Rechargeable and Nonrechargeable Implantable Pulse Generators for Deep Brain Stimulation in Parkinson’s Disease: Long-Term Experience
Abstract
Objectives: The study’s objective is to assess long-term experience with rechargeable (r-IPG) and nonrechargeable implant pulse generators (nr-IPGs) for deep brain stimulation (DBS) in Parkinson’s disease (PD).
Material and Methods: Qualitative semistructured interviews, clinical outcomes, and care load estimations were retrospectively collected for a PD-DBS population implanted at our center from 2006 to 2022.
Results: Thirty-seven nr-IPG patients (follow-up 85.3 ± 32.0 months) and 43 r-IPG patients (follow-up 73.1 ± 7.7 months) were analyzed. Long-term satisfaction was sustained in both groups (100% of r-IPG carriers and 75.7% of nr-IPGs, p = 0.001). In r-IPGs, 97.7% recharged the battery easily, and recharging time did not impact everyday life. The percentage of malfunctioning problems (32.6%) in the r-IPG group was in line with previous observations on short-term follow-ups. The size of the IPG was considered too big for 16.2% and 4.2% for nr-IPGs and r-IPGs (p = 0.086), and concerns of interventions for IPG replacements were still present in the nr-IPG group (48.6%). The total amount of days of hospitalization (19.6 ± 9.9 vs. 9.3 ± 4.8, p < 0.001) and the number of complications after the first implant (13 vs. 5, p < 0.05) and during subsequent admissions for IPG substitutions (4 vs. 0, p < 0.05) were higher for the nr-IPGs.
Conclusions: The overall level of long-term satisfaction with IPGs is consistent over time regardless of type. R-IPGs reported no discomfort with recharging even in the long-term evaluation. IPG replacement surgeries and sizes are still a concern, especially for the nr-IPG carriers, but did not affect a high level of sustained satisfaction. Resource burden remains higher for nr-IPGs even in the long term.
1. Introduction
Deep brain stimulation (DBS) is an established treatment for managing motor symptoms in advanced Parkinson’s disease (PD) [1]. Recent advancements in battery technology have expanded the options for implantable pulse generators (IPGs) used in DBS, offering both rechargeable (r-IPGs) and nonrechargeable (nr-IPGs) devices [2]. Nr-IPGs are equipped with fixed-life batteries, typically lasting between 2 and 6 years depending on the intensity of stimulation and must be periodically replaced by a surgical intervention [3]. In contrast, r-IPGs require to be regularly recharged, but the potential lifespan exceeds 20 years (9–25 years) with proper maintenance. In clinical practice due to the absence of shared guidelines, the selection between the two is variable and depends on (1) patient-specific factors, such as lifestyle, overall health condition, cognitive status, age at intervention, and presence of a caregiver [4]; (2) clinicians’ expertise and personal attitude [4]; (3) DBS application such as the amount of current delivered [5]; and (4) economic factors [6]. Among all these, individual patient preferences play the most meaningful role in the shared decision-making process regarding IPG selection [5]. While numerous studies have examined patient concerns before implantation and their short-term satisfaction postintervention [2, 5, 7], there is limited knowledge about patients’ long-term experiences and satisfaction with the available IPGs, highlighting the need for further research on this topic.
2. Methods
2.1. Study Design
This monocentric, observational, retrospective cohort study was conducted at the Movement Disorder Centre of Ferrara Hospital. Participants or their legal representatives gave informed consent, and the study protocol received approval from the local ethical committee (489/2023/Oss/AOUFe). The study conformed with the ethical standards stated in the 1964 Declaration of Helsinki and its later amendments.
2.2. Outcome Measures
To assess qualitatively overall satisfaction and concerns related to DBS and IPGs and the recharging process, a semistructured survey was applied during outpatient visits of DBS carriers. A 17-item interview with options such as “yes,” “no,” “not known,” or multiple-choice selections was administered to r-IPG carriers and a similar 9-item interview to nr-IPG carriers. All the questions were focused only on the type of device used and not on clinical outcomes. Patients completed the survey independently, and their responses were not shared with the clinician who assessed the visit. To analyze the determining factors influencing the clinician in the choice of the IPG, the physician was asked to retrospectively reconstruct the reason for not proposing the switch prior to the last substitution in patients with nr-IPG choosing one among these options: (i) advanced age, (ii) absence of caregiver-inadequate care setting, (iii) cognitive decline (MCI or dementia), and (iv) poor prognosis at 12 months.
In order to document other relevant factors for IPG, as clinical and economic matters, data on outcome and care load were also retrospectively collected.
2.3. Recruitment
One hundred and fifty-four PD patients underwent bilateral subthalamic nuclei DBS between January 2006 and December 2022. Among them, only patients who had been followed at our center were considered for the study, and 94 were selected for evaluation. PD was diagnosed according to Movement Disorder Society (MDS) criteria [8], and patients met criteria for a DBS implant [9]. After surgery, patients were evaluated monthly and once a good therapeutic response was achieved every 6 months in the first year and annually in the subsequent follow-up. At the time of the first implant, when available (since 2012 at our center), both types of IPG were presented to the patient and their family, and the choice was made by the patient on clinician’s recommendation. For subsequent replacements, unless explicitly requested by the patient, the clinician reserved the right to decide whether to propose a switch. We retrospectively collected the last follow-up survey. We included patients who had undergone comprehensive clinical evaluations both prior to the DBS implant and on the same date as the survey completion. To assess long-term experience, only patients who had been receiving DBS therapy for at least 24 months were considered. We excluded those with cognitive decline (Montreal Cognitive Assessment [MoCA] < 17) to maximize the reliability of the responses provided, depression (Beck Depression Inventory [BDI] < 16), and/or apathy (Apathy Scale < 18) at the time of the survey. Additionally, patients with incomplete evaluation data were not included.
2.4. Data Collection
Age, sex, duration of disease, ON medication/ON stimulation MDS–Unified Parkinson’s Disease Rating Scale (UPDRS) III and IV, Hoehn and Yahr (H&Y), and levodopa equivalent of daily dose (LEDD) before DBS and at the follow-up visit were retrospectively collected from discharge letters, outpatient clinical reports, and healthcare reports. When only UPDRS was available, it was converted to MDS-UPDRS according to the literature [10]. Total number of days for hospitalization, including implantations, replacements, and complications (surgery related and stimulus induced), were as well recorded. Data from patients who switched to a different device type were recorded based on the device they were using at the time of the interview.
2.5. Statistical Analysis
Data are presented as numbers or percentages for categorical variables and mean and standard deviation for continuous variables. Normal distribution was checked for each variable with Shapiro–Wilk test. Comparison for categorical variables was made using the chi-square test or Fisher’s exact test and comparison between continuous variables by Student t-test for normally distributed data or by Mann–Whitney test for not normally distributed data. p value significance was set at p < 0.05. Statistical analyses were performed with SPSS Statistics (Version 25).
3. Results
Interviews with completed clinical data were available for 80 PD-DBS patients among 94 patients followed in the time frame interval of 17 years from 2006 to 2022. Forty-three patients were r-IPG carriers, and 37 were nr-IPG carriers. Three nr-IPG of 37 carriers switched to r-IPGs after the first replacement. For the patients who switched, the interview was conducted prior to it (mean follow-up of 43 months) (flow chart, Figure 1). The survey was collected 85.3 (±32.0) months after DBS implant for nr-IPG and 73.1 (±37.7) months for r-IPG.
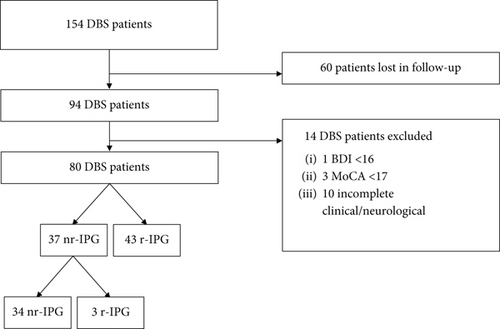
Demographic and clinical data are presented in Table 1. No significant differences were detected between groups for sex (19 males for nr-IPG vs. 30 for r-IPG), years of disease at surgery (12.4 ± 4.9 vs. 12.0 ± 4.0) and at last follow-up (19.9 ± 5.5 vs. 18.0 ± 4.8), pre-DBS On-medication MDS-UPDRS III (26.0 ± 6.3 vs. 23.2 ± 9.4) and IV (5.9 ± 3.9 vs. 6.3 ± 2.6), pre-DBS LEDD (870.0 ± 396.0 mg vs. 1053.1 ± 580.3 mg), and last follow-up LEDD (749.4 ± 276.4 mg vs. 695.5 ± 331.2 mg). Significant differences were present for age at intervention (63.6 ± 7.0 years for nr-IPG carriers vs. 54.8 ± 6.9 years for r-IPGs) and at follow-up (70.9 ± 6.7 years vs. 60.8 ± 7.2 years) and MDS-UPDRS III (25.8 ± 8.4 vs. 19.9 ± 8.1), MDS-UPDRS IV (4.5 ± 3.8 vs. 2.3 ± 2.0), and H&Y (3.3 ± 0.6 vs. 2.7 ± 0.5) at the moment of the survey.
| Nonrechargeable (N = 37) | Rechargeable (N = 43) | p value | |
|---|---|---|---|
| Male (n) | 19.0 (51.4) | 30.0 (69.8) | 0.092 |
| Age at intervention (years) | 63.6 (7.0) | 54.8 (6.9) | < 0.05 |
| Years of illness at surgery (years) | 12.4 (4.9) | 12.0 (4.0) | 0.326 |
| Follow-up (months) | 85.3 (32.0) | 73.1 (37.7) | 0.086 |
| Age (years) | 70.9 (6.7) | 60.8 (7.2) | < 0.05 |
| Years of illness (years) | 19.9 (5.5) | 18.0 (4.8) | 0.487 |
| On-MDS UPDRS III | |||
| Baseline | 26.0 (6.3) | 23.2 (9.4) | 0.110 |
| Follow-up | 25.8 (8.4) | 19.9 (8.1) | < 0.05 |
| On-MDS UPDRS IV | |||
| Baseline | 5.9 (3.9) | 6.3 (2.6) | 0.322 |
| Follow-up | 4.5 (3.8) | 2.3 (2.0) | < 0.05 |
| H&Y | |||
| Baseline | 2.3 (0.4) | 2.6 (0.4) | < 0.05 |
| Follow-up | 3.3 (0.6) | 2.7 (0.5) | < 0.05 |
| LEDD (mg) | |||
| Baseline | 870.0 (396.0) | 1053.1 (580.3) | 0.055 |
| Follow-up | 749.4 (276.4) | 695.5 (331.2) | 0.225 |
| Target STN | 37.0 (100.0) | 43.0 (100.0) | |
| Device | |||
| Abbott Infinity | 13.0 (35.1) | ||
| Medtronic Kinetra | 3.0 (8.1) | ||
| Medtronic Activa PC | 14.0 (37.8) | ||
| Boston Vercise Gevia (NR) | 1.0 (2.7) | ||
| St. Jude LibraXP | 6.0 (16.2) | ||
| Medtronic Activa RC | 3.0 (7.0) | ||
| Boston Genus | 7.0 (16.2) | ||
| Boston Vercise Gevia | 32.0 (74.4) | ||
| St. Jude Brio | 1.0 (2.3) | ||
| Outpatient accesses for programming sessions (n) | 7.5 (2.7) | 4.5 (2.5) | < 0.05 |
| Number of replacements | |||
| I | 36.0 (97.3) | 0 | |
| II | 23.0 (62.2) | ||
| III | 7.0 (18.9) | ||
| IV | 1.0 (2.7) | ||
| Switch to other type of IPG (n) | 3.0 (8.1) | 0 |
- Note: Data are expressed as mean (standard deviation) for age at intervention, years of illness at surgery, months of follow-up, age and years of illness at follow-up, baseline and follow-up On-MDS UPDRS III, On-MDS UPDRS IV, H&Y, LEDD, and outpatient access for programming sessions and as numbers (percentage) for sex, DBS target, type of device, switch to other type of IPG, and drop out from DBS.
- Abbreviations: H&Y, Hoehn and Yahr Scale; IPG, implantable pulse generator; LEDD, levodopa equivalent daily dose; MDS-UPDRS, Movement Disorder Society–Unified Parkinson’s Disease Rating Scale; N, number; STN, subthalamic nucleus.
Answers to the interview are summarized in Tables 2, 3, and 4. At the time of the survey, the majority of patients (93.0% of r-IPGs vs. 83.8% of nr-IPGs) were satisfied with their DBS implant. One hundred percent of r-IPGs and 75.7% of nr-IPGs (p = 0.001) were globally satisfied with their IPGs. Ninety-five percent of r-IPGs and 45.9% of nr-IPGs (p < 0.001) would have chosen again the same type of IPG. 97.7% of r-IPGs and 64.9% of nr-IPGs would recommend their type of IPG (p = 0.001). Furthermore, patients in both groups claim that the size of the IPG was too big (16.2% in nr-IPGs and 4.2% in r-IPGs, p = 0.086).
| Nonrechargeable (N = 37) | Rechargeable (N = 43) | p value | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Not known | Yes | No | Not known | ||
| Are you satisfied with your DBS device? | 31.0 (83.8) | 6.0 (16.2) | 0 | 40.0 (93.0) | 3.0 (7.0) | 0 | 0.192 |
| Are you satisfied with your IPG? | 28.0 (75.7) | 9.0 (24.3) | 0 | 43.0 (100.0) | 0 | 0 | 0.001 |
| Would you choose the same type of IPG today? | 17.0 (45.9) | 17.0 (45.9) | 3.0 (8.2) | 41.0 (95.3) | 2.0 (4.7) | 0 | < 0.001 |
| Would you recommend your type of IPG to other people? | 24.0 (64.9) | 9.0 (24.3) | 4.0 (10.8) | 42.0 (97.7) | 1.0 (2.3) | 0 | 0.001 |
| Is the size of the IPG acceptable? | 31.0 (83.8) | 6.0 (16.2) | 0 | 41.0 (95.3) | 2.0 (4.7) | 0 | 0.086 |
| If not, is it too big? | 6.0 (16.2) | 0 | 0 | 2.0 (4.2) | 0 | 0 | |
- Note: Data are expressed in numbers (percentage).
- Abbreviations: DBS, deep brain stimulation; IPG, implantable pulse generator.
| Nonrechargeable (N = 37) | |||
|---|---|---|---|
| Yes | No | Not known | |
| Do the periodic surgical IPG replacements bother you? | 18.0 (48.6) | 19.0 (51.4) | 0 |
| Have you ever been offered to switch to the other type of IPG? | 14.0 (37.8) | 21.0 (56.8) | 2.0 (5.4) |
| If not, would have wanted that possibility? | 10.0 (47.6) | 10.0 (47.6) | 1.0 (0.8) |
- Note: Data are expressed in numbers (percentage).
- Abbreviation: IPG, implantable pulse generator.
| Rechargeable (N = 43) | |||
|---|---|---|---|
| Is it difficult to use r-IPG? | Yes | No | Not known |
| 1.0 (2.3) | 42.0 (97.7) | 0 | |
| Do you recharge the battery by yourself? | 35.0 (81.4) | 8.0 (18.6) | 0 |
| How often do you recharge the battery? | More than once a week | Once every 2 weeks | Once a month or less |
| 8.0 (18.6) | 20.0 (46.5) | 15.0 (34.9) | |
| How long do you recharge your battery? | Less than 1 h | Between 1 and 2 h | More than 2 hours |
| 5.0 (11.6) | 29.0 (67.5) | 9.0 (20.9) | |
| Does this time bother your personal life? | Yes | No | Not known |
| 1.0 (2.3) | 42.0 (97.7) | 0 | |
| Did you ever forget to recharge the battery? | 6.0 (14.0) | 37.0 (86.0) | 0 |
| If yes, how often? | Less than three times a year | Between three times a year and once a month | More than once a month |
| 5.0 (11.6) | 1.0 (2.3) | 0 | |
| Have you ever had malfunctioning problems with your recharge? | Yes | No | Not known |
| 14.0 (32.6) | 29.0 (67.4) | 0 | |
| What kind? | Charge-IPG matching | Sudden switch off | Remote control malfunction |
| 10.0 (23.3) | 2.0 (4.7) | 9.0 (21.0) | |
| Did you ever contact the support service? | Yes | No | Not known |
| 9.0 (20.9) | 34.0 (79.1) | 0 | |
| How many times? | Once | Twice | More than twice |
| 8.0 (18.6) | 1.0 (2.3) | 0 | |
- Note: Data are expressed in numbers (percentage).
- Abbreviation: IPG, implantable pulse generator.
Regarding the long-term experience of r-IPG patients, 97.7% do not find it difficult to recharge the battery, and 81.4% claim to do so autonomously. Furthermore, 86.0% have never forgotten to recharge the battery. Most of the patients recharge the battery every 2 weeks (46.5%) for an estimated time between 1 and 2 h (67.5%). The time of the recharge did not impact on everyday life for almost the totality of the patients (97.7%). Referring to the recharge system, 32.6% experienced malfunctioning problems, with the majority concerning the charge-IPG matching (23.3%) and the remote control malfunction (21.0%). Among nr-IPG carriers, 48.6% still expressed concerns about undergoing interventions for IPG replacements.
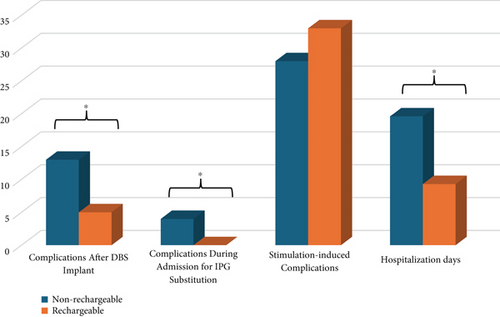
A higher number of complications after the first DBS implant (13 vs. 5, p < 0.05) and during subsequent admissions for the IPG substitutions (4 vs. 0, p < 0.05) were detected in the nr-IPG group compared to r-IPGs. Among these, infective complications of IPG (five for nr-IPGs and one for r-IPGs) and complications due to malfunction of the IPG (three for nr-IPGs and two for r-IPGs) or unpredictable battery depletion (two for nr-IPGs) were the most numerous. Among the latter, one case of withdrawal syndrome required a brief hospitalization. In seven cases among nr-IPG carriers, an early intervention of substitution was necessary. Surgical revision of the IPG was performed in six patients (three with nr-IPG and three with r-IPG), while lead drainage was carried out in three patients with nr-IPG. The overall IPG infection rate for all surgical procedures in nr-IPGs was 4.8%, and in r-IPGs, it was 2.3%. Three out of five nr-IPG infections occur after the initial implantation within a period of 28 to 50 months following surgery, while others occur within 3 months after the first replacement, which was performed regularly due to the end of life of the battery.
No infective complications of IPG were detected in the nr-IPG sample after the second or subsequent replacements (3/37 after implant, 2/36 after I replacement, and no cases for II, III, and IV replacements). Other intercurrent complications observed in the cohort (infection of the DBS leads, resurgical procedures for mispositioning, and idiopathic delayed-onset edema) do not appear to be directly attributable to the IPG. During hospitalization for IPG substitutions in the nr-IPG group, two patients developed delirium, one complicated with sepsis, and one presented an adverse reaction to prophylactic antibiotics.
The number of total amounts of hospitalization days, considering replacements and complications, was significantly higher for the nr-IPG group compared to the r-IPG group (19.6 ± 9.9 vs. 9.3 ± 4.8, p < 0.001). No differences concerning the presence of stimulation-induced complications during the outpatient monopolar revision and DBS parameter fine-tuning within the first 6 months after surgery were detected between groups (28 for nr-IPGs and 33 for r-IPGs, p = 0.911). Data about care load and complications during or after hospitalization are shown in Figure 2.
The choice between remaining with nr-IPG and switching to a r-IPG was offered before a subsequent substitution to 14 patients, and 3 of them accepted. Of the 11 remaining patients, 6 refused fearing not to be able to recharge, 3 for a personal preference, and 2 considering r-IPG incompatible with their lifestyle (many travels for business).
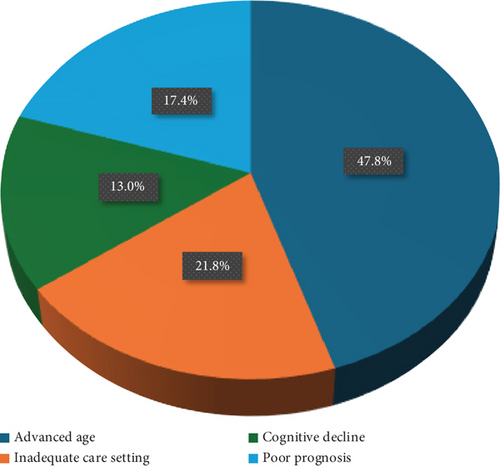
In Figure 3a, a survey on clinician perspective of switching or not to r-IPG is reported. Data are available for 23 patients out of 34. The remaining patients (11/34) underwent replacements or passed away before the r-IPG became commercially available. Advanced age (patients > 70 years old) was determinant for not proposing the switch in 47.8% (11/23) of cases examined by the clinician, while the absence of caregivers or inadequate care setting accounted for 21.8% (5/23), the presence of cognitive decline (MCI or dementia) for 13.0% (3/23), and a poor prognosis at 12 months for 17.4% (4/23). In Figure 3b, patient’s perspective on not switching is reported (data inferred from the interviews of patients who were offered to switch and choose to keep nr-IPG and those who stated they were satisfied with their current nr-IPG device and would not have wanted to switch). Fear of not being able to recharge was determinant in 57.2% (12/21) of patients, and lifestyle consisting of many travels for business in 9.5% (2/21). A personal preference for the nr-IPG device accounted for 33.3% (7/21).

4. Discussion
A significant emphasis on making choices based on individual focusing has been placed in selection criteria for proposing DBS to patients with PD to ensure the best clinical outcomes after the intervention [11]. Important in this patient-centered paradigm is the choice between r-IPG and nr-IPG proposed by physicians [4], who play a crucial role in highlighting the most significant factors (clinical, organizational, and economical) to be considered in this decision-making process [12].
Referring to patient’s perspective, many works in the literature report on patient’s satisfaction with their IPG after short-term intervals of 3–6 months [13, 14], 12 months [15], or medium-term interval (18–42 months) [2, 5, 16–18] post-DBS implant. Only one study evaluated a longer follow-up (40.3 ± 24.8 months [18]) but solely for r-IPG carriers (see Table 5 for review).
| References | Study design | Patients | R-IPG carriers (n) | Nr-IPG carriers (n) | Follow-up (months) | Data collection | Results |
|---|---|---|---|---|---|---|---|
| Timmermann et al. [13] | Prospective |
|
21 | 0 | 3 | Survey |
|
| Waln et al. [16] | Prospective |
|
26 | 0 | 12.1 | Survey |
|
| Jia et al. [15] | Prospective | - 53 PD | 53 | 0 | 12 | Survey |
|
| Jakobs et al. [17] | Retrospective |
|
31 | 0 | 21.2 ± 10 | Survey |
|
| Khaleeq et al. [7] | Prospective |
|
11 | 19 | Assessment pre-DBS | Survey |
|
| Mitchell et al. [14] | Retrospective |
|
59 | 0 | 6 | Survey |
|
| Hitti et al. [18] | Retrospective | - 102 PD | 102 | 0 | 27.6 | Survey |
|
| Furlanetti et al. [5] | Prospective |
|
11 | 15 | 18 ± 7.2 | Survey |
|
| Jakobs et al. [19] | Retrospective |
|
195 | 0 | 40 | Survey |
|
| Qiu et al. [2] | Prospective | - 220 PD | 192 | 28 | 18 | Survey |
|
- Abbreviations: DBS, deep brain stimulation; Dyt, dystonia; ET, essential tremor; IPG, implantable pulse generator; n, numbers; NR, nonrechargeable; OCD, obsessive–compulsive disorder; PD, Parkinson’s disease; R, rechargeable; Syn, syndrome.
Data on long-term experiences with r-IPG and nr-IPG are lacking [13], and to the best of our knowledge, this is the first study to assess the real-life long-term experiences and satisfaction of patients with r-IPG and nr-IPG.
Consistent with previous short- and mid-term follow-up findings [2, 13, 17], the majority of patients in our study reported high overall satisfaction with their IPG after long-term follow-up, regardless of the device type. In a Chinese cohort, Qiu et al. observed similar satisfaction levels between r-IPG users (88%) and nr-IPG users (83%) after 18 months of DBS therapy [2]. Timmerman et al. [13] and Hitti et al. [18] both found a sustained preference for r-IPGs among patients who had switched from an nr-IPG, with evaluations conducted 3 months (seven patients, 75%) and 2 years (83 patients, 70.3%) postsurgery, respectively. A widening gap in satisfaction levels over the long term, with higher satisfaction among r-IPG users (100%) and slightly lower satisfaction among nr-IPG users (75.7%), has been detected in our survey. It is unclear whether this difference reflects a gradual divergence over time or is due to differing characteristics between the two populations which could significantly affect the results (see Table 1).
Our findings on the long-term experience of using r-IPGs align with previous studies that assessed the sustainability of the recharging process and the complications encountered during short-term follow-up [2, 15, 18]. Recharging time and frequency are consistent with Jakobs et al.’s findings (IPG recharge every 10 ± 8 days for 97 ± 57 min), showing stability even over the longer term [19]. Furlanetti et al. demonstrated that patients who were well-informed and had realistic expectations before surgery did not experience a change in concerns about recharging postoperatively [5]. Our results confirm that this trend continues even many years after implantation, indicating that fears of discomfort with the recharging process are not borne out in patients’ long-term experiences. Differently from previous suggestions, increasing time burden of recharging over the years might not impact patient satisfaction [18]. The incidence of technical device-related issues in our r-IPG cohort mirrors that of a previous study by Mitchell et al. (32.6% vs. 35.6%) [14], suggesting that the rate of hardware-related adverse events for r-IPGs remains stable over time. Finally, in contrast to Qiu et al. [2], who reported that 89% of r-IPG users abstained from work and 73% from travel due to the recharging process, our r-IPG carriers did not report any restrictions in their daily activities, including work.
Fear of multiple replacement surgeries and the size of the IPG have been identified as significant factors contributing to dissatisfaction with nr-IPGs, especially as concerns that emerge over time following implant surgery [5, 7]. However, even if these factors remain issues for our nr-IPG group, our survey demonstrated that the level of satisfaction is consistently high, thus not appearing to be affected by such concerns even if still present. Furthermore, the majority of patients in our population who were offered the possibility chose not to switch to a r-IPG still maintaining a high level of satisfaction with their nr-IPGs.
In our cohort (Figure 3a), advanced age (> 75 years) emerged as one of the most significant factors influencing the decision of clinicians not to recommend a switch to a r-IPG (47.8%), and cognitive impairment prevented the proposal of switching to an r-IPG in 13.0% of cases involving nr-IPG carriers. Indeed, supporting these positions, satisfaction was sustained among those who retained the nr-IPG without being offered to switch.
On the other side, Timmermann et al. suggested a role of age in r-IPG satisfaction, detecting a lower degree of satisfaction among elderly patients about the recharging process [13]. However, studies in r-IPGs have not identified a direct role of age on the adaptability to the recharge [2, 19], and these data also appear to be supported by our observations over the clinical follow-up period, considering the adherence and ease of handling of the recharging process as reported by patients. Differently, it is not possible to draw any indication of the effect of cognitive decline in this decision-making process because all the available studies conducted on IPG carriers, as our work, enrolled those patients who preserved global cognitive functions during follow-ups [18, 19] and real data in patients with severe cognitive decline are lacking. Regardless of cognitive impairment, akin to Qiu et al. [2], our r-IPG carriers who declared recharging process concerns were successfully assisted by a caregiver without reported decreased satisfaction in this choice.
Another crucial factor in choosing between different types of devices for DBS carriers is the clinical and ethical considerations regarding DBS in end-stage PD [20]. In our observation, poor prognosis at 12 months was a key reason (17.4% of cases) for maintaining a nr-IPG. Patients who have reached advanced stages of the disease or developed significant comorbidities may still benefit from DBS, and abruptly discontinuing treatment could lead to serious consequences [21]. For that reason, the management of IPG replacement in these cases is still debated, and therapy discontinuation strategies are not always feasible. Traditionally, advanced age and late-stage PD with dementia have been reasons to prefer nr-IPGs to reduce the caregiving burden on patients. To corroborate this, evidence shows that surgical replacement has proven to be relatively safe at this stage [22]. On the other hand, previous research has demonstrated that caregivers can effectively handle the recharging of r-IPGs without affecting satisfaction levels or complication rates [19]. Therefore, a robust social and healthcare support network could make r-IPGs as well beneficial for patients in the later stages of the disease, potentially reducing the risk of multiple surgeries.
It is important to emphasize that the data and considerations presented here do not aim to establish the superiority of one type of IPG over the other, but rather to align with current trends in DBS of promoting careful case-by-case assessment rather than strict indications [11] even in the IPG choice. The majority (89.2%) of nr-IPG users in our study had their first DBS implantation between 2006 and 2011 when r-IPGs were not yet available in our country (r-IPGs became available at our hospital in 2012); for this reason, despite the aforementioned limitations, the data appear to be more reliable only for assessing satisfaction in absolute terms. These patients were likely offered the opportunity to eventually switch to r-IPGs during subsequent replacements, but by that time, they were older and less independent, which probably influenced their decision to stick with the IPG type they were familiar with, as demonstrated by the fact that concerns about the recharging process were a key factor for 57.2% of nr-IPG patients who chose not to switch (Figure 3b). Furthermore, the two samples from the DBS population were not homogeneous in certain baseline aspects. The age at the time of intervention and the disease severity were significantly lower in the r-IPGs group; this is likely due to a gradual shift in eligibility criteria for DBS together with a progressive increase in expertise, which resulted in the selection of patients at less advanced stages of the disease. In particular, it is highly plausible that age at the time of implantation may have influenced patients’ expectations and, consequently, their level of satisfaction, as well as contributed to a subjective variation in what is perceived as acceptable when assessed at follow-up compared to baseline. Moreover, although there are no differences in LEDD at baseline and follow-up between groups, a more pronounced reduction is observed in the r-IPG group, and its possible contribution to a higher degree of satisfaction cannot be excluded. Finally, a possible subjective preference of patients for different manufacturers was not assessed. These elements indeed make it impossible to assess a fully reliable comparison of the satisfaction levels reported between the two IPGs, which, in any case, is not among the objectives of the study.
In our cohort, IPG infection was the most meaningful complication after DBS implant with an incidence during all surgical procedures of 4.08%, as in line with previous studies (ranging from 2.01% to 6.4%) [19, 23–26]. Comparing the IPG types, akin to Frizon et al. [26], we confirmed a lower infection rate in r-IPG than in nr-IPG, although the latter showed a decreasing trend during the subsequent IPG replacements. These data are not in line with previous reports which have shown an increasing rate of infections with the number of replacement procedures [23–25, 27] Similarly, a higher rate of complications during hospitalization for IPG replacements was detected in nr-IPG groups (4 vs. 0). A limitation due to the progressive improvement of surgical techniques, care practices, and technologies over the years constrains the interpretation of the analyses reported here. Although part of the surgical procedures and complications analyzed in the study occurred after both types of IPG were introduced to the market, some of the initial implant procedures took place during a period when only one type of IPG (NR) was available, lacking a control sample. Taking these limitations into account, our data on long-term follow-up seems to confirm literature data of more favorable outcomes in r-IPG over nr-IPG because of fewer clinical risks (withdrawal syndrome after IPG exhaustion or sudden failure), less infective risk, and less hospitalization stay and cost [28–30].
In addition to the retrospective nature of this study, the monocentric design restricted the number of patients analyzed. Lastly, patient satisfaction and experience were assessed from a subjective qualitative point of view due to the absence of a validated scale for these evaluations, and the progressively increasing dropout rate among Nr-IPG carriers prior to the commercial availability of rechargeable devices may have affected the results.
5. Conclusion
Long-term observations show that the overall satisfaction with IPGs among DBS carriers remains stable, although r-IPG users report higher satisfaction. The discomfort associated with recharging is likely to be less of a factor when evaluating IPG choices, and the need for multiple surgeries for battery replacements and the size of the device, even if it could still be an issue, do not affect satisfaction with nr-IPGs over time. Whenever possible, optimal shared decision-making involves offering patients a choice between r-IPGs and nr-IPGs to enhance patient satisfaction and adherence to treatment. Additionally, r-IPGs are associated with fewer complications and shorter hospital stays, even in long-term evaluations.
Further studies on the long-term assessment of IPG carriers are needed to better identify individuals who may benefit more from different types of IPG, and observational analysis on long-term experience is required to determine whether clinicians should consider factors like age, cognitive decline, and support networks holistically rather than in isolation when advising on IPG options.
Ethics Statement
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Conflicts of Interest
Mariachiara Sensi receives speaking honoraria from AbbVie, Boston Scientific, and Bial. She receives advisory board fees from Bial. All other authors report no conflict of interest or disclosures.
Author Contributions
Conceptualization: Pietro Antenucci, Mariachiara Sensi; data collection and methodology: Pietro Antenucci, Fabiana Colucci; analysis and interpretation of results: Pietro Antenucci, Ilaria Casetta, Mariachiara Sensi; writing—original draft preparation: Pietro Antenucci; writing—review and editing: Mariachiara Sensi, Ilaria Casetta; supervision: Fabiana Colucci, Mariachiara Sensi. All authors reviewed the results and approved the final version of the manuscript.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




