L1CAM and p65 as Predictive Markers of ZFTA Fusion in Ependymomas
Abstract
Purpose: In the 2021 World Health Organization (WHO) classification, ependymomas were classified according to their anatomical localization and molecular features. The “RELA fusion-positive ependymoma” group, which was the first defined molecular subtype in the previous (2016) classification, was included as “ZFTA fusion-positive ependymoma” in the final classification. We aimed to determine ZFTA fusion-positivity in supratentorial (ST) and posterior fossa (PF) ependymomas and to investigate the correlation with both L1CAM and p65 immunoreactivity.
Methods: The study included 17 ST and 9 PF cases with Grades 1, 2, and 3 ependymomas. The presence of the ZFTA fusion was evaluated using the fluorescence in situ hybridization (FISH) technique. L1CAM and p65 antibodies were applied for immunohistochemical analysis. The immunoreactivity for L1CAM and p65 was correlated with ZFTA fusion as assessed by FISH. Prognostic significance of the same was evaluated using the Kaplan–Meier survival analysis.
Results: ZFTA fusion-positivity was detected in 7 of 12 (58%) ST-localized Grade 3 ependymoma cases; however, it was not observed in PF-localized cases or in ST-localized Grade 1 subependymoma (SE) and Grade 2 ependymoma cases. Six of the seven ZFTA fusion-positive cases exhibited clear cell morphology. All ZFTA fusion-positive cases showed L1CAM immunohistochemical positivity, and six of them also demonstrated nuclear p65 positivity. Moreover, we identified a new FISH pattern, which we termed the “short break-apart.”
Conclusion: Together, these data indicate a strong correlation between FISH and immunohistochemistry results. However, a more reliable assessment on this matter could be accomplished through a multicentric study involving a larger number of cases.
1. Introduction
Ependymomas are relatively rare glial tumors, represent approximately 6.8% of central nervous system tumors (CNSTs), and are the third most common CNSTs in children [1]. Ependymomas comprise small, round ependymal cells within the fibrillar matrix, forming characteristic pseudorosettes, or true ependymal rosettes [2].
Although glial fibrillary acidic protein (GFAP) immunoreactivity generally exhibits pseudorosette structures, variable immunoreactivity is detected in other tumor elements such as rosette and papillar structures [3]. Reactivity with an antiepithelial membrane antigen (EMA) antibody is detected as perinuclear dot-like or cytoplasmic ring-like structures that occur on the luminal surface of some ependymal rosettes as well [4].
Tumor grade is assigned through evaluation of the mitotic count, microvascular proliferation, and necrosis. The argument that histological grade is not related to biological behavior and survival is gaining increasing support [5–7].
The variable clinical course of ependymoma depends primarily on the surgical removal of the tumor, adjuvant radiotherapy (RT), patient’s age, tumor location, and the newly defined molecular groups [8–10]. Tumor location is an important prognostic factor, and ST ependymomas, particularly those of children, are associated with a higher survival rate compared with that of PF neoplasms, whose surgical resection may be critical [11].
ST and infratentorial (IT)-located ependymomas are classified into the following molecular subgroups: (1) ST-located tumors include ZFTA fusion-positive (ST-EPN-ZFTA) and YAP-1 fusion-positive (ST-EPN-YAP1), and (2) IT tumors include PF Groups A (PF-EPN-A) and B (PF-EPN-B) [9]. Remarkably, in 70% of childhood ST-located ependymomas, the C11orf95 (now called ZFTA) gene located on chromosome 11 is fused to RELA that encodes a component of the NF-κB pathway [9, 12]. In subsequent studies, it was identified that while the most frequent fusion partner of the ZFTA gene is RELA, there are also other fusion partners besides RELA [13]. Consequently, in the 2021 classification, this group was named as “ZFTA fusion-positive ependymoma” [13]. This fusion is undetectable in ependymomas located in the PF and spinal (SP) areas or in Grade 1 tumors [9, 12].
ST-EPN-ZFTAs exhibit the standard structural and cytological features of ST ependymomas as well as the clear cell change, a distinctive vascular pattern of branching capillaries, or both [13]. Such tumors are immunohistochemically positive for GFAP and EMA, as are other ependymomas; but unlike other ependymomas, they show cytoplasmic and membranous immunostaining of L1CAM and nuclear immunostaining of p65 [12, 13]. However, other tumors may express L1CAM [14].
The ZFTA-RELA fusion is the most common structural variant in ependymomas, and one of the methods for detecting this fusion involves using fluorescence in situ hybridization (FISH) technique with a break-apart probe to formalin-fixed, paraffin-embedded (FFPE) tissues [9, 12, 15]. ST-EPN-ZFTAs are associated with the worst clinical course among the ST molecular groups [9]. However, some recent studies have reported that ST-EPN-ZFTAs may have a good prognosis [16–18].
We focused on ZFTA fusion-positive ependymomas in our study as we believe that the development of targeted therapies for this condition in the future will be beneficial. We also aimed to examine its association with immunohistochemical L1CAM and p65 positivity to evaluate whether L1CAM and/or p65 could be used as an alternative method to investigate ZFTA fusion-positivity.
2. Materials and Methods
2.1. Case Selection
The locations and rates of 103 cases of ependymoma which were diagnosed between 2011 and 2019 at Istanbul University - Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Pathology, were as follows: 28 (27%) ST, 16 (16%) PF, 58 (56%) SP, and 1 (1%) inguinal lymph node. Intracranially located cases such as ST and PF were included, and SP or other regional ependymomas were excluded. FFPE blocks of seven of the 28 resected tissues with ST location and five of the 16 resected tissues with PF location could not be retrieved. Among the remaining 21 ST-located resection and 11 PF-located resection materials, there were two recurrence materials of two cases and one recurrence material of two cases, respectively. Resection materials of relapses were included as well, and 26 cases (17 ST and 9 PF localizations) were included in the study (Table 1).
Of the 17 ependymoma cases with ST localization, 4 (24%) were Grade 1 SE, 1 (5%) was Grade 2 ependymoma, and 12 (71%) were Grade 3 ependymoma. Of the 9 ependymoma cases with PF localization, 1 (11%) was Grade 1 SE, 6 (67%) were Grade 2 ependymoma, and 2 (22%) were Grade 3 ependymoma. Grades were assigned according to the histomorphological criteria of the WHO 2021 CNST classification.
The study was ethically approved by the local Ethics Committee of Istanbul University - Cerrahpasa, Cerrahpasa Faculty of Medicine (protocol number: 53648, date: 04.04.2019).
2.2. FISH Analysis
The ZFTA fusion was evaluated using commercial probes (RELA Break Apart FISH Probe Kit, CytoTest Inc., Rockville, United States). To analyze 4-μm-thick sections from selected FFPE blocks, sections were placed on positively charged slides and dried overnight at 56°C to adhere to the slide. After the slides were deparaffinized, hybridization was performed, and the slides were evaluated using a fluorescence microscope (Olympus BX61; Olympus Optical, Japan) equipped with a digital camera (XLMM, DageMTI, Indiana, United States) and imaging software (Duet, Bioview Ltd., Israel). The RELA break-apart was analyzed using a dual-color probe (green and orange) to detect the chromosome 11q13.1 region (RELA Break Apart FISH Probe Kit, CytoTest Inc., Rockville, United States). The orange and green signals indicate hybridization to 3 ′- or 5 ′-terminal sequences, respectively. Two yellow signals are observed in normal (negative) cells. However, in the setting of a break-apart, one yellow signal and separate orange and green signals are detected.
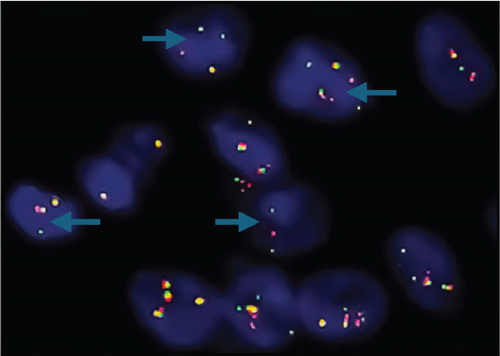
Shaoyan et al. examined the break-apart using FISH as follows: Nonoverlapping cells (n = 50) were individually selected, and detection of the break-apart in ≥ 15% of the cells was defined as RELA fusion-positive [19]. Further, Pagès et al. evaluated 100 selectable tumor cells, which were scored as RELA fusion-positive when ≥ 20% of the cells exhibited a break-apart signal [18]. Here, we selected 100 evaluable tumor cells and assigned the cut-off value of 15% as RELA fusion-positive. A break-apart is indicated when the distance between the orange and green signals is twice the size of a FISH signal [20]. We used this interval to diagnose our cases as ZFTA fusion-positive. In addition, in most of our positive cases, we observed another pattern that we call the “short break-apart” in which the green and orange signals were distinct, although the distance between them was less than that of a standard break-apart signal.
A subsequent FISH analysis was conducted on the ST Grades 2 and 3 ependymoma cases at the pathology department of Marmara University, School of Medicine, in which we did not detect RELA fusion positivity. For this study, the C11orf95 break-apart probe (ZFTA Break Apart FISH Probe Kit, CytoTest Inc., Rockville, United States) was used. The aim was to identify the rarer fusion partners of ZFTA outside of RELA.
2.3. Immunohistochemistry
Immunohistochemistry was performed using an automated instrument (BenchMark XT IHC/ISH Staining Module, Ventana Medical Systems Inc., Medical Systems, Tucson, Arizona, United States). Sections (2–3 μm thick) prepared from FFPE blocks were placed on positively charged slides at 40°C overnight. After deparaffinization, the slides were rehydrated using a series of alcohol solutions of decreasing concentrations and then with distilled water. Slides were incubated in 10 mmol/L buffered citrate at 36°C for 30 min to restore antigenicity. A commercially available anti-L1CAM (CD171) antibody (monoclonal, UJ127.11, Thermo Fisher Scientific, Rockford, United States) and an anti-NF-κB p65 antibody (polyclonal, AF5006, Affinity Biosciences, United States) were applied to tissue sections at a dilution of 1/100 and incubated for 88 min. Immunostaining was visualized using 0.1% hematoxylin. Cases exhibiting diffuse and strong cytoplasmic and membranous immunoreactivity for L1CAM were considered positive. A normal kidney parenchyma sample, as recommended by the antibody manufacturer, served as the control tissue for L1CAM staining. For p65 staining, human breast cancer tissue was used as the positive control. Nuclear positivity observed in more than 50% of tumor cells was considered positive for p65. The immunopositivity exhibited a heterogeneous staining pattern, varying from weak to strong intensity.
In addition to our cases, immunohistochemical analysis of L1CAM expression was applied to randomly selected cases as follows: one case each of astrocytoma, IDH-mutant (AS), oligodendroglioma, IDH-mutant and 1p/19q-codeleted (OD), and glioblastoma, IDH-wildtype (GBM), which were diagnosed in 2019.
Two different researchers, who were fully blinded to the results of the FISH analysis, assessed the L1CAM and p65 results.
2.4. Statistical Analysis
21.0 version of SPSS (Statistical Package for the Social Sciences, Chicago, Illinois, United States) program was used for statistical analysis of the data. Pearson’s chi-square test and Fisher’s exact chi-square test were performed in order to evaluate whether there is a statistically significant relationship between the FISH results (ZFTA), and immunohistochemical L1CAM and p65 expression; a p value < 0.05 was considered to show statistical significance.
The Kaplan–Meier method was applied for survival analysis, and Log rank analysis (Mantel Cox) was preferred to compare survival curves between groups. Survival analysis was formed according to tumor location (ST + PF), histopathological diagnosis/grade, integrated diagnosis (ZFTA), age, and gender.
3. Results
3.1. Clinical and Histological Features of Cases
Among the 26 cases included in our study, 13 (50%) were male, and 13 (50%) were female, ranging in age from 1 to 57 (mean age, 24.9; median age, 21.5) years. For ST-located cases, ages ranged from 1 to 57 (mean age, 23.3; median age, 13) years. For PF-located cases, ages ranged from 2 to 57 (mean age, 27.9; median age, 28) years. There were 17 ependymomas that originated within the ST area, nine in the PF; and 14, 7, and 5 cases were diagnosed as Grades 3, 2, and 1, respectively. There were two cases with two relapses in the ST location (Case Nos. 7a, b, c, and 11a, b, c), and two cases that relapsed once in the PF location (Case Nos. 18a, b and 23a, b). Clear cell morphology was exhibited by 10 cases, seven of which were in the ST location and three in the PF location. However, papillary morphology was observed in one case with the ST location.
Among the 22 available cases, the mean follow-up period was 78 months. Eight patients died, two within the first year and six within the first 5 years. Six of the deceased cases had tumors with ST location, two with PF location; 10 cases received RT + chemotherapy after surgery, and six received RT only (Table 1).
| Case number | Age (years) | Sex | Grade | Location | Morphology | Prognosis | Treatment | FISH (ZFTA) | L1CAM | P65 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | F | 3 | ST | N/A | NED | CT + RT | N | N | N |
| 2 | 7 | M | 3 | ST | N/A | NED | CT + RT | P | P | P |
| 3 | 13 | M | 3 | ST | N/A | DOD | RT | N | N | N |
| 4 | 1 | F | 3 | ST | PA | N/A | N/A | N | N | N |
| 5 | 46 | F | 1 | ST | SE | NED | N | N | N | N |
| 6 | 41 | F | 1 | ST | SE | NED | N | N | N | N |
| 7a,b,c | 11 | M | 3 | ST | CL | PD | CT + RT | P | P | P |
| 8 | 21 | F | 3 | ST | N/A | N/A | N/A | N | N | N |
| 9 | 47 | F | 1 | ST | SE | NED | N | N | N | N |
| 10 | 29 | F | 2 | ST | CL | DOD | CT + RT | N | N | N |
| 11a,b,c | 34 | M | 3 | ST | CL | DOD | RT | P | P | P |
| 12 | 13 | F | 3 | ST | N/A | DOD | N/A | N | N | N |
| 13 | 7 | M | 3 | ST | CL | DOD | CT + RT | P | P | N |
| 14 | 57 | M | 3 | ST | CL | NED | N | P | P | P |
| 15 | 8 | M | 3 | ST | CL | NED | CT + RT | P | P | P |
| 16 | 57 | M | 1 | ST | SE | N/A | N/A | N | N | N |
| 17 | 1 | F | 3 | ST | CL | DOD | CT + RT | P | P | P |
| 18a,b | 28 | F | 1 | PF | SE | PD | RT | N | N | N |
| 19 | 54 | M | 2 | PF | N/A | N/A | N/A | N | N | N |
| 20 | 8 | M | 3 | PF | CL | NED | CT + RT | N | N | N |
| 21 | 33 | M | 2 | PF | N/A | NED | RT | N | N | N |
| 22 | 39 | F | 2 | PF | N/A | NED | RT | N | N | N |
| 23a,b | 8 | F | 3 | PF | N/A | DOD | CT + RT | N | N | N |
| 24 | 22 | F | 2 | PF | CL | NED | RT | N | N | N |
| 25 | 57 | M | 2 | PF | N/A | NED | N | N | N | N |
| 26 | 2 | M | 2 | PF | CL | DOD | CT + RT | N | N | N |
- Abbreviations: CL, clear cell; CT, chemotherapy; DOD, dead of disease; F, female; M, male; N/A, not available; N, negative; NED, no evidence of disease; P, positive; PA, papillary; PD, progressive disease; PF, posterior fossa; RT, radiotherapy; SE, subependymoma; ST, supratentorial.
3.2. FISH and Immunohistochemistry
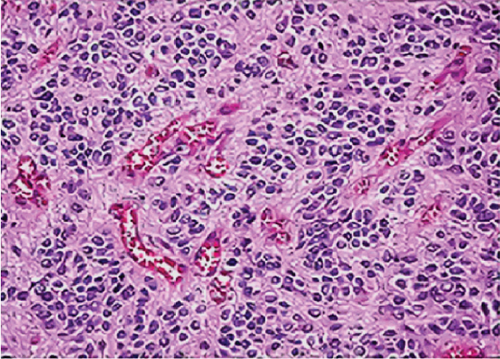
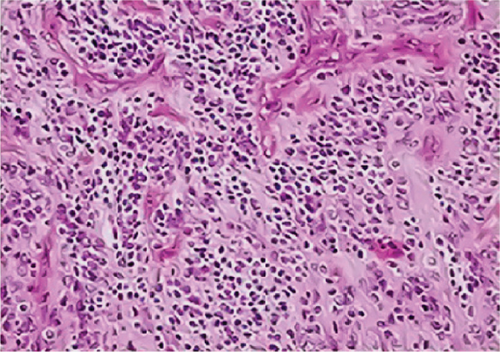
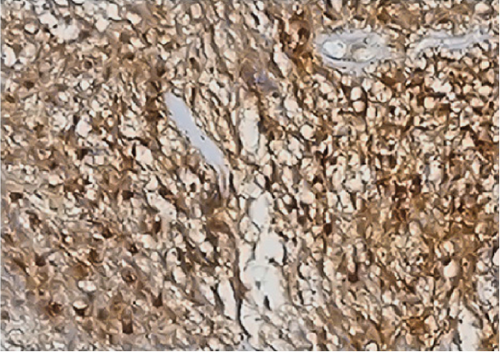
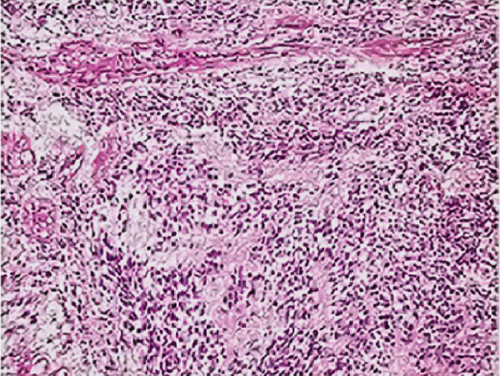
Neither ZFTA fusion-positivity nor immunohistochemical L1CAM and p65 positivity was observed in the four ST-located Grade 1 SE cases, one ST-located Grade 2 ependymoma case, and 9 PF-located cases (Figures 1m, 1n, 1o, and 1p). ZFTA fusion-positivity was detected in seven of 12 (58%) ST-located Grade 3 ependymoma cases (Figure 1d,h). Moreover, in four of these seven cases, the short break-apart pattern was detected regardless of our 15% break-apart threshold (Figure 1l). All ZFTA fusion-positive cases were cases showing ZFTA-RELA fusion. We had no cases showing positivity with other fusion partners of ZFTA.


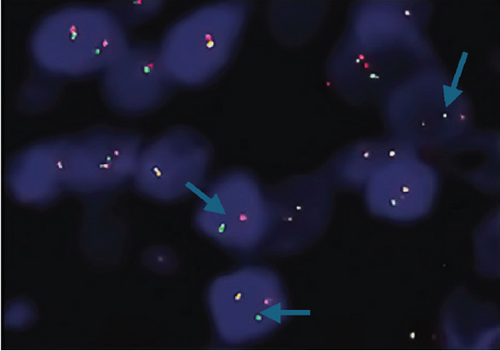

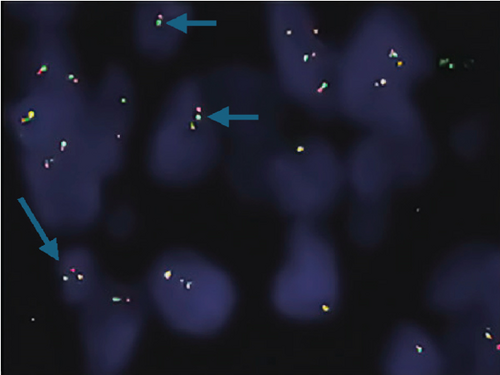
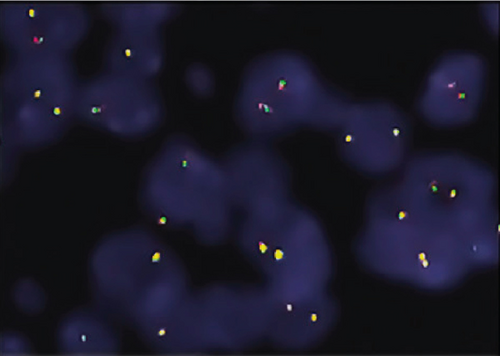
The ages of ZFTA fusion-positive cases ranged from 1 to 57 (mean age, 17.9; median age, 8) years. Among them, 5 of 7 (71%) cases were pediatric, and 2 were adults (29%). 6 of 7 (86%) of ZFTA fusion-positive cases were male, and 1 (14%) was female. There was a statistically positive and significant relationship between ZFTA results and male gender (p = 0.015).
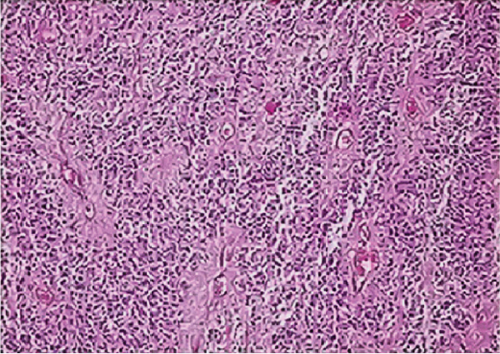
The seven ZFTA fusion-positive cases (100%) were Grade 3 ependymoma with ST location (Figures 1a, 1e, and 1i). However, the majority of our supratentorial ependymoma cases were Grade 3 ependymoma.
In one of our ZFTA fusion-positive cases, no specific morphological subtype was selected, and clear cell changes were observed in all of the remaining cases (86%). A significant positive correlation was found between ZFTA fusion-positive cases and clear cell morphology (p = 0.029). Clear cell morphology was present in only 1 of the ZFTA fusion-negative ST Grades 2 and 3 ependymomas (Figure 1m).
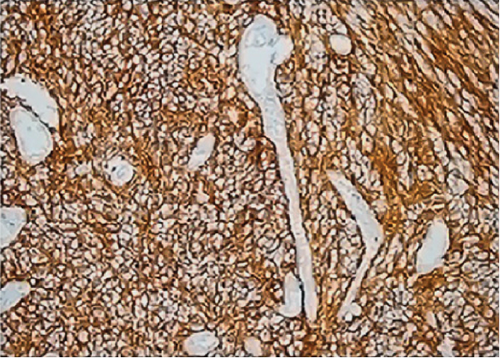
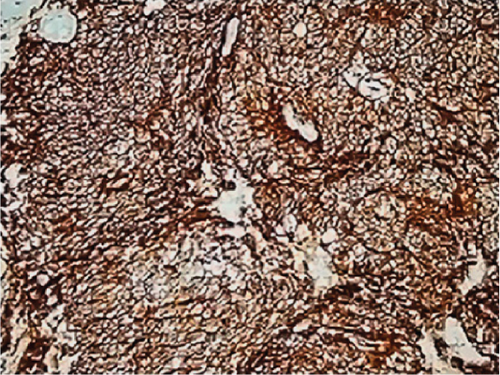
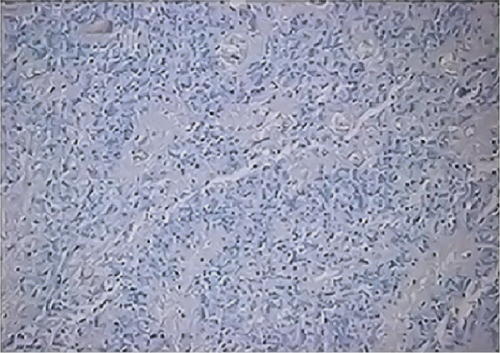
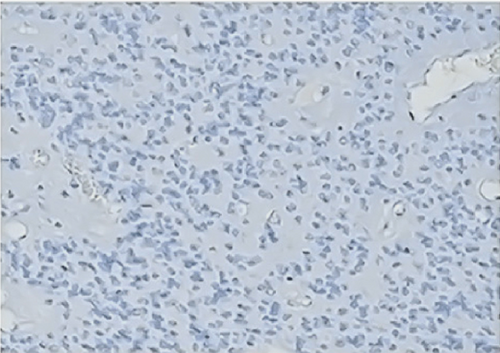
All ZFTA fusion-positive cases exhibited diffuse, strong, cytoplasmic, and membranous positivity for L1CAM (Figures 1b, 1f, and 1j). Nuclear p65 positivity in more than 50% of tumor cells was observed in six of these cases (Figures 1c, 1g, and 1k). A statistically strong and significant correlation was found between FISH results and both the L1CAM and p65 results (p = 0.00000421 and p = 0.0000457, respectively) (Table 1). Neither L1CAM nor p65 expression was detected in ZFTA fusion-negative cases (Figure 1n,o). At the same time, among randomly selected (1 each) AS, OD, and GBM cases, staining was undetectable in the GBM case, and weak and strong staining was observed in the AS and OD cases, respectively.
3.3. Prognosis and Survival According to Histomorphology and FISH Results
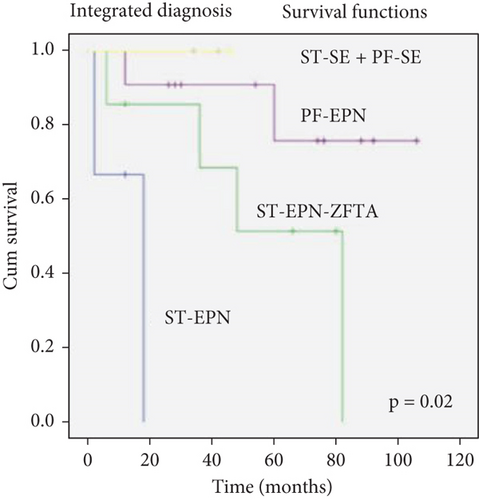
We grouped cases according to follow-up data as follows: one PF-SE, three ST-SE, seven ST-EPN-ZFTA, four ZFTA fusion-negative ependymomas (ST-EPN), and seven PF-located Grades 2 + 3 ependymomas (PF-EPN). The ST-SE and PF-SE groups experienced the highest survival rates. Among the available cases, 4 of 7 ST-EPN-ZFTA cases (57%) were alive. However, only 1 of 4 ST-EPN cases (25%) was alive. The survival rates of ZFTA fusion-positive cases were higher compared with those of ZFTA fusion-negative cases (Table 1). A statistically significant relationship was found between the survival rates of these diagnostic groups (log rank = 11,717, p = 0.02) (Figure 2).
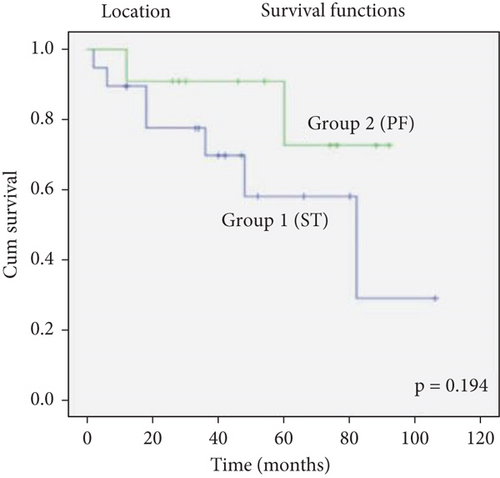
The survival rates of patients with ST location were lower than those with PF location, but there was no statistically significant difference (log rank = 1.686, p = 0.194) (Figure 2).
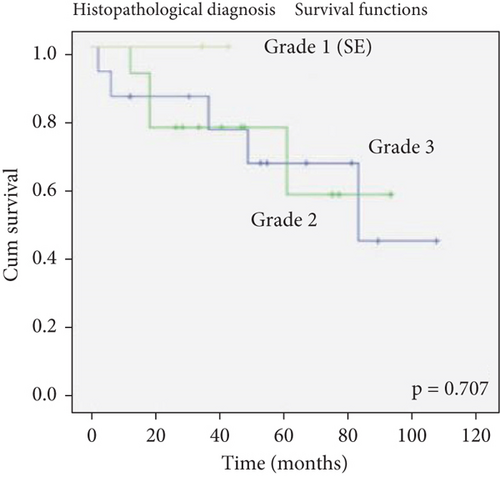
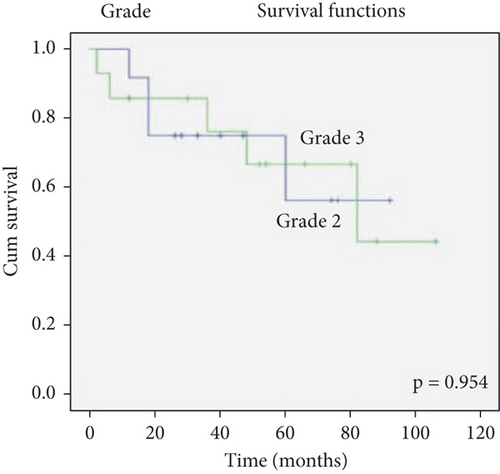
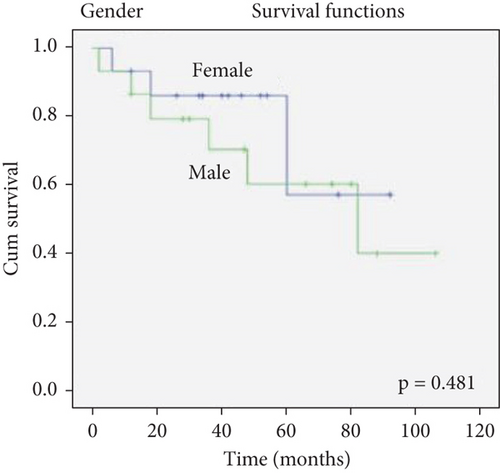
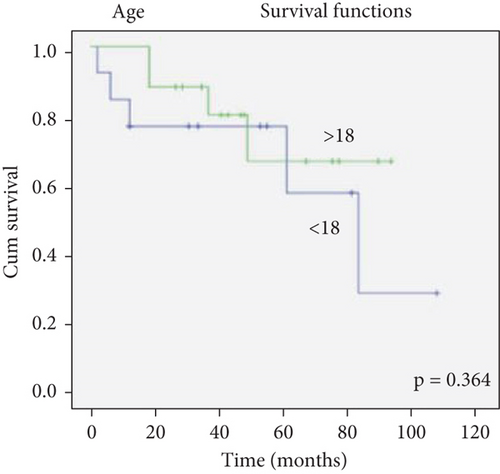
There was no effect of histopathological diagnosis, histological grade, gender, and age on survival (Figure 2).
4. Discussion
Although the present study included patients treated between 2011 and 2019, we included 26 cases (17 ST and 9 PF). The number of cases included in the study was low since they consisted only of single-center cases. Cases older than 2011 were not included in the study as problems could arise in molecular practice and immunohistochemical staining in long-standing FFPE blocks. This situation constitutes one of the limitations in our study. However, the number of cases with ST location in the literature ranges from 8 to 47 (average, 30 cases) [12, 14, 15, 17, 18, 21–23]. Therefore, we believe our findings represent a valuable contribution to our understanding of the phenotypes of ependymomas.
In similar studies, the ZFTA fusion-positivity rates range from 40% to 74% in cases of ST-located ependymomas including Grades 2 and 3 [12, 14, 15, 18, 21–23]. Consistent with literature, we detected ZFTA fusion-positivity in seven of 12 (58%) ST-located Grade 3 ependymoma cases. We had just one ST-located Grade 2 ependymoma case that did not show ZFTA fusion-positivity.
In line with the studies of Parker et al. [12] and Malgulwar et al. [21], ZFTA fusion-positivity was not observed in our nine PF-located cases. Further, in accordance with the WHO 2021 CNST classification [13] and the findings of Malgulwar et al. [21], we did not detect ZFTA fusion-positivity in four ST-located Grade 1 SE cases. The seven ST-EPN-ZFTA cases were Grade 3 ependymoma. Fukuoka et al. [22] found that all cases with ZFTA fusion-positivity were Grade 3 ependymoma, and Gessi et al. [23] and Malgulwar et al. [21] found that most ST-EPN-ZFTA cases were Grade 3 ependymoma (94% and 81.5%, respectively).
Clear cell ependymomas mainly occur in the ST region [13]. Seven (70%) of our ependymoma cases with clear cell changes were located in the ST region, and three (30%) were in the PF region. Further, 1 of our 7 ST-EPN-ZFTA cases did not exhibit a specific morphology, although the other six (86%) had clear cell morphology. Similarly, Parker et al. observed clear cell morphology in all cases with the C11orf95-RELA translocation [12]. Pietsch et al. [15], Sasaki et al. [14], and Malgulwar et al. [21] detected clear cell morphology in most of their ZFTA fusion-positive cases; Gessi et al. [23] detected ZFTA fusion-positivity in cases with clear cell changes, particularly in the pediatric group; Neumann et al. [24] reported a significant relationship between ST-located clear cell ependymomas and ZFTA fusion-positivity.
Our present findings were in agreement with data showing that ZFTA fusion-positivity occurs in infants and children as well as in adults [25]. Further, the ages of our present patient population aligned with the mean age reported for ZFTA fusion-positive cases by Malgulwar et al. [9 years (81.4%)] [21]. Moreover, in the study of Sasaki et al. [14], patient ages ranged from 1 to 64 (median age, 13) years, including 44% adults. In the present study, 86% of the ZFTA fusion-positive cases were male. The ST-EPN-ZFTA cases of Pajtler et al. [26] and Gerstner et al. [25] were predominantly male as well.
We found a strong association between FISH positivity and both the L1CAM positivity (100% sensitivity, 100% specificity) and p65 positivity (86% sensitivity, 100% specificity). Similarly, Sasaki et al. [14] reported that ZFTA fusion-positive cases detected using a break-apart FISH probe showed positive immunostaining with L1CAM, with 100% sensitivity, but 95% specificity). In contrast, Malgulwar et al. [21] detected L1CAM immunoreactivity in 79% of fusion-positive cases, and Gessi et al. [23] reported 94% sensitivity and 76% specificity for L1CAM. Additionally, Matsumoto et al. [17] detected L1CAM expression in four of five ZFTA fusion-positive cortical ependymoma cases. Chinnam et al. [27] also emphasized that L1CAM had the highest sensitivity (92.3%) for detecting ZFTA fusions. Regarding p65 staining, Sasaki et al. observed nuclear positivity in all five ZFTA fusion-positive cases, as well as in two of seven ZFTA fusion-negative cases, corresponding to a sensitivity of 100% and a specificity of 28.5%. Similarly, Gessi et al. identified ZFTA fusion positivity in 17 of 19 p65-positive ependymoma cases and reported no p65 positivity among the 23 fusion-negative cases, resulting in a sensitivity of 100% and a specificity of 92%.
Diagnosing ZFTA fusion-positive ependymomas according to immunohistochemical L1CAM and/or p65 positivity without a molecular study showing gene fusion involving ZFTA may therefore be inaccurate. However, laboratories that are unable to perform any of the molecular studies can use a preliminary immunohistochemical analysis and refer the patient to a laboratory that can confirm the diagnosis applying a molecular technique like FISH. In contrast, immunohistochemical L1CAM positivity is detected CNSTs such as AS, GBM, and gliomatosis cerebri [14, 28, 29]. Consistent with these observations, here, we used immunohistochemistry to detect the expression of L1CAM when applied to randomly selected controls. Therefore, L1CAM immunoreactivity is not specific for ependymomas, and this antibody, therefore, cannot be applied to confirm the diagnosis of ependymoma.
The short break-apart pattern was defined as the “short split pattern” in a study of the anaplastic lymphoma kinase gene rearrangement [30]. Since C11orf95 and RELA genes reside on the same chromosome, if breakage occurs in this region, it is likely that the separate green and orange signals in some sections will appear closer in the FISH evaluation. Further, evidence indicates that the separation of green and orange FISH signals represents positivity, although the specific distance is unreported [1, 13, 14, 18, 19, 22]. The short break-apart pattern was not taken into account when diagnosing ZFTA fusion-positive ependymoma. We suppose that this situation should be confirmed using other molecular methods and thus may contribute to the literature.
Fukuoka et al. [22] re-evaluated 113 ependymoma cases located in the ST and PF regions and reclassified a total of 12 cases (9 ST and 3 PF) as nonependymal tumors. Due to discrepancies between our survival outcomes and those reported in some previous studies, we also re-examined all our cases. All of our ST and PF cases were consistent with the diagnosis of ependymoma based on both their morphological features (e.g., perivascular pseudorosettes) and immunohistochemical staining results (GFAP, EMA). In all of our Grade 2 and Grade 3 ST and PF cases, GFAP positivity and paranuclear dot-like EMA immunoreactivity were observed. All SEs in both ST and PF regions were GFAP-positive; however, 1 of the 4 ST-SE cases and the PF-SE case showed no reactivity with the EMA antibody. Nevertheless, the localization and morphological features of these cases were consistent with the diagnosis of SE.
ZFTA fusion-positivity among ST-located Grades 2 and 3 ependymomas is associated with a more aggressive malignant phenotype, and such patients experience a lower survival rate (5- and 10-year survival rates, 75% and 49%, respectively) [1, 9, 21, 24, 31, 32]. However, Lillard et al. [16] reported higher survival rates associated with ZFTA fusion-positivity and suggested that this may be explained by extensive (total/subtotal) surgical resection. Matsumoto et al. [17] found that the survival rates of cortical-located ependymoma cases were much higher compared with those of other ST-located ependymoma cases, which they linked to extensive surgical resection and adjuvant RT administration. We conclude that the low risk of opening to the ventricle of cortical ependymomas and causing metastasis (seeding) may explain these findings.
Pagès et al. [18] did not find a significant difference in overall survival between ZFTA fusion-positive, YAP1 fusion-positive, and ZFTA and YAP1 fusion-negative groups. Here, we show that the ST-EPN-ZFTA group experienced better prognosis compared with those with fusion-negative ST-EPNs. Data regarding surgical resection (total/subtotal) of our present cases are missing, preventing us to reach conclusions about the significance of these data subject. Nevertheless, the studies cited question the value of prognostic factors for ependymomas [16, 17]. In order to make a more accurate comment on this issue, many studies with a larger number of cases need to be conducted.
Since this study primarily focused on ST ependymomas, PF ependymomas were included without subclassification into Group A or B. It is well known that PF-EPN-A cases tend to follow an aggressive clinical course and are associated with poor prognosis [9, 13, 33]. The fact that our survival analysis did not reflect this likely results from not stratifying the PF cases into their molecular subgroups.
The determination of the histological grade of a case depends on many factors such as the number of samples available of each case or the pathologist making the diagnosis. Further, although an increased mitotic index is required for the diagnosis of Grade 3 ependymoma, the lack of an exact value causes subjectivity [1, 13, 18]. Therefore, the influences of histopathological diagnosis and grade on survival are controversial, and currently, clinicians do not consider the histological grade in determining the treatment of Grade 2/3 ependymomas [6, 7]. In the present study, among the available cases, 2 out of 6 Grade 2 ependymoma cases (33%) were deceased, whereas 6 out of 12 Grade 3 ependymoma cases (50%) were deceased. Notably, the majority of our deceased cases were Grade 3 ependymomas. Hence, we conclude that it would be inappropriate to outright dismiss the correlation between histological grade and survival.
5. Conclusion
This study highlights the intricate interplay between tumor location, histological grade, morphology, and immunohistochemical L1CAM and p65 expression in predicting ZFTA fusion-positivity in ependymomas. While these parameters offer valuable preliminary insights, a definitive diagnosis of ZFTA fusion-positivity relies on robust molecular techniques such as FISH. Given the complex biological behavior of ZFTA fusion-positive ependymomas, further large-scale, multicenter studies are essential to unravel the prognostic and therapeutic implications of this molecular subtype. Our data underline the necessity of individualized treatment strategies, particularly in light of the survival disparities associated with ZFTA fusion-positivity and the potential for targeted therapies in the future. By advancing our understanding of these tumors, we take a critical step toward improving patient outcomes and tailoring more effective clinical interventions.
Ethics Statement
The study was ethically approved by the local Ethics Committee of Istanbul University - Cerrahpasa, Cerrahpasa Faculty of Medicine (protocol number: 53648; date: 04.04.2019).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Concept: C.T.S., S.B., N.C., and B.O.; design: C.T.S. and B.O.; data collection and/or processing: C.T.S., S.B., N.C., and B.O.; analysis and/or interpretation: C.T.S., S.U.B., N.C., B.O., and K.E.A.; literature search: C.T.S. and B.O.; writing: C.T.S. and A.M.O.
Funding
This work was supported by the Istanbul University - Cerrahpasa Scientific Research Projects Coordination Unit under Grant Number 33752.
Acknowledgments
This work was supported by the Istanbul University - Cerrahpasa Scientific Research Projects Coordination Unit under Grant Number 33752.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




