up and treatment; and were monitored at the neuromuscular outpatient clinic during the specified period. A total of 190 patients who met these criteria were included in the study.
2.3. Data Collection
Demographic characteristics, follow-up duration, clinical symptoms at diagnosis and during follow-up, treatment agents and related adverse effects, presence of autoimmune diseases, comorbidities, number of Es, plasmapheresis administration, intensive care unit admissions, AChR and MuSK antibody positivity, and AChR antibody titers were retrospectively collected from patient medical records.
2.4. Subgroup Definitions and Classification Criteria
- •
Muscle involvement: Patients with symptoms limited to extraocular muscles were classified as having OMG, while those with involvement of additional muscle groups were classified as having GMG.
- •
Antibody profile: Based on serological test results, patients were classified as AChR-positive, MuSK-positive, or single/double seronegative (AChR/MuSK).
- •
Age at onset: Patients diagnosed before the age of 50 were classified as EOMG, and those diagnosed at or after 50 years of age were classified as LOMG.
2.5. Electrophysiological Evaluation
Electrophysiological evaluations were performed as indicated using RNS and/or SFEMG.
2.6. Clinical Assessment and Outcome Measures
Disease severity at the time of diagnosis was assessed using the MGFA clinical classification. Treatment response and long-term prognosis were evaluated according to the MGFA-PIS criteria. Based on this classification, pharmacological remission (PR) and minimal manifestations (MM1, MM2, and MM3) were considered to indicate a favorable prognosis, whereas statuses defined as unchanged (U), worsened (W), exacerbated (E), or deceased (D) were accepted as indicators of poor prognosis.
Corticosteroid-related complications such as cataracts and osteoporosis/osteopenia were documented based on clinical and imaging findings. The presence of autoimmune diseases was determined by the evaluation of antinuclear antibodies (ANAs), thyroid autoantibodies, and lupus antibodies.
2.7. Ethics Approval
This study was approved by the Non-Interventional Clinical Research Ethics Committee of Tekirdağ Namık Kemal University on December 26, 2023, under protocol number 2023.213.12.05. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and good clinical practice standards.
2.8. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics Version 25.0 (IBM Corp., Armonk, New York, United States). The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess the normality of data distribution. Continuous variables were expressed as median and interquartile range (IQR), while categorical variables were presented as frequencies and percentages. For nonnormally distributed data, the Mann–Whitney U test was used for two-group comparisons, and the Kruskal–Wallis test was applied for comparisons among more than two groups. The chi-square (χ2) test was used to examine associations between categorical variables.
A p value of < 0.05 was considered statistically significant for all analyses.
3. Results
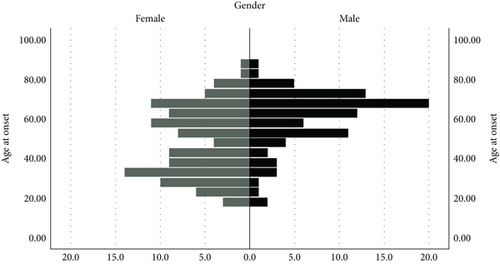
When MG classification was examined based on age at onset, 78.3% of patients in the EOMG subgroup were female, whereas 57.9% of patients in the LOMG subgroup were male (p < 0.001). The EOMG group demonstrated a significantly higher rate of seronegativity than the LOMG group (p < 0.001). The median AChR antibody level was found to be significantly higher in the LOMG group compared to the EOMG group (p < 0.001) (Table 1, Figure 1).
| Variable | EOMG | LOMG | p value |
|---|---|---|---|
| Sex | < 0.001a | ||
| Female | 54 (78.3) | 51 (42.1) | |
| Male | 15 (21.7) | 70 (57.9) | |
| Antibody type | < 0.001a | ||
| AChR | 36 (52.2) | 103 (85.1) | |
| MuSK | 3 (4.3) | 1 (0.8) | |
| Seronegative | 30 (43.5) | 17 (14) | |
| ICU admission | 8 (11.6) | 14 (11.6) | |
| Plasmapheresis | 4 (5.8) | 5 (4.1) | 0.726 |
| Electrophysiological findings | 32 (71.1) | 61 (71,8) | |
| AChR (nmol/L) | 0.26 (0.01–4.24) | 3.3 (0.53–9.70) | < 0.001b |
| Median follow-up (years) | 5 (3-8) | 5 (2-8) | 0.4532 |
- Note: Data are presented as median (Q1–Q3) or number (n) and percentage (%). Statistically significant results are shown in bold (p < 0.05).
- Abbreviations: AChR: acetylcholine receptor; EOMG: early-onset myasthenia gravis; ICU: intensive care unit; LOMG: late-onset myasthenia gravis; MuSK: muscle-specific kinase.
- aChi-square test.
- bMann–Whitney U test.
When treatment approaches were compared between the EOMG and LOMG groups, pyridostigmine was the most frequently used agent in both. However, significant differences were identified in the use of azathioprine (AZA), as well as in both current and prior corticosteroid use (p = 0.006, p = 0.002, and p < 0.001, respectively) (Table 2). In the LOMG group, AZA was the most frequently administered immunosuppressive agent, whereas corticosteroids were more commonly used in the EOMG group (Figure S1).
| Characteristic | EOMG | LOMG | p value |
|---|---|---|---|
| Azathioprine | 20 (29%) | 60 (49.6%) | 0.006a |
| Pyridostigmine | 62 (89.9%) | 111 (91.7%) | 0.863a |
| Maintenance IVIG | 8 (11.6%) | 22 (18.2%) | 0.322a |
| Previously used corticosteroids | 17 (24.6%) | 65 (53.7%) | < 0.001a |
| Currently using corticosteroids | 35 (50.7%) | 33 (27.3%) | 0.002a |
| Rituximab | 1 (1.5%) | 2 (1.7%) | 0.936a |
- Note: Data are presented as median (Q1–Q3) or number (n) and percentage (%). Statistically significant results are shown in bold (p < 0.05). IVIG: intravenous immunoglobulin.
- aChi-square test.
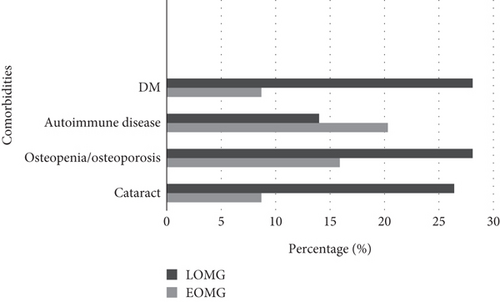
In the analysis of comorbidities, diabetes, osteopenia/osteoporosis, and cataract were more frequently observed in the LOMG group (p = 0.003, p = 0.086, and p = 0.006, respectively). Although autoimmune comorbidities were more frequent in the EOMG group, the difference was not statistically significant (p = 0.360) (Figure 2).
According to the MGFA classification, Class I was the most common clinical form in both groups, followed by Class IIb (p = 0.397). In the MGFA-PIS classification, the majority of patients in both groups were classified as MM3. The rate of E was 8.7% in the EOMG group and 1.7% in the LOMG group. Death (D) occurred only in the LOMG group, with a rate of 4.1% (p = 0.188) (Tables 3 and 4).
| MGFA class | EOMG | LOMG | p value |
|---|---|---|---|
| Class I | 35 (50.7) | 60 (49.6) | |
| Class II A | 4 (5.8) | 3 (2.5) | |
| Class II B | 17 (24.6) | 27 (22.3) | |
| Class III A | 4 (5.8) | 4 (3.3) | |
| Class III B | 6 (8.7) | 21 (17.4) | |
| Class IV A | 1 (1.4) | 0 (0) | |
| Class IV B | 1 (1.4) | 5 (4.1) | |
| Class V | 1 (1.4) | 1 (0.8) | |
| Total | 69 (100) | 121 (100) | 0.397 |
- Note: Data are presented as number (n) and percentage (%).Statistically significant differences are shown in bold (p < 0.05). Chi-square test used.
- Abbreviation: MGFA: Myasthenia Gravis Foundation of America.
| MGFA-PIS | EOMG | LOMG | p value |
|---|---|---|---|
| PR (pharmacologic remission) | 1 (1.4) | 0 (0) | |
| MM1 (minimal manifestation 1) | 5 (7.2) | 7 (5.8) | |
| MM2 | 16 (23.2) | 24 (19.8) | |
| MM3 | 30 (43.5) | 65 (53.7) | |
| I (improved) | 5 (7.2) | 7 (5.8) | |
| U (unchanged) | 4 (5.8) | 7 (5.8) | |
| W (worsened) | 2 (2.9) | 4 (3.3) | |
| E (exacerbation) | 6 (8.7) | 2 (1.7) | |
| D (death) | 0 (0) | 5 (4.1) | |
| Total | 69 (100) | 121 (100) | 0,188 |
- Note: Data are presented as number (n) and percentage (%). Statistically significant differences are shown in bold (p < 0.05). Chi-square test used.
- Abbreviation: MGFA-PIS: Myasthenia Gravis Foundation of America classification Post-Intervention Status.
Categorical evaluation of prognosis according to MGFA-PIS revealed that neither gender nor MG classification based on age at onset (EOMG vs. LOMG) had a statistically significant effect on prognosis (p = 0.131 and p = 0.650, respectively,)(Figures S2 and S3).
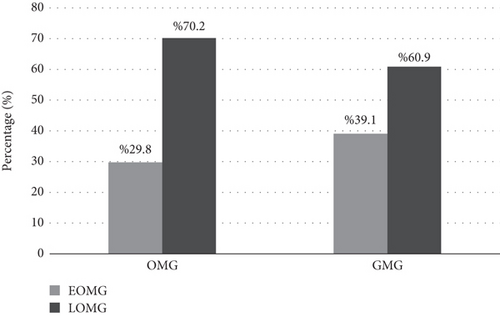
In the MG classification based on muscle involvement, LOMG was present in 70.2% of patients in the OMG group and in 60.9% of those in the GMG group (p = 0.292) (Figure 3). The median age at onset was 60 years in the OMG group and 56 years in the GMG group (p = 0.118) (Table 5). The GMG group had a significantly longer median follow-up duration (p = 0.048). Of the patients included in the study, 47 were identified as seronegative, 21 of whom were double seronegative for both AChR and MuSK antibodies (Table 5).
| Variable | OMG | GMG | p value |
|---|---|---|---|
| Age at onset | 60 (44–68.5) | 56 (36.5–67) | 0.118a |
| Age | 65 (50.5–75) | 63 (47–72.5) | 0.364a |
| Follow-up duration (years) | 4 (2–7) | 5 (3–8) | 0.048a |
| AChR antibody value (nmol/L) | 2.58 (0.31–5.66) | 2.43 (0.14–8.79) | 0.799a |
| Female sex | 30 (52.6%) | 75 (56.4%) | 0.633b |
| Antibody type | 0.112b | ||
| AChR | 47 (82.5%) | 92 (69.2%) | |
| MuSK | 0 (0%) | 4 (3.0%) | |
| Seronegative | 10 (17.5%) | 37 (27.8%) |
- Note: Data are presented as median (Q1–Q3) or number (n) and percentage (%).Statistically significant results are shown in bold (p < 0.05).
- Abbreviations: AChR: acetylcholine receptor; GMG: generalized myasthenia gravis; ICU: intensive care unit; OMG: ocular myasthenia gravis.
- aMann–Whitney U test.
- bChi-square test.
Among patients initially diagnosed with OMG based on presenting symptoms, the progression rate to GMG during a median 5-year follow-up period was 41.2%.
At admission, ptosis was the most commonly observed symptom. Unilateral ptosis, either right or left, was significantly more common in the OMG group (p = 0.022 and p < 0.001, respectively) (Figure S4).
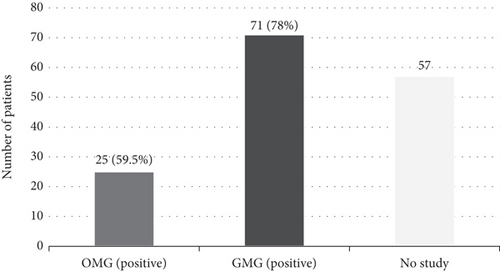
Electrophysiological testing revealed a positivity rate of 78% in the GMG group and 59.5% in the OMG group. This difference was statistically significant (p = 0.045) (Figure 4).
4. Discussion
The findings of this study were evaluated within the framework of clinical subtypes, serological profiles, comorbid conditions, and treatment strategies in MG. Results were interpreted in comparison with existing literature and analyzed in terms of prognosis using the MGFA and MGFA-PIS classifications.
Considering the gender distribution in EOMG and LOMG, the bimodal distribution in women and male predominance in the late-onset group were consistent with previous epidemiological studies [16–22]. The higher rate of autoimmune diseases in young women may help explain the predominance of female patients in the EOMG group. The regulatory effect of estrogen on the immune system has been suggested as one possible explanation for this bimodal distribution in females [1, 23].
A statistically significant difference in AChR positivity favoring the LOMG group was observed, consistent with the findings of Živković et al. [24].
Regarding immunosuppressive treatment profiles, active corticosteroid use was more frequent in the EOMG group, whereas AZA use was higher in the LOMG group. This difference may reflect age-related comorbidities and a preference to avoid corticosteroid-associated complications in older patients. These findings underscore the importance of individualized treatment strategies based on MG subtypes.
The higher frequency of autoimmune diseases in the EOMG group points to potential genetic and immunologic susceptibility. Although this difference did not reach statistical significance in our study, previous reports have linked autoimmune diseases accompanying EOMG with thymic hyperplasia and the HLA-B8-DR3 haplotype. This suggests that interpopulation variability may be attributable to ethnic differences [25].
The clinical management of LOMG patients may differ from that of EOMG, as comorbidities such as cataracts, diabetes, and osteopenia/osteoporosis are more frequent in older patients. These factors may influence both treatment response and tolerance, indicating a need for closer monitoring in this group. In this context, stratifying MG patients by age at onset is not only relevant for epidemiological purposes but also critical for guiding treatment decisions and clinical management.
According to the MGFA classification, approximately half of both the EOMG and LOMG subgroups were categorized as Class I, consistent with literature findings that ocular symptoms are most common at presentation [4, 7]. Under the MGFA-PIS classification, the majority of patients in both groups fell under the MM3 category, suggesting that a large proportion of patients remained symptomatic and required immunosuppressive therapy. The higher E rate in the EOMG group and the higher D rate in the LOMG group may be explained by increased proinflammatory immune activity associated with thymic hyperplasia in EOMG and age-related comorbidities in LOMG [26, 27]. MGFA-PIS, therefore, appears to be a valuable tool both as a means of assessing disease severity and for tracking treatment response.
The predominance of LOMG cases in both the OMG and GMG groups reflects the increasing frequency of MG diagnoses in older age. This reflects increased clinical recognition and diagnostic vigilance among healthcare professionals and within the general population. Literature also supports a notable increase in LOMG incidence in recent decades, attributed to improvements in diagnostic capabilities, aging populations, and greater clinical attention to MG in elderly individuals [28].
In our study, the age at onset was lower in the GMG group than in the OMG group. The median age in the GMG group was comparable to that reported by Karni et al. but was higher than in the study by Grob et al. [11, 29]. These differences may reflect ethnic variability across study populations.
In clinical practice, radioimmunoassay (RIA) is used for antibody detection, but this method has lower sensitivity for clustered antibodies compared to cell-based assays. Previous studies have demonstrated that cell-based assays improve antibody detection rates in seronegative MG cases [30]. In our study, a considerable number of seronegative patients were identified. This supports the idea that cell-based assays may serve as complementary diagnostic tools when conventional antibody tests yield negative results.
In our cohort, 41.2% of patients initially diagnosed with OMG developed GMG during follow-up. This rate is consistent with the 37.8% reported by Guéguen et al. in their 10-year follow-up study [31]. In contrast, Grob et al. reported a much higher conversion rate of 80% [11]. Such discrepancies may be attributed to differences in patient selection criteria, follow-up duration, timing of diagnosis, and, importantly, treatment strategies. Evidence in the literature suggests that early initiation of immunosuppressive therapy reduces the risk of progression from OMG to GMG [31–34]. The relatively low conversion rate in our study may reflect the early and widespread use of corticosteroids in our clinical practice. This finding emphasizes that early immunomodulatory treatment may not only control symptoms but also modify the disease course. Therefore, patients diagnosed with OMG should be evaluated not only through regular monitoring but also in terms of proactive treatment strategies to prevent generalization. Our observed conversion rate may support the potential protective effect of early immunosuppressive therapy in our center, although prospective studies are warranted.
Electrophysiological tests play a crucial role in the diagnosis of MG, particularly in seronegative patients. In our study, electrophysiological abnormalities were more frequently detected in the GMG group compared to the OMG group. Previous studies have reported higher RNS sensitivity in GMG patients, especially when proximal muscle groups are evaluated [6]. This may be due to the more widespread muscle involvement in GMG, making abnormalities more readily detectable. In contrast, the limited muscle involvement in OMG and the restricted number of muscles tested may reduce test sensitivity. Therefore, integrating electrophysiological data with clinical and serological findings is essential to minimize false negatives. In particular, using more sensitive tests such as SFEMG in seronegative cases may improve diagnostic accuracy.
In summary, our study identified distinct clinical, serological, and therapeutic differences between EOMG and LOMG subtypes. These differences are consistent with previously reported MG subtype characteristics and offer clinically relevant insights for personalized treatment planning in MG.
4.1. Impact of Regional Healthcare Context
The Thrace region, where this study was conducted, is located in the northwestern part of Türkiye and is characterized by relatively better access to healthcare services. All patients included in the study were regularly followed at a tertiary neuromuscular center, which may have minimized delays in diagnosis and treatment planning. This may partly explain certain findings, such as the lower-than-average rate of progression from OMG to GMG, which could be associated with a more favorable prognosis. In this context, early access to healthcare and follow-up in specialized centers appear to play a critical role in the diagnosis and management of MG. Future multicenter studies would benefit from a more comprehensive evaluation of how healthcare accessibility and regional disparities influence the clinical course of MG.
4.2. Study Limitations
This study has several limitations. First, its retrospective and single-center design may have introduced selection and data access biases, potentially limiting the generalizability of the findings. In addition, the low number of MuSK antibody–positive patients hindered the ability to draw robust conclusions about the clinical characteristics and symptoms of this subgroup. The lack of histopathological data from patients who underwent thymectomy prevented the evaluation of the relationship between thymic pathology and clinical course. Furthermore, the absence of electrophysiological testing in all patients represented an additional limitation in confirming neuromuscular transmission abnormalities. For these reasons, the findings should be supported by future prospective, multicenter studies involving larger patient cohorts.
5. Conclusion
MG is a clinically, serologically, and prognostically heterogeneous disease that necessitates individualized diagnostic and therapeutic approaches. In this study, the effects of antibody profiles, clinical subtypes, and treatment responses on the disease course were evaluated in a multidimensional manner. The findings demonstrate that MG subtypes play a decisive role not only in diagnosis but also in disease severity and treatment response, highlighting the importance of personalized patient management. Notably, the generalization rate of 41.2% observed in patients with ocular onset and the treatment-related factors influencing this process support the preventive role of early immunosuppressive therapy.
Accurate identification of MG-specific antibodies using sensitive techniques is particularly important for properly classifying patients who are otherwise labeled as seronegative. The broader implementation of standardized antibody assays nationwide could contribute to a more unified approach in the diagnosis and follow-up of MG. Moreover, with the integration of newly defined antibodies and cell-based assays into clinical practice, the underlying pathophysiological mechanisms of MG may be better elucidated, thereby providing a more solid foundation for treatment decision-making.
This study represents one of the few comprehensive, single-center investigations from Türkiye and thus contributes unique and region-specific data to the international literature.
Nomenclature
-
- MG
-
- myasthenia gravis
-
- OMG
-
- ocular myasthenia gravis
-
- GMG
-
- generalized myasthenia gravis
-
- AChR
-
- acetylcholine receptor
-
- MuSK
-
- muscle-specific kinase
-
- LRP4
-
- Low-Density Lipoprotein Receptor–Related Protein 4
-
- RNS
-
- repetitive nerve stimulation
-
- SFEMG
-
- single-fiber electromyography
-
- IVIG
-
- intravenous immunoglobulin
-
- MGFA
-
- Myasthenia Gravis Foundation of America
-
- MGFA-PIS
-
- Myasthenia Gravis Foundation of America Post-Intervention Status
-
- EOMG
-
- early-onset myasthenia gravis
-
- LOMG
-
- late-onset myasthenia gravis
-
- ICU
-
- intensive care unit
-
- CT
-
- computed tomography
-
- ANA
-
- antinuclear antibody
-
- RTX
-
- rituximab
-
- CS
-
- corticosteroid
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no funding.
Acknowledgments
We gratefully acknowledge the late Prof. Dr. Nilda Turgut for her invaluable contributions to the conceptualization and drafting of this manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section. The contents of the supporting information file (PDF), prepared to support and elaborate upon the data presented in the main manuscript, are summarized below. All figures and detailed analytical procedures are included within the file.
Open Research
Data Availability Statement
All relevant data supporting the findings of this study are included in the manuscript.




