Efficacy and Safety of Thalamotomy for Focal Hand Dystonia: A Systematic Review
Abstract
Background: Focal hand dystonia (FHD) has a significant impact on the hand motor function, especially for writers and musicians. Recently, many neurosurgeons have used thalamotomy to treat refractory FHD; this systematic review is aimed at assessing the efficacy and safety of thalamotomy for FHD.
Methods: PubMed, Medline, and Embase were searched to select relevant studies concerning thalamotomy for FHD. Demographic characteristics, surgical parameters, efficacy, and safety were extracted.
Results:A systematic review was performed including 254 patients among 15 studies. The writer’s cramp rating scale (WCRS), writing movement scores (WMS), and symptom severity scores (SSS) were assessed for hand dystonia disability. Besides, Tubiana musician’s dystonia scale (TMDS) and task-specific focal hand dystonia’s scale (TSFD) were assessed for hand motor performance. Transient complications were reported in 48 patients (18.9%) with permanent complications occurring in nine cases (3.5%).
Conclusion: Thalamotomy is an alternative option for FHD. Thalamotomy can alleviate hand dystonia disability and improve hand motor function. Although thalamotomy has certain complications, the incidence of permanent complications is relatively low. Thalamotomy needs to be fully evaluated before surgery to ensure safety.
1. Introduction
Focal hand dystonia (FHD) is a neurologic condition that affects abnormal hand muscle contractions, leading to movement disorder or posture disorder [1, 2]. FHD often manifests as focal task-specific dystonia (TSFD), a condition performing uncontrolled activation of muscle groups associated with specific, frequently overpracticed tasks, which would affect daily life and professional career [3]. It can be classified as writers’ dystonia and musicians’ dystonia [4]. Although the prevalence of FHD was relatively low which was about 8–9 per 100,000 individuals, it could have a significant impact on the professional career of writers and musicians, placing significant physical and psychological burdens on patients [5–8].
FHD treatment includes oral medications, botulinum toxin, and surgery [9]. Botulinum toxin was reported to be effective in 70% of patients with FHD [10]. However, botulinum toxin is often limited by hypotonia of the muscle spindle, and lesion surgery and DBS are choices for refractory FHD [9, 11–15]. In recent years, some neurosurgeons have used lesion surgery to treat refractory FHD and have achieved quite good therapeutic effects, especially thalamotomy [16–30]. Seven patients with writer’s dystonia having difficulty in performance received ventrooralis (Vo) thalamotomy, and all patients had a significant reduction in dystonia symptoms persistently [18]. A study consisting of 171 consecutive patients with TSFD who underwent Vo thalamotomy yielded similar results [23]. Nevertheless, apart from a review that briefly summarized thalamotomy for FHD, no detailed description of the systematic review was retrieved to provide a detailed description of thalamotomy for FHD [9]. Therefore, we performed this systematic review to assess the efficacy and safety of thalamotomy in patients with FHD.
2. Materials and Method
2.1. Search Strategy
Followed by the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a systematic review was conducted among thalamotomy for FHD. The systematic search and data extraction were performed by R.Y. and B.X. independently. The search strategy was designed using the keywords “thalamotomy,” “lesion,” “ablation,” and “hand dystonia” and linking words “AND” or “OR” among databases PubMed, Medline, and Embase, to include relevant articles published up to May 8, 2024.
2.2. Study Selection
To identify the desirable articles related to thalamotomy for FHD, R.Y. and B.X. made the study selection independently. After the removal of duplicate articles, titles and abstracts were screened to determine whether the full texts of articles should be checked. The articles would be reserved if they met (I) human patients diagnosed with FHD including writers’ dystonia and musicians’ dystonia and (II) these received thalamotomy. The articles would be deleted if they met the following: (I) not English; (II) secondary dystonia or not hand dystonia; (III) not thalamotomy; (IV) not about the therapy; (V) meet, book, conference, review, etc.
2.3. Data Extraction and Statistical Analysis
Interesting data was extracted by two reviewers independently: (I) demographic characteristics: age, duration of dystonia, inclusion criterion of patients, rating scales; (II) surgery parameters: surgery location, thalamotomy methods; (III) surgery efficacy and complications.
We have attempted to perform a meta-analysis to explore the efficacy of thalamotomy for FHD. Nevertheless, Begg’s test (p < 0.05) and Egger’s test (p < 0.05) indicated the big bias of the included sample by using StataMP 17 software. Limited by the lack of randomized controlled trials and the bias of the sample, the meta-analysis was inapposite, so a systematic review was performed.
3. Results
3.1. Search Selection
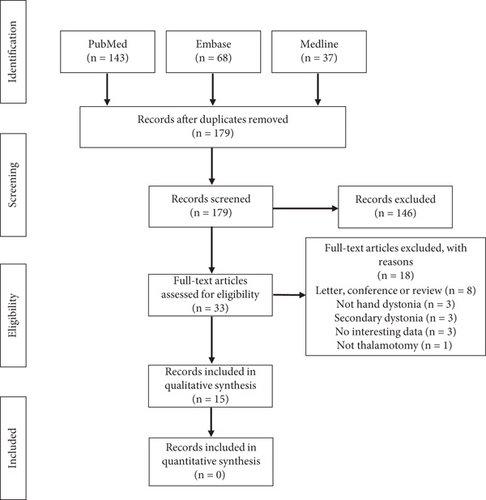
A total of 248 studies were yielded through search databases (PubMed: 143, Embase: 68, Medline: 37). After removing duplicates, 179 articles remained. When screening titles and abstracts, 146 papers did not satisfy the inclusion criteria. After screening full texts of 33 remaining articles, 18 studies that met the exclusion criteria above have been deleted: eight reported the content of the letter, conference, or review; four did not describe hand dystonia or thalamotomy; three summarized the secondary dystonia; and three did not contain interesting data. Finally, 15 studies met the inclusion criteria and were included in the systematic review (Figure 1).
3.2. Demographic Characteristics
The basic demographic characteristics are shown in Table 1. The 15 remaining articles published between 2003 and 2021 showed 254 patients with FHD who received thalamotomy. The included patients were diagnosed with FHD, focal task-specific dystonia, musician’s dystonia, and writer’s cramp, and their conditions were evaluated by writer’s cramp rating scale (WCRS), Tubiana musician’s dystonia scale (TMDS), writing movement scores (WMS), symptom severity scores (SSS), unified dystonia rating scale (UDRS), and task-specific focal hand dystonia’s scale (TSFD). Besides, one study reported hand dystonia by using neurological conditions and another one just did not describe the hand dystonia in detail.
| Author | Year | Sample | Age | Duration | Inclusion criterion | Rating scales | Surgery location | Surgery methods | Complication |
|---|---|---|---|---|---|---|---|---|---|
| Asahi, T. | 2014 | 2 | 23.62 | 5.10 | Refractory writer’s cramp | Writer’s cramp rating scale | Vo thalamotomy | Thermocoagulation at 70°C for 30 s | None |
| Asahi, T. | 2018 | 1 | 37 | 15 | Refractory focal task-specific dystonia | Tubiana musician’s dystonia scale | Vo thalamotomy | Thermocoagulation at 70°C for 30 s | None |
| Doshi, P.K. | 2017 | 7 | 31.71 | 4.36 | Refractory focal hand dystonia | Writing movement scores, symptom severity scores | Vo thalamotomy | Thermocoagulation at 70°C for 60 s | None |
| Doshi, P.K. | 2017 | 5 | 32 | 3.7 | Refractory focal hand dystonia | Writer’s cramp rating scale, symptom severity scores | Vo thalamotomy | Thermocoagulation at 70°C for 60 s | None |
| Hirato, M. | 2018 | 8 | 43.5 | 12.75 | Focal arm and hand dystonia | Unified dystonia rating scale | VIM-VO thalamotomy | Thermocoagulation at 65°C for 10–20 s | Transient dysarthria |
| Horisawa, S. | 2016 | 2 | 50,48 | 7.3 | Musician’s dystonia | Tubiana musician’s dystonia scale | Vo thalamotomy | NA | Transient dysarthria, verbal recall disturbance, hypophonia |
| Horisawa, S. | 2016 | 4 | 37.25 | 4.25 | Hairdresser’s task-specific dystonia | Neurological conditions | Vo thalamotomy | Thermocoagulation at 70°C for 30 s | Permanent and transient dysarthria |
| Horisawa, S. | 2019 | 171 | 37.1 | 7.9 | Task-specific focal hand dystonia for more than 1 year | Task-specific focal hand dystonia scale | Vo thalamotomy | Thermocoagulation at 70°C for 30 s | Permanent dysarthria, weakness(foot), dysesthesia (hand), verbal recall disturbance, etc. |
| Horisawa, S. | 2013 | 15 | 41.5 | 8.3 | Refractory task-specific focal hand dystonia | Tubiana musician’s dystonia scale | Vo thalamotomy | Thermocoagulation at 75°C for 30 s | Permanent hemiparesis |
| Horisawa, S. | 2021 | 10 | 43.2 | 9.8 | Severe focal hand dystonia (TMDS < 3, WCRS > 8) | Writer’s cramp rating scale, Tubiana musician’s dystonia scale, arm dystonia disability scale | Vo thalamotomy | At least two focused ultrasound sonications up to 55°C | Permanent dysarthria, transient unsteady gait, transient facial palsy, etc. |
| Horisawa, S. | 2018 | 1 | 35 | 9 | Musician’s dystonia | Tubiana musician’s dystonia scale | Vo thalamotomy | 13 focused ultrasound sonications at 63°C | None |
| Kim, M.J. | 2008 | 4 | 35.5 | NA | Focal hand dystonia | NA | Vo thalamotomy | Thermocoagulation at 70°C for 70 s | Transient left leg tingling sense |
| Shimizu, T. | 2018 | 4 | 25–44 | 7 | Focal hand dystonia for > 2 years and was refractory, aged at 18–80 years, affected careers | Writing movement scores | Vo thalamotomy | Thermocoagulation at 70°C for 60 s | Transient paresthesia of the lower limbs |
| Taira, T. | 2003 | 8 | 32.1 | 4 | Refractory hand dystonia cramp | Writer’s cramp rating scale | Vo thalamotomy | Thermocoagulation at 75°C for 30 s | Transient paresis, dysesthesia |
| Taira, T. | 2003 | 12 | 33.1 | 4.5 | Refractory hand dystonia cramp | Writer’s cramp rating scale | Vo thalamotomy | Thermocoagulation at 75°C for 30 s | Transient hemiparesis and dysarthria |
3.3. Surgical Parameters
In 14 studies, ventral oral nucleus (Vo) thalamotomy was performed for FHD. Besides, one study showed ventralis intermedius nucleus (VIM)–ventralis oralis nucleus (Vo) thalamotomy. In addition, thalamotomy was performed by stereotactic thermocoagulation at 65°C–75°C among 12 studies. The duration of the thermocoagulation ranged from approximately 10 to 70 s. Moreover, in two studies, stereotactic focused ultrasound sonications were administered to the Vo nucleus. One study did not contain the detailed process of thalamotomy.
3.4. Efficacy of Thalamotomy
The efficacy of thalamotomy for FHD is shown in Table 2. WCRS, WMS, and SSS were assessed for hand dystonia disability preoperatively and postoperatively. WCRS of 36 patients among five studies had a significant improvement [16, 19, 25, 29, 30]. Especially in a study published in 2003, eight patients experienced complete remission on WCRS after approximately 1 year of follow-up. Moreover, WMS and SSS were evaluated on 11 patients in two studies and 12 patients in two articles [18, 19, 28]. These patients had an important reduction in scores of WMS and SSS after 1–2 years of follow-up. Besides, in a 4.7-year follow-up study, UDRS scores of eight patients decreased from 3.1 to 0.4, indicating a significant reduction in hand dystonia [20]. In addition, eight patients showed varying degrees of improvement in hand dystonia in two studies [22, 27].
| Author | Year | Follow-up | Rating scales | N preoperatively | Scores preoperatively | SD preoperatively | N postoperatively | Scales postoperatively | SD postoperatively |
|---|---|---|---|---|---|---|---|---|---|
| Asahi, T. | 2014 | NA | Writer’s cramp rating scale | 2 | 6 | 1 | 2 | 0 | 0 |
| Asahi, T. | 2018 | 1 | Tubiana musician’s dystonia scale | 1 | 3 | 0 | 1 | 5 | 0 |
| Doshi, P.K. | 2017 | 2.57 | Writing movement scores | 7 | 11.14 | 7.77 | 7 | 2.29 | 2.25 |
| Symptom severity scores | 7 | 14.71 | 10.57 | 7 | 2.43 | 2.61 | |||
| Doshi, P.K. | 2017 | 2.23 | Writer’s cramp rating scale | 5 | 20.4 | 4.22 | 5 | 2.6 | 2.24 |
| Symptom severity scores | 5 | 29.8 | 2.48 | 5 | 12 | 2.1 | |||
| Hirato, M. | 2018 | 4.7 | Unified dystonia rating scale | 8 | 3.1 | 0.6 | 8 | 0.4 | 0.7 |
| Horisawa, S. | 2016 | 1, 3.33 | Tubiana musician’s dystonia scale | 2 | 2, 2 | 0 | 2 | 5, 5 | 0 |
| Horisawa, S. | 2016 | 1.42 | Neurological conditions | 4 |
|
NA | 4 |
|
NA |
| Horisawa, S. | 2019 | 3.95 | Task-specific focal hand dystonia scale | 171 | 1.72 | 0.57 | 72 | 4.39 | 1.07 |
| Horisawa, S. | 2013 | 2.57 | Tubiana musician’s dystonia scale | 15 | 2.7 | 0.6 | 15 | 4.6 | 0.8 |
| Horisawa, S. | 2021 | 1 | Writer’s cramp rating scale | 9 | 6.1 | 2.9 | 9 | 1.8 | 3.3 |
| Tubiana musician’s dystonia scale | 5 | 1.4 | 0.5 | 5 | 5 | 0 | |||
| Arm dystonia disability scale | 10 | 58.70% | 14.30% | 10 | 81.60% | 22.90% | |||
| Horisawa, S. | 2018 | 1 | Tubiana musician’s dystonia scale | 1 | 1 | 0 | 1 | 5 | 0 |
| Kim, M.J. | 2008 | 9 | NA | Two patients’ dystonia disappeared; two patients’ dystonia significantly reduced | |||||
| Shimizu, T. | 2018 | 1.25 | Writing movement scores | 4 | 22.5 | 2.18 | 4 | 0 | 0 |
| Taira, T. | 2003 | 1.09 | Writer’s cramp rating scale | 8 | 13.1 | 6.3 | 8 | 0 | 0 |
| Taira, T. | 2003 | 13.1 | Writer’s cramp rating scale | 12 | 13.1 | 6.3 | NA | 0.8 | NA |
TMDS and TSFD were assessed for hand motor performance. Twenty-four patients in five studies were evaluated for hand performance using the TMDS [17, 21, 24–26]. Patients were likely able to play normally postoperatively compared to stop playing due to blockage preoperatively. Besides, in a study involving 171 patients, TSFD scores increased from 1.72 to 4.39, showing normal hand function after thalamotomy [23].
3.5. Safety and Complications
Transient and permanent complications were reported in 55 patients (21.7%), and the specific details of complications are shown in Table 3. Transient complications were defined as those resolving within the follow-up period [25]. Forty-eight patients experience transient complications including dysarthria, verbal recall disturbance, hypophonia, body weakness, facial palsy, unsteady gait, intracranial hemorrhage (ICH), leg tingling sense, paresthesia, dysesthesia, and surgery-related complications. Besides, nine patients experience permanent complications including dysarthria, foot weakness, hand dysesthesia, verbal recall disturbance, and hemiparesis.
| Author | Year | Patients | Transient complications | Permanent complications |
|---|---|---|---|---|
| Hirato, M. | 2018 | 1 | Transient dysarthria (n = 1) | |
| Horisawa, S. | 2016 | 2 | Transient dysarthria and verbal recall disturbance (n = 1), hypophonia (n = 1) | |
| Horisawa, S. | 2016 | 2 | Transient dysarthria (n = 1) | Mild dysarthria (n = 1) |
| Horisawa, S. | 2019 | 34 | Dysarthria (n = 10), verbal recall disturbance (n = 8), hemibody weakness (n = 6), foot weakness (n = 3), facial palsy (n = 2) | Dysarthria (n = 2), foot weakness (n = 2), hand dysesthesia (n = 1), verbal recall disturbance (n = 1) |
| Horisawa, S. | 2013 | 1 | Permanent hemiparesis (n = 1) | |
| Horisawa, S. | 2021 | 5 | Dysarthria (n = 2), unsteady gait (n = 1), dysarthria and unsteady gait (n = 1), facial palsy (n = 1) | Dysarthria (n = 1) |
| Kim, M.J. | 2008 | 2 | Left thalamus bleeding(n = 1), left leg tingling sense (n = 1) | |
| Shimizu, T. | 2018 | 4 | Transient paresthesia of the lower limbs (n = 4) | |
| Taira, T. | 2003 | 2 | Transient paresis and dysesthesia (n = 1), air embolism during surgery (n = 1) | |
| Taira, T. | 2003 | 2 | Mild right hemiparesis and dysarthria (n = 2) |
4. Discussion
This systematic review evaluated the efficacy and safety of thalamotomy for hand dystonia. Our results showed that thalamotomy could alleviate hand dystonia disability and improve hand motor function to varying degrees. Although our study suggested that thalamotomy was associated with varying degrees of complications, most were transient compared to a few permanent complications. Our research mainly included case series and case reports but lacked randomized controlled studies, so our study had a certain risk of bias. Therefore, we conducted a systematic review instead of a meta-analysis.
Bilateral pallidotomy has been reported in other studies of lesional surgery for dystonia. In a systematic review of bilateral pallidotomy for dystonia, 96% of patients showed improvement in the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS), and 15 of 21 patients showed improvement in disability scores [31]. In another article concerning pallidotomy for primary dystonia, unilateral pallidotomy had significant improvement in the BFMDRS scores from 11.2 preoperatively to 5.4 at the last follow-up (51.8% improvement) when 14.6 preoperatively to 3.8 at the last follow-up (74.0% improvement) for bilateral pallidotomy [32]. In our study, 254 patients with FHD had varying degrees of symptom improvement after thalamotomy. The patient’s hand dystonia disability showed significant improvement after surgery using the CRS, WMS, and SSS scales, and the hand’s motor performance ability went from cessation of performance preoperatively to normal performance postoperatively using the TMDS and TSFD scales. These results demonstrate the efficacy of lesional surgery for FHD.
The mechanism of thalamotomy for FHD is currently unclear. Some scholars speculated that the reorganization of structural connections such as the cerebellum, thalamus, basal ganglia, parietal lobe, and cingulate cortex areas leads to destructive changes in sensorimotor, resulting in FHD [33–35]. In particular, changes in sensory perception in the supplementary motor area (SMAp) may explain abnormal contraction of antagonist muscles and abnormal posture [36]. Functional connections were weakened in the left lateral premotor cortex, left thalamus, and left/right pallidum through fMRI, suggesting extensive changes in the basal ganglia thalamic cortical motor circuit [37–39]. Furthermore, the changes in these circuits may be due to abnormal time windows altering the action selection process [40]. Thalamotomy may have damaging effects on these circuits, thereby improving FHD. Therefore, neurosurgeons always seem to use the thalamus as a target for FHD treatment rather than other targets due to the corticobasal ganglia-thalamo-cortical circuit [23].
In our study, 55 of 254 patients (21.7%) experienced transient or permanent complications. Nine patients (3.5%) had permanent complications including dysarthria, foot weakness, hand dysesthesia, verbal recall disturbance, and hemiparesis. Although the incidence of complications is relatively high, the incidence of permanent complications is relatively low. The result is similar to the complications of other lesion surgeries. Complications were reported in 20 of 100 patients, of which eight were permanent adverse events [31]. For ICH, a retrospective study, analyzing 721 procedures for movement disorders, reported an overall ICH rate of 5.1%, with symptomatic ICH occurring in only 1.1% of cases. For Vo thalamotomy specifically, the symptomatic ICH rate was 1.3%, and the prolonged deficit rate was 0.4% [41]. Overall, lesion surgery is relatively safe, but lesion surgery must be performed with caution to further reduce the probability of these complications.
Recurrence, defined as the deterioration of FHD scales after transient improvement, is also receiving increasing attention, as it directly affects clinical efficacy. Due to insufficient or incorrect coagulation, some patients with recurrence have significant therapeutic effects after the second thalamotomy. One article analyzing 171 patients reported 18 patients who experienced recurrence and 9 underwent a second thalamotomy of which 7 achieved improvement [23]. Other cases have also shown the same results [16, 22, 25, 29, 30]. The location and range of the target, as well as the thermocoagulation, have a significant impact on recurrence, so a careful surgical plan must be developed preoperatively.
In recent years, more and more neurosurgeons have been paying attention to deep brain stimulation (DBS) to treat dystonia. In a systematic review containing 35 articles, authors analyzed 319 patients who received pallidus internus (GPi)–DBS and 113 patients who received subthalamic nucleus (STN)–DBS. Results showed that both GPi and STN stimulation improved dystonia to varying degrees (standardized mean difference = 1.56 for GPi-DBS and standardized mean difference = 2.06 for STN-DBS) [42]. Similar results were seen with DBS for Meige syndrome [43]. One hundred and eighty-eight patients with Meige syndrome received GPi-DBS, and 110 received STN-DBS. In BFMDRS-M, the mean difference was 10.57 for GPi-DBS and 8.59 for STN-DBS. Besides, in BFMDRS-D, the mean difference was 5.96 for GPi-DBS and 4.71 for STN-DBS. However, there is no systematic evidence in the current literature to clarify the efficacy and safety of DBS treatment for FHD [44–47]. Furthermore, while DBS is reversible therapeutics, its long-term feasibility must also be considered. Since FHD often manifests at a relatively young age, typically in the 40s, factors such as device-related complications, long-term management, battery replacements, and costs highlight the importance of thalamotomy as a crucial treatment option [44].
Our study has several limitations. The major limitation is that only case series and case reports were available for inclusion in our study, which inevitably led to high positive results and publication bias. More randomized controlled trials with large samples are required to support the results. In addition, different assessment methods were used in different studies, and some assessment methods are highly subjective, which might lead to bias. A detailed and standardized scoring tool was recommended for evaluating FHD.
In conclusion, based on this systematic review, thalamotomy is an alternative option for FHD. Thalamotomy can alleviate hand dystonia disability and improve hand motor function. Thalamotomy has certain complications and it needs to be fully evaluated before surgery to ensure safety.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
W.W. raised the manuscript and managed the trial. R.Y. searched the database, performed data extraction, and drafted the manuscript. B.X. searched the database, performed data extraction, and revised the manuscript. X.S. did data analysis and prepared table. X.H. prepared figures and revised the manuscript. R.Y. and B.X. contribute equally to this work.
Funding
This work was supported by 135 Project of Outstanding Development of West China Hospital, Sichuan University (ZY2017307).
Acknowledgments
This work was supported by 135 Project of Outstanding Development of West China Hospital, Sichuan University (ZY2017307).
Open Research
Data Availability Statement
All relevant data are included in the manuscript.




