Prehospital Identification of Patients With Stroke—mNIHSS Versus FAST
Abstract
Introduction: Early identification of stroke is important. The Face Arm Speech Test (FAST) and the modified National Institutes of Health Stroke Scale (mNIHSS) are two tools for stroke identification.
Aim: The aims of this study are to investigate (a) whether the use of the mNIHSS in an emergency medical service (EMS) setting improves stroke/transient ischaemic attack (TIA) identification compared with the FAST, (b) to what extent “code stroke” is activated, and (c) which neurologic deficits/symptoms affect stroke identification.
Methods: The method used is a retrospective pre–post-implementation study. EMS stroke identification was examined before and after the introduction of the mNIHSS, replacing the FAST, for EMS stroke screening. The FAST was replaced with the mNIHSS on 1 December 2022. Patients ≥ 18 years of age, diagnosed with stroke/TIA from 1 January 2021 to 31 May 2023 and under the care of the EMS, not more than 72 h before hospital care, were included. Data was manually extracted from EMS medical records regarding whether the FAST or mNIHSS was performed and if stroke/TIA was identified by the EMS personnel. The association between the applied stroke screening tool and EMS identification of stroke/TIA was then studied.
Results: A total of 1849 EMS missions with a hospital-confirmed diagnosis of stroke/TIA were included. The most common diagnosis was ischaemic stroke, 59.4%. Haemorrhagic stroke constituted 10.8%, and TIA 29.8%. Stroke/TIA was identified in 82.5% of cases. When the mNIHSS was used for stroke assessment, stroke/TIA was identified in 87.6% of cases. The corresponding figure for the FAST was 88.4%. For patients in whom neurological symptoms were unassessed, or a method for assessment other than the mNIHSS/FAST was applied, the identification rate for stroke/TIA was 41.6%. When a physician was consulted, “code stroke” was activated in 58.6% of all cases. The corresponding figure for the mNIHSS was 57.2% and for the FAST 59.9%.
Conclusions: The stroke identification rate does not appear to differ between the FAST and mNIHSS. The FAST and mNIHSS result in “code stroke” activation to an equal extent. Speech impairment and arm or leg paresis appear to improve EMS stroke identification. Conversely, impaired balance, convulsions, and vertigo/dizziness are associated with a lower identification rate. Both initial EMS suspicion of stroke and the subsequent application of a stroke scale appear to facilitate stroke identification.
1. Introduction
There are 12 million incidents of stroke world-wide annually. Stroke is one of the most common causes of death with approximately seven million deaths per year globally [1, 2]. In Sweden, approximately 20,000 patients are diagnosed with stroke yearly. The corresponding number for transient ischaemic attack (TIA) is 9000 [3].
Stroke is classified into ischaemic or haemorrhagic stroke. Haemorrhagic stroke consists of intracerebral or subarachnoid haemorrhage, while ischaemic stroke is caused by an embolism or thrombosis [4]. Stroke is a time-sensitive condition, and especially in the case of ischaemic stroke, “time is brain” [5]. Treatment modalities for haemorrhagic stroke vary depending on the type of haemorrhage. Two potential approaches include open surgery and blood pressure management, often in combination, with minimally invasive coiling [6]. For ischaemic stroke, a favourable patient outcome is substantially reduced when reperfusion treatment is delayed or absent [7]. Acute stroke management needs, therefore, to be streamlined to minimise treatment delay [8, 9]. A cornerstone in acute stroke management is the activation of stroke teams when stroke is suspected, allowing immediate neuroimaging and, thereafter, initiation of reperfusion therapy, if indicated [5, 10]. In Sweden, about 80% of patients with stroke arrive at a hospital by ambulance [3, 11]. Prehospital identification of stroke and prenotification, activating “code stroke” and the hospital stroke team, improve several outcome measures, such as onset/door-to-needle time, thrombolysis/thrombectomy rate, and mortality [10, 12]. Furthermore, prehospital stroke identification allows direct transport to hospitals capable of stroke treatment [9, 10, 13] or thrombectomy when suspecting large vessel occlusion (LVO), bypassing hospitals not offering adequate capacity [9]. However, recent studies suggest that direct transport to thrombectomy-capable hospitals may not always provide a clear advantage [14, 15].
Thus, stroke identification is of the utmost importance early on in an emergency medical service (EMS) setting. However, not all strokes are identified by the EMS personnel [3, 11], and some patients with stroke/TIA are not even transported to a hospital [16]. Identification of stroke by the EMS personnel on scene varies from 59% to 80% [11, 12, 17, 18]. Several stroke scales are used for the identification of stroke, such as the Face Arm Speech Test (FAST) and the National Institutes of Health Stroke Scale (NIHSS) [19]. There is also a modified version of the NIHSS, mNIHSS, in which items lacking interrater reliability have been excluded. This favours prehospital use and reduces the time needed to perform the test [20, 21]. There is no unequivocal evidence as to which stroke scale is best for identifying stroke [13, 19]. Investigation is thus needed to show whether or not the use of different stroke scales affects EMS stroke identification.
The aim of this study was to investigate whether the use of the mNIHSS in the EMS setting improves stroke/TIA identification compared with the FAST, to what extent “code stroke” is activated, and which neurologic deficits/symptoms affect stroke identification. The hypothesis is that the use of the mNIHSS improves stroke/TIA identification and increases “code stroke” activation compared with the FAST.
2. Materials and Methods
2.1. Design
This is a retrospective pre–post-implementation study.
2.2. Study Setting
Patients were recruited from the region of Halland, Sweden, with 330,000 inhabitants and an area of 5500 km2. About 33,000 patient-related EMS missions are carried out annually (transports between care facilities excluded). There are approximately 20 ambulances operating daily. The EMS is staffed with at least one registered nurse, in most cases with a 1-year master’s level specialist training in prehospital emergency care. They commonly work with another nurse, but in some cases with an emergency medical technician (EMT). The EMS nurse is always in charge of patient assessment and care. Henceforth, the term “EMS personnel” will be used to describe those providing care and assessment, regardless of personnel category. There are two emergency hospitals, both capable of handling EMS “code stroke” with computed tomography (CT) and thrombolysis treatment. Thrombectomy cannot be performed at any of the hospitals in Halland. Therefore, patients deemed suitable for thrombectomy are transported to hospitals in adjacent regions.
If EMS personnel suspect stroke, an in-hospital physician is consulted and patient characteristics and symptoms are described. The physician then decides whether “code stroke” should be activated or not. If activated, a hospital stroke team is allocated to the radiology department. The EMS transports the patient directly to the radiology department, meeting up with the stroke team and bypassing the emergency department (ED). On arrival to the radiology department, a CT scan is performed, and if indicated, thrombolysis carried out. If thrombectomy is deemed appropriate, the patient is transported to a hospital with thrombectomy capabilities in an adjacent region.
Until November 2022, EMS regional guidelines stated that the FAST should be used for stroke assessment. The FAST has been used by the regional EMS for more than 10 years. On 1 December 2022, the guidelines were changed and the use of the mNIHSS was instead advocated. One month prior to this change in EMS guidelines, the EMS personnel received verbal information regarding the introduction of the mNIHSS at ambulance station work meetings. EMS personnel also underwent a 10-min digital training programme in which the application of the mNIHSS was described using video instructions produced by two consultant physicians at the regional neurology clinic.
- •
Facial weakness—can the patient smile? Facial droop?
- •
Arm weakness—can the patient raise both arms equally?
- •
Speech—can the patient repeat the sentence “The weather is nice today”?
- •
Time—when did symptoms first appear?
In the mNIHSS, items with lower interrater reliability in the original NIHSS have been excluded, such as ataxia, facial paralysis, level of consciousness, and dysarthria [20]. Thus, it has become simpler and faster to use, while improving validity and reliability [21].
When the mNIHSS was introduced in Halland, it was incorporated as a checklist in the EMS digital record system, accessible during patient assessment (Table 1).
| Category | Question/observation | Further instructions | Score |
|---|---|---|---|
| Level of consciousness—questions |
|
|
|
| Level of consciousness—commands |
|
|
|
| Gaze | Horizontal extraocular movements | First, observe eye position; then, test eye movements to the right and left by having the patient follow the examiner’s finger |
|
| Visual fields | Introduce visual stimulus or threat to patient’s visual field quadrants | Face to face, the patient looks at the examiner’s nose. Hold up your hands simultaneously in both visual fields and wave with one hand only and then with both hands repeatedly. If cooperation is lacking, try moving your hand “threateningly” towards the patient’s eye from the side |
|
| Motor drift—arm | Patient in a lying position. Lift each arm 45° and hold for 10 s | Count out loud |
|
| Motor drift—leg | Patient in a lying position. Straight leg raise on each side to 30 , hold for 5 s | Count out loud |
|
| Sensory | Examine with a firm “pinch” on the right and left arm and leg |
|
|
| Language and dysarthria | Assess during the conversation | If necessary, ask the patient to name simple objects |
|
| Total score: | |||
- •
Patient fully conscious.
- •
Positive item in the FAST, one point or more for the mNIHSS.
- •
Symptom onset within 24 h.
The decision to activate “code stroke” is then made by the physician based on information provided by the EMS regarding symptoms, age, comorbidities, duration, etc.
2.3. Data Collection
EMS missions carried out from 1 January 2021 until 31 May 2023 were included in the study.
- •
≥ 18 years of age
- •
International Classification of Diseases (ICD) 10 Diagnosis Codes I60, I61, and I63 (stroke) and G45.0–G45.3 or G45.8–9 (TIA) at the ED or at hospital discharge according to the attending physician
- •
EMS care not more than 72 h prior to hospital care
Data was collected using automated data extraction from the Region Halland data warehouse [22]. This technique has been used previously with reliable results [23, 24]. Data extraction identified 1998 eligible EMS missions. A data sample extracted from 20 EMS missions was compared with the original data source, that is, prehospital and hospital medical records, to ensure accuracy. Automated data extraction was used for the following variables: patient age and sex, EMS mission priority and time stamps, vital signs, EMS stroke/TIA identification, mortality, and ICD 10 diagnosis code at the ED and at hospital discharge.
Automated data extraction was complemented by manual data collection in which EMS and hospital medical records were scanned. Data was extracted manually regarding whether the FAST or mNIHSS was performed, which neurological defects were discovered according to the EMS personnel, whether symptoms had occurred more than 24 h earlier, if the hospital physician had been contacted, and if “code stroke” had been activated. Data on neurological symptoms was acquired from either the mNIHSS checklist, being integrated in the EMS digital record, or by analysing free text when using FAST, or if the mNIHSS checklist had not been correctly completed. During manual data extraction, the accuracy of the automated data extraction was further validated. Manual data extraction was preceded by thorough discussions in the research group on how data and variables should be interpreted to ensure correct data extraction. When uncertainty arose, those specific cases were discussed in the research group for consensus.
If stroke/TIA was indicated as the reason for EMS contact in the EMS triage system (Rapid Triage and Treatment System (RETTS)) [25, 26], this was equated with EMS identification of stroke/TIA. Cases in which stroke/TIA were not identified by the EMS personnel were carefully checked in the hospital records to ensure that stroke/TIA symptoms had not debuted during the hospital stay. If so, the case was excluded. Cases in which ICD10 stroke diagnoses were made only at the ED, and not at discharge from the hospital ward, were also extensively checked to ensure diagnostic accuracy by the ED physician. Patients with an ED diagnosis of stroke/TIA, unconfirmed by a main diagnosis of stroke/TIA on hospital discharge but still included in the study, referred to patients sent directly from the ED to a hospital outside the county. Likewise, patients receiving another main diagnosis on hospital discharge due to, for example, an in-hospital accident causing a hip fracture were still diagnosed with stroke/TIA.
In total, 1998 EMS missions concerning patients diagnosed with stroke/TIA within 72 h were identified. After manual data extraction, 149 EMS missions were excluded. A total of 1849 EMS missions remained. Of these, 370 were assessed with the mNIHSS and 1253 with the FAST. There were also 226 EMS missions, which either lacked a stroke assessment or were neurologically assessed without using the FAST or mNIHSS. The latter group was excluded from the comparative analyses of the stroke identification rate of the FAST versus mNIHSS (Figure 1).
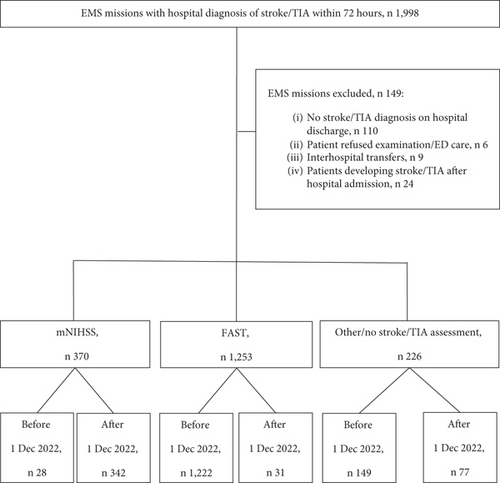
2.4. Endpoints
- •
EMS identification of stroke/TIA. Defined as patient assignment to RETTS triage code “suspicion of stroke” by the EMS personnel.
- •
Emergency Medical Dispatch Centre (EMDC) identification of stroke/TIA. Defined as suspected stroke/TIA indicated as reason for ambulance dispatch.
- •
Physician consulted regarding “code stroke” activation. Defined as a phone call between EMS personnel and hospital physician being denoted in the EMS medical record.
- •
“Code stroke” activation. Defined as direct transport to the radiology department for a computer tomography scan following a decision by a hospital physician.
2.5. Sample Size
A power calculation was performed to determine the necessary sample size for effectively addressing the study’s primary goal: to assess whether the mNIHSS improves EMS identification of stroke/TIA compared with the FAST. However, the current rate of stroke/TIA identification by EMS in Halland using FAST is unknown. A recent study from an adjacent county found that EMS personnel identified 80% of stroke/TIA cases when using the mNIHSS. It was hypothesised that the FAST would have a lower identification rate than the mNIHSS. To demonstrate a difference of seven percentage points in stroke identification rate—specifically, an increase from 73% with the FAST to 80% with the mNIHSS—a sample size with a 2:1 ratio was calculated. This required 418 EMS missions in the mNIHSS group (the intervention group) and 878 EMS missions in the FAST group (the control group). The analysis was aimed at a statistical power of 80% with a Type I error rate of 5%.
2.6. Data Analysis
For descriptive analyses of categorical variables, frequencies and percentages are presented. Continuous variables are presented by mean, standard deviation, median, and quartiles (Q1–Q3). For group comparison of categorical data, Pearson’s chi-square, Fisher’s exact test, and logistic regression were used. For continuous data, independent t-test or Mann–Whitney’s U-test was applied.
Cases in which both the FAST and mNIHSS were used by EMS personnel were considered mNIHSS cases, since mNIHSS includes most items assessed in FAST. It can thus be considered an extended FAST. For the variables “EMS time on scene” and “EMS transportation time,” values of less than 1 min were excluded. This is because such short time intervals are unrealistic and are a known error linked to incorrect status reports by EMS personnel.
p values below 0.05 were considered statistically significant. IBM SPSS Statistics for Windows version 29 (IBM Corp., Armonk, New York, United States) was used for all analyses.
2.7. Ethics
The study was approved by the Swedish Ethical Review Authority (Dnr 2022-06745-01).
3. Results
3.1. Cohort Characteristics
Of the initial 1998 missions, 149 were excluded after manual review (no stroke/TIA diagnosis at hospital discharge, n 110; patient refused examination/ED care, n 6; interhospital transfers, n 9; and patients developing stroke/TIA after hospital admission, n 24) (Figure 1). In total, 1849 EMS missions concerning 1694 unique patients were included in the study. Median age was 79 years, and 55.7% were men (Table 2).
| All (%) (n) | mNIHSS (%) (n) | FAST (%) (n) | Other (%) (n) | |
|---|---|---|---|---|
| All | 100 (1849) | 20.0 (370) | 66.8 (1253) | 12.2 (226) |
| Age | ||||
| Median (Q1, Q2) | 79 (72, 85) | 79 (71, 85) | 79 (72, 85) | 80 (73, 86) |
| Mean (±SD) | 77.5 (±11.5) | 77.0 (±11.7) | 77.4 (±11.4) | 78.7 (±11.9) |
| Sex | ||||
| Men | 55.7 (1029) | 58.1 (215) | 55.7 (698) | 51.3 (116) |
| Women | 44.3 (820) | 41.9 (155) | 44.3 (555) | 48.7 (110) |
| Priority by EMDC | ||||
| Priority 1 | 81.6 (1508) | 80.6 (298) | 84.3 (1056) | 68.2 (154) |
| Priority 2 | 17.3 (320) | 17.8 (66) | 14.8 (186) | 30.0 (68) |
| Priority 3 | 1.1 (21) | 1.6 (6) | 0.9 (11) | 1.8 (4) |
| Symptoms debut ≤ 24 h before EMS arrival | 91.3 (1689), 14a | 93.5 (344), 2a | 92.9 (1160), 5a | 81.9 (185), 7a |
- Abbreviations: EMDC = Emergency Medical Dispatch Centre; FAST = Face Arm Speech Test; mNIHSS = modified National Institutes of Health Stroke Scale.
- aMissing.
3.2. Diagnosis and EMS Stroke Identification
The most common diagnosis was ischaemic stroke, accounting for 59.4% of cases. Haemorrhagic stroke made up 10.8%, and TIA accounted for 29.8% (Table 3). When excluding TIA cases, ischaemic stroke represented 84.7%, and haemorrhagic stroke 15.3%.
| Stroke screening tool | EMS stroke/TIA identification ∗ | |||||
|---|---|---|---|---|---|---|
| Diagnosis at hospital discharge | All (%) (n) | mNIHSS (%) (n) | FAST (%) (n) | Other (%) (n) | Yes | No |
| All | 100 (1849) | 20.0 (370) | 66.8 (1253) | 12.2 (226) | 82.5 (1526) | 17.5 (323) |
| TIA | 29.8 (551) | 35.1 (130) | 30.7 (385) | 15.9 (36) | 90.6 (499) | 9.4 (52) |
| Ischaemic stroke | 59.4 (1099) | 55.7 (206) | 60.3 (756) | 60.6 (137) | 81.2 (892) | 18.8 (207) |
| Haemorrhagic stroke | 10.8 (199) | 9.2 (34) | 8.9 (112) | 23.5 (53) | 67.8 (135) | 32.2 (64) |
- Abbreviation: TIA = transient ischaemic attack.
- ∗Pearson’s chi2 two-sided p value < 0.001.
In 82.5% of cases, stroke/TIA was indicated as the reason for EMS contact. Other common reasons for EMS contact included vertigo/dizziness (5.7%) and nonspecific symptoms (2.6%) (Supporting Information 1). When excluding not fully conscious patients as being difficult to assess for other neurological symptoms, 83.4% were identified as a having stroke/TIA. The median time on the scene was shorter when stroke/TIA was identified by the EMS, 22.3 min compared to 24.3 min, when another reason for EMS contact was indicated.
The EMDC indicated that 72.1% of ambulance dispatches were due to suspected stroke/TIA. Additionally, 4.4% were dispatched for vertigo/dizziness, and 3.5% for trauma. When the EMDC indicated the dispatch as stroke/TIA, the EMS correctly identified stroke/TIA in 92.9% of these cases (Supporting Information 2). If the EMDC indicated a different reason for the dispatch, the EMS suspected stroke/TIA in 55.6% of the cases.
The EMS stroke/TIA identification rate was lower in haemorrhagic stroke, 67.8% compared with 81.2% for ischaemic stroke (Table 3).
In 37 cases, the patients were not transported by ambulance to the hospital. Of these, seven were referred to self-care, 13 to a primary healthcare centre, and 17 to an alternative mode of transportation to the ED. Ten patients were evaluated using the mNIHSS, 15 were evaluated with the FAST, four were evaluated with other methods, and eight received no neurological assessment. Thirteen patients were identified as having a stroke/TIA; eight with nonspecific symptoms, six with vertigo/dizziness, and three with headaches. Among these 37 cases, seven were later diagnosed with TIA.
3.3. Neurological Symptoms
Arm paresis (OR 4.6), speech/language impairment (OR 4.5), and sensory impairment (OR 4.4) were the symptoms most strongly associated with stroke/TIA identification by the EMS. In contrast, the presence of vertigo/dizziness (OR 0.2), convulsions (OR 0.3), and impaired balance (OR 0.6) significantly reduced the likelihood of stroke/TIA being identified (Table 4).
| Symptom assessed by EMS (%) (n), n = 1849 | Symptom present when assessed (%) (n), n = 1849 | EMS stroke/TIA identification if symptom is present, % (n), n = 1849 | EMS stroke/TIA identification OR (CI 95%)a | Symptom registered in FAST patients (%) (n), n = 1253 | Symptom registered in mNIHSS patients (%) (n), n = 370 | p valueb | |
|---|---|---|---|---|---|---|---|
| Arm paresis | 90.7 (1677) | 40.4 (677) | 95.7 (648) | 4.6 (3.1–6.8) | 42.5 (532) | 32.4 (120) | < 0.001 |
| Speech/language impairment | 94.0 (1738) | 43.6 (757) | 95.5 (723) | 4.5 (3.3–6.2) | 43.1 (540) | 45.7 (169) | 0.379 |
| Sensory impairment | 40.0 (740) | 34.9 (258) | 96.1 (248) | 4.4 (2.4–8.0) | 13.7 (172) | 20.3 (75) | 0.002 |
| Leg paresis | 90.0 (1665) | 35.6 (593) | 95.3 (565) | 3.7 (2.6–5.4) | 37.0 (463) | 29.5 (109) | 0.008 |
| Gaze palsy | 55.4 (1025) | 15.1 (155) | 94.2 (146) | 2.6 (1.4–4.8) | 6.6 (83) | 15.7 (58) | < 0.001 |
| Vision impairment | 40.1 (741) | 28.5 (211) | 88.2 (186) | 1.5 (1.1–2.1) | 8.9 (111) | 24.9 (92) | < 0.001 |
| Impaired understanding | 95.6 (1767) | 16.9 (298) | 87.2 (260) | 1.5 (1.1–2.0) | 15.2 (192) | 14.9 (55) | 0.829 |
| Confusion | 95.9 (1774) | 27.5 (487) | 83.0 (404) | 1.1 (0.9–1.4) | 24.0 (301) | 30.3 (112) | 0.015 |
| Impaired balance | 89.1 (1648) | 46.1 (759) | 80.8 (613) | 0.6 (0.5–0.8) | 43.7 (547) | 40.0 (148) | 0.212 |
| Convulsions | 96.5 (1758) | 1.5 (27) | 55.6 (15) | 0.3 (0.1–0.5) | 0.9 (11) | 0.5 (2) | 0.522 |
| Vertigo/dizziness | 89.3 (1652) | 17.4 (288) | 54.9 (158) | 0.2 (0.1–0.2) | 15.8 (198) | 14.9 (55) | 0.662 |
- Abbreviations: CI = confidence interval, FAST = Face Arm Speech Test, mNIHSS = modified National Institutes of Health Stroke Scale, OR = odds ratio.
- aLogistic regression.
- bChi2 FAST versus mNIHSS.
Arm and leg pareses were more commonly identified in patients assessed with the FAST, whereas sensory impairment, gaze palsy, vision impairment, and confusion were more frequently identified in patients assessed with the mNIHSS. Although these latter symptoms are not included in the FAST, they were still often evaluated in patients assessed with the FAST (Table 4). Among patients with a negative FAST, 54.1% had a positive finding for any of the items included in the mNIHSS but not in the FAST. In patients with negative FAST, but at least one positive mNIHSS-specific item, 65.5% were identified as having a stroke/TIA by the EMS.
3.4. mNIHSS Versus FAST
The mNIHSS was used in 370 EMS missions, while the FAST scale was applied in 1253 missions. In 226 cases, either neurological symptoms were not assessed, or an assessment method other than mNIHSS or FAST was used.
When the mNIHSS was used for stroke assessment, stroke or TIA was identified in 87.6% of cases, while the identification rate for the FAST was 88.4%. There was no statistically significant difference between the two methods. Excluding patients who were not fully conscious did not substantially change these identification rates (Table 5). For patients in whom neurological symptoms were not assessed at all or who underwent an assessment method other than the mNIHSS or FAST, the identification rate for stroke/TIA was 41.6%.
| mNIHSS (%) (n) | FAST (%) (n) | p valuea | Confidence interval (CI), 95%b | |
|---|---|---|---|---|
| All | 22.8 (370) | 77.2 (1253) | ||
| EMS stroke identification, all cases | 87.6 (324) | 88.4 (1108) | 0.647 | |
| EMS stroke identification, patients not being fully conscious excluded | 87.1 (305) | 88.5 (1036) | 0.508 | |
| EMS time on scene | ||||
| Median (Q1, Q3) | 24 (18.29) | 21 (16.28) | ||
| Mean (±SD) | 24.9 (±10.1) | 22.0 (±10.4) | (−4.0 to −1.7) | |
| Missing, n | 10 | 15 | ||
| Total EMS time: Dispatch to hospital arrival | ||||
| Median (Q1, Q3) | 56 (43.69) | 54 (42.68) | ||
| Mean (±SD) | 58.3 (±19.8) | 56.2 (±19.5) | (−0.2–4.4) | |
| Missing, n | 10 | 17 | ||
- aFisher’s exact test (two-sided).
- bTwo-sample independent t-test.
Time from EMS dispatch to hospital arrival was 2 min longer in the mNIHSS group compared with the FAST group: However, this difference was not statistically significant. Additionally, the time spent on scene was 3 min longer for the mNIHSS group than for the FAST group, with median times of 24 and 21 min, respectively (Table 5).
3.5. “Code Stroke” Activation
In 62.1% of all cases, a hospital physician was consulted for the activation of “code stroke.” When excluding patients not fully conscious, who had symptom onset more than 24 h prior, or were diagnosed with TIA at discharge, that is, those not eligible for “code stroke,” a physician was consulted in 74.6% of cases. In the mNIHSS group, a physician was contacted in 80.9% of cases, compared with 80.0% in the FAST group. When a physician was consulted, “code stroke” was activated in 58.6% of all cases, with 57.2% in the mNIHSS group and 59.9% in the FAST group. Excluding ineligible cases, the activation rate increased to 65.0%, 64.6% for mNIHSS and 65.1% for FAST (Table 6).
| mNIHSS (%) (n) | FAST (%) (n) | p valueb | |
|---|---|---|---|
| 22.8 (370) | 77.2 (1253) | ||
| Physician consulted, all cases (missing = 3) | 67.6 (250) | 68.5 (857) | 0.751 |
| Physician consulted, cases eligible for “code stroke”a | 80.9 (161) | 80.0 (571) | 0.841 |
| “Code stroke” activation, all cases | 38.6 (143) | 40.9 (513) | 0.434 |
| “Code stroke” activation, cases with physician contact only | 57.2 (143) | 59.9 (513) | 0.465 |
| “Code stroke” activation, cases with physician contact and eligible for “code stroke”a | 64.6 (104) | 65.1 (372) | 0.926 |
- aPatients not fully conscious, symptoms making their debut > 24 h, or TIA diagnosis on hospital discharge excluded.
- bFisher’s exact test two-sided.
For patients presenting with vertigo/dizziness, a physician was consulted in 45.8% of the cases, and “code stroke” was activated in 48.5% of these consultations. In comparison, for all cases combined, a physician was consulted in 62.1% of cases. The mNIHSS score was significantly associated with “code stroke” activation, with higher score increasing the probability for “code stroke” activation. For patients scoring ≥ 5 points, indicating eligibility for thrombectomy according to local guidelines, “code stroke” was activated in 79.3% of the cases, compared with 21.2% for patients scoring 0–4 points.
4. Discussion
In this study, the overall prehospital identification rate for stroke/TIA was 82.5%. There was no significant difference between mNIHSS (87.6%) and FAST (88.4%). When neither FAST nor mNIHSS was applied, the stroke/TIA identification rate was 41.6%. No association was observed between mNIHSS and FAST in relation to “code stroke” activation. Therefore, the hypothesis that using mNIHSS would improve stroke/TIA identification and increase “code stroke” activation compared with FAST was not supported by the results.
The overall prehospital stroke/TIA identification rate of 82.5% in our study is slightly higher than that reported by Magnusson et al. [12], who found an 80.4% identification rate in a similar Swedish EMS context. It is also notably higher than the rates reported in studies from the United States (74%) [17] and England (62%) [18].
When the EMDC indicated stroke/TIA at the time of ambulance dispatch, the EMS identification rate was 92.9%. It is reasonable to assume that cases noted as stroke/TIA by the EMDC are those with more obvious and distinctive stroke symptoms [27, 28], making them easier to identify both for the dispatchers and EMS personnel on scene. When stroke/TIA is indicated at dispatch, EMS personnel probably already suspect stroke/TIA, which increases the likelihood of applying a stroke scale.
Leg/arm paresis and speech/language impairment were commonly present in patients identified as having a stroke/TIA by the EMS personnel, partially corroborating findings from previous research [12, 17, 29]. Also, sensory impairment was overrepresented in patients identified as having a stroke by the EMS personnel. A finding that has not been widely reported. While there is no obvious explanation for this, it may be suspected that sensory impairment is a symptom which EMS personnel strongly associate with neurological damage, as this is commonly evaluated in the EMS setting when caring for patients with potential spinal injuries.
Sensory impairment, gaze palsy, vision impairment, and confusion were logically more commonly identified in patients assessed with the mNIHSS compared to those assessed with the FAST. As these signs and symptoms are not covered by any of the FAST items. Consequently, the use of the FAST carries a risk of missing these signs and symptoms. Our findings also showed that 54.1% of patients with a negative FAST had at least one mNIHSS-specific finding. This could be seen as support for the use of the mNIHSS. However, since the stroke identification rate surprisingly did not differ between the mNIHSS and FAST, it cannot be concluded that these symptoms are decisive for EMS stroke identification.
It was unexpected that the use of the mNIHSS did not appear to improve EMS stroke identification, given that the mNIHSS includes more assessment items and provides a more comprehensive neurological examination than the FAST. A similar finding was reported by Fothergill et al. [30], where the use of the Recognition of Stroke in the Emergency Room (ROSIER) score did not improve stroke identification by paramedics compared to the FAST. The reason why more comprehensive scores such as the mNIHSS or ROSIER do not seem to enhance EMS stroke identification remains unclear. One possible explanation is that EMS personnel may not always perform a full or accurate assessment of all mNIHSS items, leading to missed stroke symptoms, possibly due to lack of necessary skills in detecting neurological deficits. Another possibility is that when using the FAST, EMS personnel remain attentive to other neurological symptoms beyond those explicitly included in the FAST, resulting in a similar overall neurological evaluation regardless of whether the FAST or mNIHSS is used. This is supported by our finding that sometimes, mNIHSS-specific items were also assessed in patients evaluated with the FAST. Thus, it appears that neurological assessment is only partially guided by the chosen stroke screening tool and that EMS personnel may also be influenced by other factors when screening for stroke. Future research is needed to better understand these mechanisms and optimise prehospital stroke identification.
Vertigo/dizziness and haemorrhagic stroke were both associated with decreased stroke/TIA identification rate. Haemorrhagic stroke symptoms may differ from those caused by ischaemic stroke [31, 32]. Rathore et al. [32] report that arm paresis, speech/language impairment, and sensory impairment are more common among patients with ischaemic stroke than among those with haemorrhagic stroke. Since these symptoms seem to enhance EMS stroke/TIA identification, this may explain why haemorrhagic strokes are more frequently overlooked by EMS personnel. Patients with haemorrhagic stroke have also been reported to present with altered levels of consciousness more often than those with ischaemic stroke, a finding confirmed in our study. An altered level of consciousness complicates the assessment and examination of neurological symptoms, which may further explain why haemorrhagic strokes are less likely to be identified by EMS [33].
Vertigo/dizziness has previously been reported to impair stroke identification [11, 17, 33, 34]. Neither mNIHSS nor FAST includes vertigo/dizziness as a specific item. Kerber et al. [34] found that less than 1% of ED patients presenting with vertigo/dizziness were diagnosed with stroke/TIA, a trend similarly observed by Magnusson et al. [35] in the EMS setting. Ad hoc analyses of the study period show that 3054 EMS missions in Halland were categorised as vertigo/dizziness, of which 106 cases were later diagnosed with stroke/TIA, representing 3.5% of these patients. With the low incidence of stroke/TIA among vertigo/dizziness patients and vertigo/dizziness not being included as an item in either the mNIHSS or FAST, stroke/TIA is often missed in this group.
However, it remains crucial for EMS personnel to recognise that vertigo/dizziness can be a symptom of stroke/TIA and should not be disregarded. Consideration should be given to including vertigo/dizziness in prehospital stroke screening tools. A structured examination may help differentiate between stroke-related and nonstroke-related vertigo/dizziness [36].
When caring for patients with stroke, time is often of the utmost importance [5]. It is therefore concerning to observe that median time on scene was 3 min longer when mNIHSS was used compared to FAST, even though the total EMS mission time did not differ significantly. mNIHSS is more comprehensive than FAST, with a greater number of assessment items, which may have contributed to the extended time on scene. This finding aligns with Guterud’s et al. [37] study, where the mNIHSS and FAST were compared. In our study, the use of the mNIHSS was examined in the months immediately following implementation. The limited experience of EMS personnel with the mNIHSS may have prolonged the time on scene when using this tool.
It is also possible that the time on scene is related to the personnel’s judgement of urgency. A higher proportion of patients in the mNIHSS group were discharged with a TIA diagnosis, and mortality was lower. This suggests that the mNIHSS cohort may have been less critically ill, for whom time may not have been a crucial factor. If a larger proportion of patients in the mNIHSS group were not considered “code stroke” cases, the pace of care could have been slower, potentially explaining the observed time difference. This is further supported by the fact that “code stroke” was slightly more frequently activated in the FAST group than in the mNIHSS group. One could speculate that EMS personnel use the mNIHSS in less specific cases, where time is deemed less critical, whereas the FAST, being quicker and more familiar, is used for clear and urgent cases. This is also supported by the finding that most patients assessed with the mNIHSS received a low total mNIHSS score.
Although the use of the mNIHSS may extend the time spent on scene, there are at least three reasons why its prehospital use can still be advocated. Firstly, the NIHSS is the primary stroke scale used in hospital settings [38, 39]. Utilising the same tool for stroke assessment in both prehospital and hospital environments enhances the ability to track symptom development over time, even after the transition from prehospital to in-hospital care, and improves communication between prehospital and hospital personnel [37]. Previous studies have also shown acceptable reliability when comparing the use of the mNIHSS between EMS personnel and physicians [21, 40].
Secondly, the mNIHSS appears to be superior to the FAST in terms of EMS identification of minor strokes [37]. Finally, thrombectomy is only available at a few specialised centres, and interhospital transfers are a significant source of delay. Therefore, it is important that suitable thrombectomy patients are identified as early as possible in the prehospital setting. This would allow for direct transport to a thrombectomy-capable hospital, avoiding the time lost on interhospital transfers [9, 41]. FAST is a rougher and more arbitrary tool compared with mNIHSS, which may make it more challenging in identifying appropriate thrombectomy candidates [37]. The mNIHSS is more nuanced, and the total score, along with specific items, yields a positive result, providing better guidance regarding LVO and thrombectomy suitability, compared with FAST’s dichotomous outcome [9].
There is an ongoing debate concerning the benefits of transporting patients to the nearest hospital for initial diagnostics and stabilisation versus direct transport to a thrombectomy-capable hospital, often referred to as the “drip and ship” versus “mothership” model. There is no definitive evidence showing the superiority of either method [42]. Further research is needed, including studies focusing on prehospital LVO recognition using specific stroke scales to determine which patients would benefit from each transport approach [43, 44].
The stroke/TIA identification rate of 82.5% is, to our knowledge, the highest reported by nonphysician personnel in the EMS setting. It may be unreasonable to expect a higher level of stroke identification using the currently available stroke scales. It is important to remember that EMS personnel operate in a complex environment, encountering a broad spectrum of patients and conditions. Expecting expert-level knowledge for the assessment of every medical condition is unrealistic. While paying closer attention to symptoms like vertigo/dizziness might slightly improve the identification rate, it would probably lead to overtriage, given the low incidence of stroke/TIA among patients with these symptoms.
Modern technology may offer additional ways to enhance prehospital stroke identification. For example, prehospital video triage with in-hospital neurologists [45], artificial intelligence-based decision support tools [46], microwave-based stroke diagnostics [47], and mobile prehospital computer tomography scanners [48]. However, these technologies still rely on EMS personnel suspecting stroke/TIA in the first place.
The proportion of cases in which a physician was consulted was relatively low. This could be due to several factors. Firstly, EMS personnel must suspect stroke to seek consultation. Secondly, symptoms may have regressed before EMS arrival, or the patient’s condition may not have resulted in positive FAST/mNIHSS scores. Thirdly, the patient may be unstable due to failing vital signs or time-critical injuries. Finally, physician consultations may not always be recorded in the EMS medical record.
“Code stroke” was activated in 65.0% of eligible cases, a figure that one might expect to be higher. In the study by Andersson et al. [11], the corresponding figure was 70%. The somewhat lower activation rate may be explained by several factors that can exclude patients from thrombolysis before reaching the hospital, such as comorbidity, minimal symptoms, or having passed the thrombolysis time window. In fact, most stroke patients arrive more than 4.5 h after symptom onset, which disqualifies them from thrombolysis [8]. The strategies proposed above for improving prehospital stroke/TIA identification may also be beneficial for increasing “code stroke” activation.
4.1. Strengths and Limitations
The main strength of this study is its focus on the routine change in standard care using real-world data, specifically the transition from FAST to mNIHSS for prehospital stroke assessment. This approach minimises the potential bias that may arise when EMS personnel or physicians are aware of participating in a study, which could influence their assessments, behaviour, or decision-making.
The primary limitation of the study is its retrospective, observational pre–post-implementation design, which prevents drawing causal conclusions. The observational design, combined with potential bias, may distort the composition of the cohorts. As discussed earlier, it is possible that the FAST is used for more obvious cases, while the mNIHSS is applied in more unspecific cases. We anticipated a clearer transition from the FAST to mNIHSS following the change in local EMS guidelines on 1 December 2022. However, this was not entirely the case. FAST was still used in some instances after that date, and some EMS personnel employed mNIHSS before the official guideline change. In some cases, both FAST and mNIHSS were used. These overlaps complicate the comparative analysis, and as a result, the findings should be interpreted with caution.
Another limitation is that the study relies on medical records, which vary in quality. This compromises the validity of the data and, consequently, reliability of the results.
Additionally, the study lacks data on stroke severity, the prevalence of LVO, and the frequency of primary and secondary transfers to stroke centres for reperfusion therapy or thrombectomy. This gap hinders analyses of patients suitable for thrombectomy and limits the ability to assess potential disparities in stroke severity between cohorts.
In the analysis of the association between specific neurological symptoms and stroke/TIA identification, not all symptoms were assessed in every patient, particularly vision and sensory impairment, as these symptoms are not included in the FAST scale. This limitation complicates the interpretation of the results. Additionally, the lack of these data makes it difficult to determine why the use of mNIHSS instead of FAST does not appear to improve EMS stroke identification, despite including more neurological assessment items.
The study is underpowered, whereby 95 cases are missing in the mNIHSS group. This is primarily due to two factors. Firstly, the FAST continued to be used after 1 December 2022, despite the guideline change. Secondly, cases were unexpectedly lost due to discrepancies between the initial stroke/TIA diagnosis in the ED and the final diagnosis at hospital discharge. This increases the risk for Type I errors.
The total cohort of 1849 cases can be considered large, particularly in the EMS setting. However, generalisations should be made with caution due to variations in staffing and competencies between different EMS organisations, which probably influence stroke/TIA identification. In this study, stroke assessments were predominantly conducted by nurses, most of whom were specialised in prehospital emergency care. Given that our findings align with those of other studies conducted in Swedish EMS context [12, 16], we conclude that the results are reliable and applicable to Swedish EMS care and potentially applicable to other nurse-based EMS organisations. Nonetheless, any application of these results to different settings should be approached with caution.
5. Conclusions
The stroke identification rate does not appear to differ between the FAST and mNIHSS. The FAST and mNIHSS result in “code stroke” activation to an equal extent. Speech impairment, as well as arm or leg paresis, seems to enhance the EMS stroke identification rate. Conversely, impaired balance, convulsions, and vertigo/dizziness are associated with a lowered identification rate. Initial EMS suspicion of stroke and subsequent application of a stroke scale, regardless of which one, appear to facilitate stroke identification. Further improvement in prehospital stroke identification and “code stroke” activation will require advanced technical support, such as video consultation with a neurologist or prehospital neuroimaging.
Disclosure
The funding bodies had no role in the design, conduct, interpretation, or writing of the report on this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study has been funded by the Department of Ambulance and Prehospital Care, Region Halland, and the Scientific Council of Region Halland (HALLAND-991532).
Acknowledgments
We want to thank Ola Lövenvald for data extraction and Margaret Myers and Mark Rosenfeld for language editing.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data is available on reasonable request. The datasets generated and analysed during the current study are not publicly available due to the integrity of patient privacy but are available from the corresponding author on reasonable request and if approved by the Swedish Ethical Review Authority.




