The Effects of miR-425-3p and Seminal Microbiota on Semen Parameters: Insights Into Male Infertility
Abstract
Male infertility is a multifaceted condition influenced by various factors, resulting in diverse phenotypic expressions. MicroRNAs (miRNAs), oxidative stress, and microbiota are potential contributors to compromised semen quality. This study explores the relationship between miR-425-3p, seminal microbiota, and oxidative stress in relation to impaired semen parameters. Semen samples were collected from 39 individuals with sperm motility <20% and 31 individuals with sperm motility >20%. Microbial cultures were performed to analyze the microbiota in the samples. DNA extraction from seminal cultured colonies, followed by PCR amplification targeting the 16S rRNA gene was conducted for sequencing, while RNA extraction from seminal plasma facilitated RT-qPCR analysis of miR-425-3p. Additionally, oxidative lipid degradation was evaluated using a thiobarbituric acid reactive species (TBARS) assay, comparing malondialdehyde (MDA) levels between samples with good and poor sperm motility. Our analysis revealed a diverse semen microbiota, predominantly consisting of Staphylococcus, Enterococcus, Bacillus, Micrococcus, and Neisseria. Less prevalent genera included Streptococcus, Acinetobacter, Corynebacterium, Actinomyces, and Escherichia coli. Higher bacterial loads were positively correlated with improved sperm motility, averaging 20,373 CFU/mL in the group with sperm motility >20% compared to 1247 CFU/mL in group with sperm motility <20% (p = 0.03). Conversely, miR-425-3p expression was significantly elevated in group with sperm motility <20%, showing a fourfold increase (p = 0.034). Reduced miR-425-3p expression was observed in samples abundant in Bacillus, particularly when Neisseria was absent. MDA levels, a marker of oxidative stress, were significantly higher in the less motile group (9.46 ± 5.07 nmol/mL) compared to the motile group (5.25 ± 3.32 nmol/mL, p = 0.038). The analysis revealed distinct microbial profiles associated with varying sperm motility levels. These findings establish a connection between increased miR-425-3p expression, elevated seminal oxidative stress, and decreased sperm motility. Our data provide insights into the roles of seminal microbiota and microRNA in the pathogenesis of impaired semen quality.
1. Introduction
Male infertility is defined as a man’s inability to conceive a child with a fertile partner, persisting for a minimum of 1 year of unprotected sexual intercourse [1]. It is estimated that male factors contribute to approximately 40%–50% of all infertility cases, highlighting the significance of male reproductive health [2]. The underlying causes of male infertility can be multifaceted, often arising from a combination of genetic and epigenetic abnormalities, hormonal imbalances, oxidative stress, and microbiota imbalances [3–5]. These factors can adversely affect sperm quality, motility, and overall reproductive capacity, necessitating a comprehensive understanding of their roles in male infertility.
Microbiota imbalances, which refer to the diverse group of microorganisms inhabiting a specific environment [6], have been implicated in various health issues, including infertility [7]. Their effects can manifest in several ways, such as direct adherence to sperm, which may lead to increased semen viscosity and subsequent impairment of normal sperm motility [8]. Research has shown that numerous microorganisms exhibit an inverse relationship with semen parameters, mitochondrial function, and DNA integrity in sperm cells [9]. However, the molecular mechanisms by which certain bacteria influence semen quality and contribute to infertility remain inadequately understood, indicating a critical area for further investigation.
Recent discoveries indicate that the host organism plays a crucial role in facilitating the propagation of microorganisms by releasing various factors, including microRNAs (miRNAs) [10]. miRNAs are small, noncoding RNA molecules, typically ranging from 20 to 23 nucleotides in length, produced in the nucleus and subsequently transported to the cytoplasm. They exert their regulatory influence by inducing gene silencing through binding to the 3′-untranslated regions (UTRs) of their target mRNAs, resulting in mRNA degradation or inhibition of translation [11]. The impact of specific miRNAs on mammalian spermatogenesis has been examined [12], revealing that abnormal expression of certain miRNAs is linked to male infertility [13]. For instance, miR-425-3p has been identified as highly expressed in the seminal plasma and in extracellular microvesicles derived from the seminal plasma of men with oligoasthenozoospermia [14, 15]. In addition, the expression of miR-425-3p is reduced in the sperm samples of subfertile men when compared to those of fertile men [16]. Furthermore, this microRNA has been demonstrated to affect the diversity of microbial species in human stool [17]. In addition, the seminal plasma of approximately 30%–40% of infertile males exhibits elevated levels of reactive oxygen species (ROS), leading to oxidative stress [18]. Oxidative stress occurs when there is an imbalance between ROS production and antioxidant capacity [19]. This imbalance can further disrupt cellular functions, including those involved in sperm health and motility, creating a complex interplay between oxidative stress, microRNA activity, and the microbiota present in seminal fluid.
This study aimed to determine the effect of miR-425-3p and seminal microbiota on semen parameters, as well as the influence of oxidative stress on sperm motility.
2. Materials and Methods
2.1. Patients and Sample Collection
The study received ethical approval from the Institutional Review Board (IRB) at Jordan University of Science and Technology (Reference No. 2022/151/28). Seventy semen samples were obtained from patients visiting in vitro fertilization (IVF) clinics at Prince Rashed Ben Al-Hasan Military Hospital in Irbid, Jordan. The samples were processed for routine semen analysis following the 2010 WHO guidelines for semen analysis. Semen samples were collected via masturbation following a minimum of 3 days of sexual abstinence. Samples from individuals with diabetes mellitus or any history of testicular injury were excluded from the study. Additionally, participants who had taken corticosteroids or any medication for urinary tract infections within 3 months prior to sample collection were also excluded to ensure more accurate results regarding bacterial distribution. The ages of the participants ranged from 23 to 49 years.
Research suggests that a sperm concentration of at least 10 million spermatozoa per milliliter, with 15% exhibiting progressive motility, is essential for achieving satisfactory fertilization rates during IVF or insemination therapy [20]. In this study, the threshold for sperm motility was set at 20%. Accordingly, we classified the samples based on their motility into two categories: “progressively motile sperm” (fast and forward-moving) and “nonprogressively motile sperm” (slow or sluggish). We then divided the samples into two groups based on motility as follows: 31 samples had sperm motility >20%, while 39 samples had sperm motility <20%. The characteristics of these two groups are presented in Table 1.
| Characteristic | Sperm motility >20% | Sperm motility <20% | p-Value |
|---|---|---|---|
| Age (years) | 33.35 ± 5.6 | 32.96 ± 6.14 | 0.790 |
| Ejaculate volume (mL) | 3.5 ± 1.5 | 3.502 ± 1.13 | 0.993 |
| Sperm concentration (106/mL) | 74.58 ± 29.85 | 42.68 ± 29.67 | <0.0001 |
| Sperm motility (%) | 24.88 ± 3.15 | 7.029 ± 4.91 | <0.0001 |
| Morphology (%) | 10.12 ± 3.5 | 5.73 ± 3.84 | <0.0001 |
- Note: Sperm motility >20%, n = 31; Sperm motility <20%, n = 39. Mean values were reported with their corresponding standard deviations (SDs). Statistical significance was determined using a nonparametric Mann–Whitney U test, with a significance threshold set at p < 0.05. Morphology (%) refers to the percentage of sperm in the semen sample that exhibit normal shape and structure, as assessed through microscopic evaluation in accordance with the WHO guidelines for semen analysis. Motility (%) refers to the percentage of spermatozoa that show progressive movement. Specifically, this includes both Type 1 and Type 2 motility categories, which represent sperm moving actively forward and the percentage of fast, sluggish-moving sperm.
2.2. Semen Culture and DNA Extraction
To determine the bacterial load, 0.1 mL from each of the 70 semen samples was serially diluted in tenfold increments, and 0.1 mL from each dilution was spotted on duplicate LB agar plates. The agar plates were incubated at 37°C for 48 h. After incubation, colonies were counted to calculate the colony-forming unit (CFU) for each sample. Also, representative colonies were selected and transferred to LB broth and incubated overnight at 37°C in a shaking incubator. Bacterial cells were collected by centrifugation and stored at −20°C for DNA extraction. DNA was extracted using the phenol–chloroform method, and the DNA concentration was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA).
2.3. Polymerase Chain Reaction (PCR)
PCR amplifications for each microbial DNA were conducted in a total reaction volume of 25 μL, comprising 12.5 μL of 2x PCR Master Mix (Intron i-Tag, South Korea), 9.5 μL of nuclease-free water, 1 μL of each universal primer, and 1 μL of the DNA template. Each amplification run included negative controls (without DNA) to detect contamination. To target the 16S rRNA gene fragment of the bacterial genomic DNA, universal 16S rRNA primers were utilized. The forward primer used was 5′-AGAGTTTGATCCTGGCTCAG-3′, paired with the reverse primer 5′-GGTTACCTTGTTACGACTT-3′. After PCR, 5 μL of the product was loaded onto a 1% agarose gel stained with ethidium bromide. Gel electrophoresis was performed at 120 V for 45 min, and the bands were visualized using a UVP GelDoc-It2 310 Imaging System (Fisher Scientific). A 1 kb DNA ladder was used to estimate the size of the fragments.
2.4. 16S rRNA Sanger Sequencing
A specific region of the bacterial 16S rRNA gene was selectively amplified using universal 16S rRNA bacterial primers. The forward primer used was 5′-AGAGTTTGATCCTGGCTCAG-3′, paired with the reverse primer 5′-GGTTACCTTGTTACGACTT-3′. PCR was conducted under optimized thermal cycling conditions. Sequencing was performed by Macrogen Inc. (South Korea) using the forward primer. The resulting sequences were analyzed using the BLASTN algorithm at NCBI.
2.5. RNA Extraction and Quantitative Reverse Transcription PCR (RT-qPCR)
RNA was extracted from 70 fresh seminal plasma samples using the Direct-zol RNA MiniPrep Kit (Zymo Research, USA) from human seminal plasma, and DNase I treatment was applied to prevent DNA contamination, following the manufacturer’s protocol. Following RNA extraction, purity and concentration were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) to ensure suitability for downstream applications. After assessing RNA quality, 65 samples were included in the study, with five samples excluded due to poor RNA quality. Of these, 29 samples had sperm motility >20% (n = 29), while 36 samples had sperm motility <20% (n = 36), bringing the total to 65 samples included in RT-qPCR analysis. A total of 50 ng of isolated RNA was then reverse transcribed into complementary DNA (cDNA) utilizing the Mir-X miRNA First-Strand Synthesis Kit (Takara, USA). For the quantitative real-time PCR (RT-qPCR) reaction, a total volume of 20 μL was prepared. The reaction mixture consisted of 2 μL of cDNA, 4 μL of RNase-free water, About, 2 μL of a specific forward primer for miR-425-3p, and the mRQ 3′ universal tag sequence as the reverse primer (using the Mir-X miRNA First-Strand Synthesis Kit), along with 10 μL of 2x QuantiTect SYBR Green PCR Master Mix (Qiagen, Germany). The qPCR amplification was performed over 40 cycles under the following thermal cycling conditions; an initial denaturation at 95°C for 15 min, followed by 40 cycles of 94°C for 15 s (denaturation), 55°C for 30 s (annealing), and 70°C for 30 s (extension). The RT-qPCR procedure was performed in triplicate, incorporating negative controls (no template control and no reverse transcriptase control) for each sample to verify the specificity and reliability of the RT-qPCR results. To ensure accurate quantification of relative gene expression, the small nuclear RNA RNU6B was employed as an internal control for normalization.
2.6. Assessment of Oxidative Stress via Lipid Peroxidation Measurement
The evaluation of oxidative stress was conducted by measuring lipid peroxidation levels through the detection of thiobarbituric acid reactive species (TBARS) [21], specifically quantifying malondialdehyde (MDA), a key end product of lipid peroxidation and a reliable marker for ROS levels. From the remaining seminal plasma samples, a total of 30 were used for oxidative stress analysis. Of these, eight samples had sperm motility >20% (n = 8), while 22 samples had sperm motility <20% (n = 22). The upper organic layer containing the TBARS was carefully extracted and transferred to a sterile Eppendorf tube. Subsequently, 2.5 mL of either blank control (without MDA) or test samples was pipetted into a quartz cuvette. The absorbance of each sample was measured at 535 nm using a UV–Vis spectrophotometer (Thermo Fisher Scientific, USA).
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 9 for Windows (GraphPad Software, San Diego, CA, USA) and R software (v4.2.1, R Core Team). A Student’s t-test was applied to evaluate associations between variables with normally distributed data, while the Mann–Whitney U test was used for comparing differences between groups with nonnormally distributed data. ANOVA followed by Tukey’s HSD test was employed to compare the presence of specific bacteria, miR-425-3p, and oxidative stress levels. A p-value of less than 0.05 was considered statistically significant. The relative quantification of miRNA expression was performed using the 2−ΔΔCt method. The ΔCt value, representing the difference, was calculated using the formula: ΔCt = Ct (miR-425-3p) − Ct (RNU6B). Subsequently, the ΔΔCt value was determined using the formula: ΔΔCt = ΔCt (sperm motility >20%) − ΔCt (sperm motility <20%), where the group with sperm motility >20% served as the control. The relative expression levels of miRNA were then calculated using the formula 2−ΔΔCt, providing a quantitative measure of miRNA expression changes between the tested groups.
3. Results
3.1. Baseline Characteristics of the Study Subjects
The spermiogram characteristics of men with sperm motility >20% (n = 31), and those with sperm motility <20% (n = 39) are presented in Table 1. Men with sperm motility >20% showed statistically significant differences compared to men with sperm motility <20%, particularly in terms of sperm concentration (106/mL) and morphology (%) (p < 0.001). However, other parameters, such as age and semen volume, did not differ significantly between the two groups.
3.2. Bacterial Load and Sperm Motility Comparison
Bacterial isolates were identified in all 70 semen samples, with colony counts varying significantly between samples. Interestingly, the group with sperm motility >20% exhibited a notably higher bacterial load, with a mean of 20,373 CFU/mL. In contrast, the group with sperm motility <20% had a considerably lower mean bacterial count, measuring only 1247 CFU/mL. The difference in bacterial load between these two groups was statistically significant, with a p-value of 0.03, suggesting a potential association between higher bacterial load and increased sperm motility (Table S1).
DNA was successfully extracted from each individual bacterial colony, and the 16S rRNA gene, a widely conserved genetic marker in bacteria, was amplified using universal primers. The PCR amplification process yielded the expected amplicon size of approximately 1300 base pairs (bps), which aligns with the target region of the 16S rRNA gene. This result confirms the successful amplification and detection of bacterial DNA from each isolate, validating the presence of the target bacterial genetic material in all samples (Table S2).
3.3. Seminal Microbiota Composition
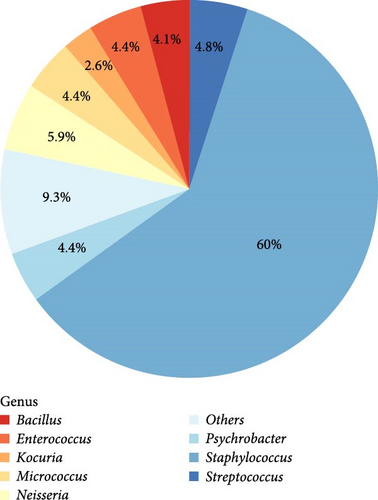
From a cohort of 70 semen samples, a total of 270 bacterial isolates were identified using 16S rRNA sequencing, showcasing a diverse range of bacterial genera based on their relative incidence. As depicted in Figure 1A, the genus Staphylococcus was predominant, accounting for 60% of the total isolates (162/270), making it the most abundant genus in the seminal microbiota. This was followed by Neisseria (5.93%, 16/270), Streptococcus (4.81%, 13/270), and Micrococcus (4.44%, 12/270), which also comprised notable portions of the microbiome. Genera such as Psychrobacter (4.44%, 12/270), Enterococcus (4.44%, 12/270), Bacillus (4.07%, 11/270), and Kocuria (2.59%, 7/270), though less abundant, still contributed to the overall microbial composition. The remaining 25 isolates (9.26%) were classified as “others,” representing a diverse array of less common genera that together enhance the microbial diversity within these samples (Table S3).
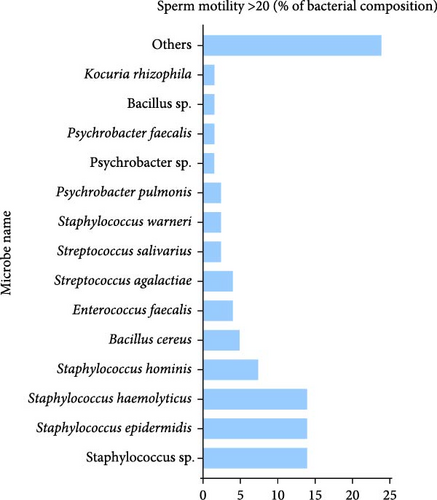
A comparative analysis of representative colonies between groups with differing sperm motility levels revealed distinct bacterial compositions. In the group with sperm motility >20%, the microbiota was predominantly composed of Bacillus cereus (4.92%) and Bacillus sp. (2.46%), which were unique to this group. Additionally, members of the Staphylococcus and Enterococcus genera were present, as illustrated in Figure 1B. Notably, Stutzerimonas stutzeri was identified, albeit at a very low frequency (0.82%).
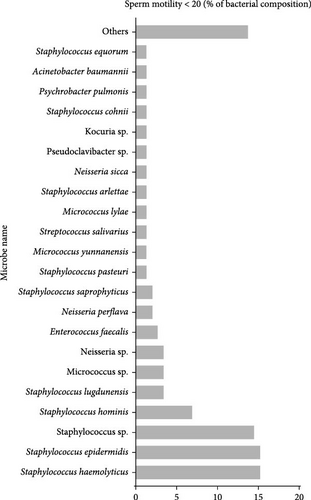
In contrast, the group with sperm motility <20% exhibited a more diverse bacterial profile, which was particularly striking. Clinically significant species, such as Acinetobacter baumannii (1.39%) and Neisseria sp. (3.47%), were highlighted due to their relevance in microbial ecology. Other bacterial species, including Corynebacterium glucuronolyticum, Pseudoclavibacter sp., Escherichia coli, and various species within the Enterococcus genus, were also detected, contributing to the group’s distinct bacterial landscape (Figure 1C).
3.4. Expression Level of miR-425-3p in Seminal Plasma
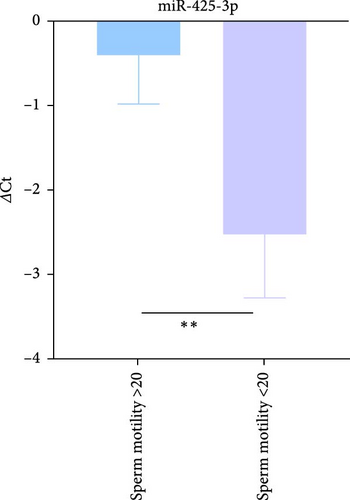
The expression levels of miR-425-3p were assessed between two distinct groups categorized by sperm motility; those with motility >20% and those with motility <20%. Using RT-qPCR, we identified a significant relative upregulation of miR-425-3p in the group with motility <20%, with a ΔCt value of −2.85. Conversely, the group with motility >20% exhibited a ΔCt value of −0.74 (indicating higher expression levels with lower ΔCt values). This difference was statistically significant, with a p-value of 0.034, as shown in Figure 2A. Furthermore, fold change analysis using the 2−ΔΔCt method demonstrated that the expression level of miR-425-3p was about fourfold (4.32) higher in the group with motility <20% compared to the group with motility >20%.
3.5. Association of miR-425-3p on the Seminal Microbiota Distributions
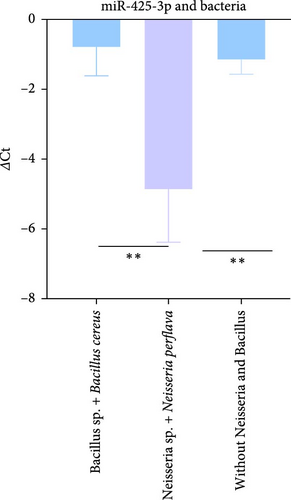
Our subsequent investigation focused on identifying potential variations in miR-425-3p expression associated with specific bacterial types. Notably, samples that were abundant in Bacillus sp. and Bacillus cereus but devoid of Neisseria demonstrated significantly reduced levels of miR-425-3p (∆Ct = −0.7886). In contrast, samples that lacked Bacillus species yet were enriched with Neisseria sp. and Neisseria perflava exhibited a markedly higher miR-425-3p expression level (lower ∆Ct = −4.84). Additionally, samples devoid of both Bacillus and Neisseria species had an intermediate expression level (∆Ct = −1.1).
The differences in miR-425-3p expression levels among these sample sets were statistically significant. Specifically, a comparison between samples abundant in Bacillus sp. and Bacillus cereus (without Neisseria) and those enriched with Neisseria sp. and Neisseria perflava yielded a p-value of 0.0164. Furthermore, the contrast in miR-425-3p expression between samples lacking Bacillus species and enriched with Neisseria and samples devoid of both Bacillus and Neisseria was also significant, with a p-value of 0.0028 (Figure 2B). These findings suggest a complex interplay between specific bacterial types and miR-425-3p expression in seminal fluid.
3.6. Seminal MDA Content
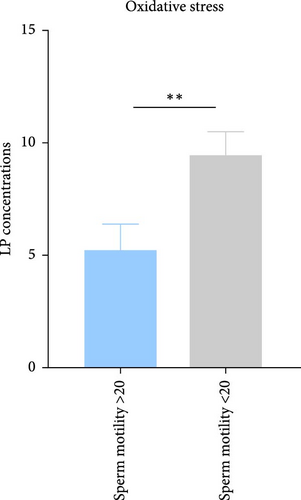
The assessment of lipid peroxidation, as indicated by MDA levels in seminal plasma, revealed significant differences between the studied groups. In individuals with sperm motility >20%, the mean MDA concentration was measured at 5.25 ± 3.32 nmol/mL. In contrast, individuals with sperm motility <20% exhibited a higher mean MDA level of 9.46 ± 5.07 nmol/mL. Statistical analysis indicated that the difference in MDA levels between the two groups was significant, with a p-value of 0.038 (Figure 2C). These findings suggest that increased lipid peroxidation may be associated with reduced sperm motility in the studied population.
3.7. Seminal Microbiota’s Contribution to Oxidative Stress
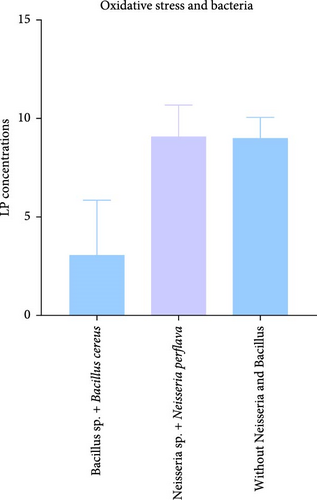
Our investigation also aimed to explore potential differences in oxidative stress associated with specific bacterial species present in seminal plasma. Notably, samples characterized by a high frequency of Bacillus species, including Bacillus cereus, and devoid of Neisseria showed lower MDA levels, averaging 3.07 ± 4.88 nmol/mL. In contrast, samples that lacked Bacillus species but were rich in Neisseria species, particularly Neisseria perflava, exhibited elevated MDA levels averaging 9.1 ± 2.2 nmol/mL. This difference approached significance, with a p-value of 0.09 (Figure 2D). Furthermore, samples abundant in Bacillus species and Bacillus cereus, while lacking both Neisseria perflava and Neisseria, demonstrated lower MDA levels of 3.07 ± 4.88 nmol/mL. In comparison, samples that lacked both Bacillus and Neisseria presented an average MDA level of 9.04 ± 4.77 nmol/mL, with a p-value of 0.0542.
4. Discussion
This study represents the first exploration into the possible involvement of miR-425-3p in shaping the seminal microbiota in relation to sperm motility. By analyzing the microbial communities present in semen samples, we identified a notable presence of bacterial genera such as Staphylococcus, Enterococcus, Bacillus, Micrococcus, and Neisseria. Lesser-known but relevant taxa like Streptococcus, Acinetobacter, Corynebacterium, Actinomyces, and Escherichia coli were also detected. The presence of these bacteria in seminal microbiota has been linked to male reproductive health in prior studies, emphasizing their relevance in the context of seminal microbial environments [22]. These bacteria are commonly associated with the human microbiome, and their presence in semen may impact male fertility. One particular bacterium, Staphylococcus haemolyticus, a significant part of the skin microbiota, has been shown to modify the sperm plasma membrane’s structure in vitro, potentially leading to male infertility [23, 24]. Additionally, Enterococcus faecalis is associated with poor semen quality [25]; however, in our study, both S. haemolyticus and E. faecalis were present in samples with sperm motility >20%. This finding suggests variability in bacterial effects, as these genera are usually linked to negative reproductive outcomes. Interestingly, Acinetobacter baumannii was detected in samples with sperm motility <20%, consistent with previous findings that exposure to A. baumannii reduces sperm motility in vitro [26] and that its presence is noted in semen samples of patients with chronic prostatitis syndrome (CPS) [27]. The presence of A. baumannii in semen may negatively affect sperm motility, possibly contributing to male infertility. Additionally, Acinetobacter, along with Pseudomonas and Escherichia, were recognized as key components of the prostate microbiome in cancer patients [28]. Some of these represent a normal flora in the reproductive tract, while their enrichment may affect reproductive health. Unique bacterial genera and species, such as Neorhizobium sp., Stutzerimonas stutzeri, Actinomyces oris, and Pseudoclavibacter, were also discovered in this investigation, which have not been previously reported in semen or associated with male infertility. These bacteria may represent novel contributors to seminal microbial composition, with unknown effects on fertility. Notably, Bacillus cereus was exclusively found in samples with sperm motility >20% and good semen quality. While there are no prior reports of Bacillus cereus in human semen, it has been found in bovine semen [29] and is used in probiotics for selenium enrichment [30].
The study observed differential expression of miR-425-3p in men with varying sperm motility. miR-425-3p was overexpressed in individuals with sperm motility <20% and underexpressed in those with sperm motility >20%. Previous research has also reported elevated miR-425-3p in individuals with oligoasthenozoospermia, as well as in men with spermatogenic impairments [14–16]. miR-425-3p may serve as a marker for impaired sperm function, with higher levels linked to poorer motility and can influence microbial composition and host health, impacting a variety of physiological systems [31]. Interestingly, our study showed that low miR-425-3p expression was associated with samples rich in Bacillus species, while high expression correlated with samples rich in Neisseria species. This suggests a relationship between miR-425-3p expression and specific bacterial profiles in semen, potentially influencing fertility outcomes. miR-425-3p is known to regulate cellular processes such as growth, survival, and proliferation, including pathways relevant to male reproductive function, such as the PI3K/AKT pathway [32]. Dysregulation of this pathway has been linked to decreased sperm motility [33]. The role of miR-425-3p in reproductive health may involve modulation of critical signaling pathways, further affecting sperm motility. miR-425-3p may target genes like mt-nd5 and MPV17 (TargetScan https://www.targetscan.org), and other genes involved in spermatogenesis and related processes [34]. Studies indicate that genetic changes in mt-nd5 disrupt complex I activity and reduce the mitochondria’s energy production capacity [35]. Furthermore, mutations in the MT-ND4 gene have been linked to idiopathic oligoasthenospermia [36]. Additionally, miR-425-3p also targets genes involved in antioxidant defense [37]. Our study found higher seminal MDA levels in men with poor motility, which aligns with previous reports [38]. The increase in oxidative stress, as evidenced by elevated MDA levels, may contribute to reduced sperm motility, underscoring the potential impact of oxidative damage on male infertility. The current study’s limitations include the absence of control over variables including food, smoking, antibiotics, and sexual activity that can alter the seminal microbiota, miRNA profiles and oxidative stress state, as well as the insufficient knowledge of the possible mechanism and connection between host miRNAs and microbiota. Additionally, human semen contains both aerobic and anaerobic bacteria, but our culture methods allowed us to detect only aerobic bacteria. Finally, we acknowledge that bacterial concentration may have greater clinical relevance than mere presence, which was not fully addressed in this study.
5. Conclusion
In conclusion, this study suggests that miRNAs, especially miR-425-3p, may affect the composition of the seminal microbiota and serve as potential biomarkers for male infertility. Additionally, exploring the probiotic use of Bacillus cereus could provide a strategy to reduce oxidative stress in semen. The interplay between seminal microbiota and miRNAs significantly influences male reproductive health; however, further research is necessary to fully elucidate the mechanisms underlying these interactions. Our findings indicate that targeting both microbiota and miRNAs could lead to innovative strategies for managing male infertility, although more investigation is needed to clarify these complex relationships.
Ethics Statement
The study received ethical approval from the Institutional Review Board (IRB) at Jordan University of Science and Technology (Reference No. 2022/151/28). Seventy semen samples were obtained from patients visiting in vitro fertilization (IVF) clinics at Prince Rashed Ben Al-Hasan Military Hospital in Irbid, Jordan.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Osamah Batiha was responsible for planning, supervision, funding acquisition, and assisting in manuscript writing. Raghad Abu Diak conducted the experiments and assisted with the manuscript writing. Mahmoud A. Alfaqih contributed the research concept and supported the planning stage. Qutaiba Ababneh managed the microbiota aspect, while Raghad Abu Al-Rub was involved in the oxidative stress experiments, and Mothanna Nawafleh handled sample collection and clinical oversight. Masood Abu-Halima supervised the qPCR work, coordinated and assisted with statistical analyses, and critically reviewed and organized the manuscript.
Funding
This study was funded by the Deanship of Research, Jordan University of Science and Technology (Grant 20220590).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting information of this article.