Shilajit Mitigates Diabetes-Induced Testicular Dysfunction in Mice: A Modulation in Insulin Sensitivity, Germ Cell-Junctional Dynamics, and Oxido-Apoptotic Status
Abstract
Diabetes is a chronic metabolic condition that causes testicular damage by high oxidative stress, rendering 35% of afflicted people infertile. Shilajit is a traditional Indian medicine known for its antioxidant, antidiabetic, and aphrodisiac properties. However, its effectiveness on diabetes-induced testicular dysfunction remains unclear. Therefore, the current investigation aimed to determine whether Shilajit could restore testicular functions in diabetic mice. Two days postpartum male Parkes mice received a single intraperitoneal injection of streptozotocin (STZ) (90 mg/kg BW) to induce diabetes. Three months postinjection, the effects of daily Shilajit (100 and 200 mg/kg BW) treatment were evaluated for one spermatogenic cycle in adult diabetic mice, using Empagliflozin (10 mg/kg BW) as a positive control. In STZ-induced diabetic mice, testicular functions were compromised due to disruptions in testosterone biosynthesis, changes in germ-cell ratios, and increased oxidative stress and apoptosis. Shilajit restored glycemic status in diabetic mice by significantly decreasing serum glucose, insulin level, and homeostasis model assessment of insulin resistance (HOMA-IR) value while increasing insulin sensitivity. These effects were comparable to those observed with conventional antidiabetic medication Empagliflozin. Further, Shilajit stimulates steroidogenesis and germ cell dynamics of diabetic mice by increasing the activity of StAR, 3β-HSD, and 17β-HSD enzymes and 1C:2C, 4C:S-Ph, and 1C:4C germ cell ratios, respectively. Shilajit also improves blood–testis barrier (BTB) functioning by increasing expression of ZO-1, Connexin-43, N-Cadherin, and β-catenin as well as oxidative status and apoptosis by modulating NF-E2–related factor 2 (Nrf-2)/heme oxygenase 1 (HO-1) signaling and Bax/Bcl-2 ratio. Subsequently, Shilajit improved the histoarchitecture of testis and epididymis in diabetic mice and recovered both qualitative and quantitative sperm parameters, as seen by higher percentages of sperm motility, viability, and normal sperm morphology as well as increased sperm numbers in cauda epididymis. In summary, Shilajit restores glycemic status, increases insulin sensitivity, stimulates steroidogenesis, and improves testicular functions through Sertoli cell and Nrf-2/HO-1 signaling in STZ-induced diabetic mice.
1. Introduction
Diabetes mellitus (DM) is a metabolic condition marked by chronic hyperglycemia that affects the normal physiology of several body functions [1]. It has been estimated that by 2045, there will be a 46% increase in diabetes cases among adults of 20–70 years of age [2]. One of the lesser-discussed but significant complications of hyperglycemia is its detrimental impact on male reproductive health [3]. DM and associated metabolic disturbances can impair steroidogenesis (the production of steroid hormones) and spermatogenesis (the production of sperm), leading to reduced fertility [4]. These impairments are often linked to oxidative stress and inflammation, which can disrupt the blood–testis barrier (BTB) and alter key signaling pathways within the testes [5]. Furthermore, oxidative stress, a common consequence of diabetes, exacerbates testicular damage by disrupting cellular functions and structures by disturbing NF-E2–related factor 2 (Nrf-2)/Kelch-like ECH-associated protein 1 (Keap-1) signaling [6].
In recent years, several medications have been used to manage diabetes, including glibenclamide, insulin, metformin (antihyperglycemic agents), aminoguanidine (an antiglycation agent), and empagliflozin [7]. However, long-term use of these conventional antidiabetic drugs is limited due to severe side effects and decreased efficacy [8]. Given the limitations of conventional antidiabetic medications, a natural supplement with both pro-fertility and antidiabetic properties could be a promising approach to managing reproductive functions in diabetes patients.
Shilajit, a natural exudate predominantly found in the Himalayas, has been used in traditional medicine for over 3000 years in different countries throughout the world, particularly India, China, Russia, Iran, etc. [9, 10]. Dibenzo-alpha pyrones (DBPs) and their metabolites, small peptides (which make up nonprotein amino acids), certain lipids, and carrier molecules (fulvic acids) make up the active ingredient of Shilajit. Also, there are at least 5%–7% DBPs in standard Shilajit [11]. Shilajit has been used as a main ingredient in most of the Ayurvedic antidiabetic formulations available. Further, it is regarded as a rejuvenator in Indian traditional medicine (Ayurveda), and contemporary systematic studies have confirmed its adaptogenic, antiaging, antidiabetic, rejuvenating, antioxidant, immunomodulatory, and antidyslipidemic properties, establishing Shilajit as a true panacea in traditional medicine [12–14]. These attributes make Shilajit a promising candidate for mitigating the adverse effects of diabetes on male reproductive health. We previously reported that Shilajit protects mouse testes from heavy metal and chemotherapeutic drug-induced reproductive dysfunction [15, 16]. Recently, it has been proven to minimize oxidative stress induced by hazardous compounds such as carboplatin, mercury chloride, and aspirin in rats [9, 17]. However, only a few research have documented the antidiabetic effects of Shilajit in diabetic rats treated with streptozotocin (STZ) and alloxan [11, 18]. And, till date, there is no evidence regarding the ability of Shilajit to ameliorate male infertility induced by diabetes despite being a well-known traditional aphrodisiac and rejuvenator along with having antidiabetic properties.
Thus, the current investigation was designed to see whether Shilajit could protect testicular functions from diabetes-induced reproductive impairment. There are several rodent models available for inducing diabetes and studying the processes underlying complications of diabetes, such as diabetes-induced reproductive dysfunction [19]. We have selected the neonatal-STZ (nSTZ) induced mouse model because it has an advantage over other models. It relies on the characteristics of pancreatic beta-cell death, which is followed by the regeneration of beta cells and subsequently glucose intolerance [20–22]. The nSTZ-treated mouse model is one of the most frequently used type-2 diabetes (T2D)-like models, with treated animals in maturity (adulthood) displaying the usual symptoms of T2D [22–24] and also mimics the pathophysiology of human diabetes [25]. Therefore, in this investigation, we assessed the effectiveness of Shilajit on testicular function in diabetic male mice using histology, immunohistochemistry, sperm analysis, western blot analysis, flow cytometric analysis, ELISA, and biochemical calorimetric estimations to elucidate the potential of Shilajit as a therapeutic agent in preserving male reproductive health amidst diabetic conditions.
2. Material and Methods
2.1. Chemicals
Unless otherwise stated, all chemicals used in this experiment were of research grade and were obtained from E. Merck or SRL India.
2.2. Experimental Materials and Their Doses
Purified Shilajit (Batch No.:20201001; Shilajit-964; containing 40% fulvic acid and 5% dibenzo-α-pyrones) was purchased from an authorized supplier [16]. Empagliflozin (Boehringer Ingelheim India Pvt. Ltd) and Shilajit were dissolved in double distilled water at dosages of 10 mg/kg body weight (BW) [26] and 100 and 200 mg/kg BW [15, 16], respectively, and administered orally for a duration of 35 days, corresponding to one spermatogenic cycle in mice.
2.3. Animal Care, Maintenance, and Experimental Design
- 1.
Control: Vehicle control (10 ml/kg BW per day orally for one spermatogenic cycle).
- 2.
DM: STZ (90 mg/kg BW, single IP injection at 2 days postpartum).
- 3.
DM + Emp.: STZ (90 mg/kg BW, single IP injection at 2 days postpartum), followed by Empagliflozin, positive control (10 mg/kg BW per day) for one spermatogenic cycle.
- 4.
DM + S100: STZ (90 mg/kg BW, single IP injection at 2 days postpartum), followed by Shilajit (100 mg/kg BW per day) for one spermatogenic cycle.
- 5.
DM + S200: STZ (90 mg/kg BW, single IP injection at 2 days postpartum) followed by Shilajit (200 mg/kg BW per day) for one spermatogenic cycle.
2.4. Sample Collection
Twenty-four hours after the last treatments, we sacrificed the experimental mice. After recording the final BW, we collected trunk blood and kept the serum at −80°C for future analysis. After being surgically removed, the testes were carefully cleansed to eliminate any last traces of blood and weighed precisely to within 0.10 mg. For sperm analysis, spermatozoa were obtained from the cauda epididymis. The distribution of testis tissue/group to perform different parameters is provided in Figure S1.
2.5. Measurement of Blood Glucose and Insulin Level
An Accu-Chek Active digital glucometer (range 10–600 mg/dl) was used to test the fasting blood glucose levels in each group both before and after they were treated with Empagliflozin and Shilajit. The insulin ELISA kit was used to test the serum insulin level in accordance with the manufacturer’s instructions (DiaMetra, Segrate, Milan, Italy).
2.5.1. Other Glycemic Parameters
Insulin sensitivity in each group was calculated as described by Ranjan et al. [27]. In brief, insulin sensitivity has been determined using the following formula: Insulin sensitivity = [14.1/(glu) (ins)] × 100. IR was calculated using the homeostasis model assessment (HOMA), as published before by Patil et al. [19]. The HOMA of insulin resistance (HOMA-IR) score was determined by the formula: HOMA-IR = [fasting blood glucose (mg/dl) × fasting serum insulin (µU/ml)/2430].
2.6. Sperm Analysis
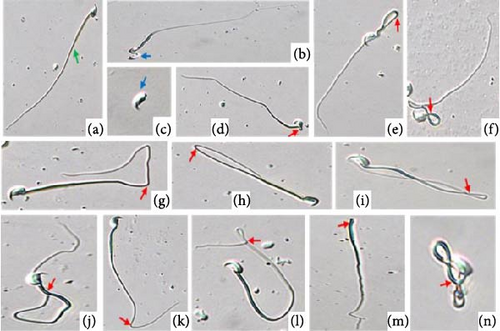
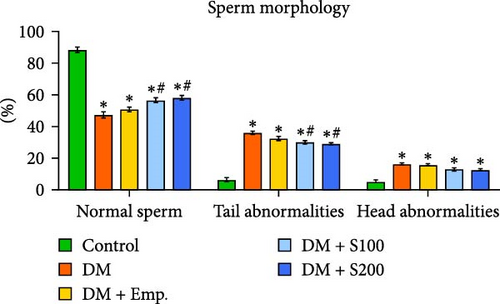
During the euthanasia procedure, spermatozoa from the cauda epididymis were collected in physiological saline. At 37°C, 0.5 ml of normal saline was used to mince the cauda epididymis in order to make sperm suspensions. The motility, morphology, viability, and concentration of the spermatozoa were then evaluated using this suspension, according to our previous study [16]. The morphological anomalies in the sperm suspension were examined using a light microscope at 400X magnification by smearing the suspension onto a clean glass slide. The standards established by Zaneveld and Polakoski [28] and Wyrobek and Bruce [29] were used to assess abnormalities in sperm. A total of 200 spermatozoa from each mouse were examined, with different fields of view covering both normal and abnormal morphology. Morphological abnormalities in spermatozoa were classified into two categories: those with tail abnormalities and those with head structure abnormalities. Concurrently, spermatozoa exhibiting normal morphological characteristics were also assessed [30].
2.7. Daily Sperm Production (DSP)
To determine the impact of Shilajit on DSP in diabetic mice, the number of 14–16 steps sonication resistant testicular spermatozoa heads was quantified. In short, the testis underwent homogenization in 1 ml of ice-cold double-distilled water, followed by a 1.5-min sonication. Subsequently, a hemocytometer was used to count the spermatozoa heads (400X phase-contrast microscope). In mouse spermatogenesis, developing spermatids in steps 14–16 requires 4.84 days. Thus, the number of spermatids (14–16 steps) was divided by 4.84 to calculate the DSP [31, 32].
2.8. Flow Cytometry
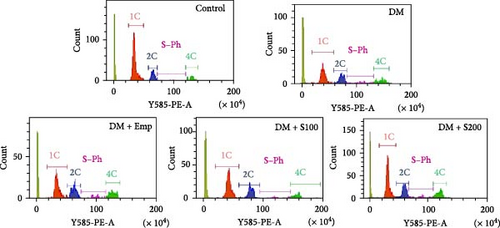
A flow cytometric analysis of germ cells was conducted to analyze the impact of Shilajit on testicular germ cell dynamics in diabetic mice [30]. In brief, the testes were placed in complete media (DMEM:Hams F12, 1:1) containing 0.25% collagenase (Type IA) (Sigma, St. Louis, USA). This mix was incubated for 15 min at a temperature of 37°C with moderate shaking after the removal of the tunica albuginea. The seminiferous tubules (ST) were washed thrice with PBS in a falcon tube to remove the interstitial cells, and then these tubules were allowed to settle for 10 min in ice. The tubules were minced using a sharp curved scissor to extract the germ cells. Tissue debris was eliminated, and a single-cell suspension was prepared by filtering the cell mixture through a 40 μm nylon mesh (Genetix Biotech Asia Pvt. Ltd.). Following centrifugation of the suspension at 3000 g for 5 min, the resulting pellet was subjected to two rounds of washing in PBS. At this stage, the cell count was assessed in each single-cell suspension. Thereafter, the cells were preserved by immersing them in chilled 70% methanol and storing them at a temperature of −20°C for a minimum of 24 h. At the time of analysis, 20-min incubation at 37°C was carried out using 0.25% RNase (Bangalore Genei, India). Following two washes in PBS, the cells were subjected to DNA staining using propidium iodide (PI; Sigma, St. Louis, USA) solution (50 µg/ml in PBS) and incubated in the dark at 4°C for 30 min. The flow cytometer captured fluorescence events at a frequency of around 150 per second. The DNA content distribution histogram was examined using CytExpert software [16].
2.9. Histopathology of Testis and Epididymis
To analyze the effect of Shilajit treatment on the histoarchitecture of testis and epididymis in normal and diabetic mice, periodic acid schiff-hematoxylin (PAS-hematoxylin) staining was performed following the methodology established by Mishra and Singh [33]. After dissecting, the mouse testis with epididymis was placed in Bouin’s solution immediately and then subjected to dehydration in a series of ethanol (50%, 70%, 90%, and absolute alcohol). About 6 µm thin sections of the testis with epididymis (embedded in paraffin wax) were cut and stained with the PAS-hematoxylin staining procedure. For this, the sections were dewaxed in xylene and gradually rehydrated using graded ethanol series (absolute alcohol, 90%, 70%, and 50%) and water. Then, the sections were treated with periodic acid. The slides were then soaked in running water for 25 min, followed by exposure to schiff’s reagent at a set temperature (4°C, 30 min). After that, the slides were treated with bisulfite solution (3 times for 2 min each) for differentiation, followed by staining with hematoxylin. Subsequently, the sections were subjected to dehydration using a series of alcohol solutions (50%, 70%, 90%, and absolute alcohol). After clearing in xylene, the slides were mounted in DPX and finally examined under a light microscope for histological analysis. At least 100 ST/group were examined and broadly categorized into three stages, I–VI, VII–VIII, and IX–XII, for presentation. For analyzing quantitative parameters such as ST diameter (STD) and germinal epithelium height (GEH), stage VII ST was used for measurement using motic software. The percentage of ST that were affected was also determined [33].
2.10. Immunohistochemistry of Proliferating Cell Nuclear Antigen (PCNA)
Immunohistochemical localization of PCNA (Table S1) was performed as outlined in our previous study [16]. The strength of the PCNA immuno-positive signals was subjectively assessed by carefully observing the PCNA-positive cells in testicular sections of each group.
2.11. Evaluation of Oxidative Stress
To examine how different treatments affect the testicular lipid peroxidation (LPO) and activity of antioxidant enzymes (superoxide dismutase [SOD] and catalase), a testis homogenate in (10% [w/v]) in 0.01 mol/l cold PBS (pH 7.4) was centrifuged for 10 min at 2600 rpm at 4°C. Half of the resultant supernatant was used to measure LPO, following the procedures outlined by Ohkawa, Ohish, and Yagi [34].
The other half of the supernatant was centrifuged again for 30 min at 4°C at 12,500 rpm. We utilized the resultant supernatant to evaluate the activity of SOD and catalase, following the methodologies described by Das, Samanta, and Chainy [35] and Sinha [36], respectively. We conducted protein estimation using Lowry’s method [37].
2.12. Testosterone and Estradiol Assay
The serum testosterone and estradiol levels were estimated using the ELISA kits (DiaMetra, Italy) following the instructions provided by the manufacturer.
2.13. Western Blot Analysis for Testicular Steroidogenic, Oxidative Stress, PCNA, Apoptotic, and BTB Markers
Western blot analysis was used to evaluate the expression of different markers, including PCNA, oxidative stress markers, steroidogenic markers, apoptotic markers, and BTB markers (Table S1). The detailed procedure of western blot analysis and estimation of proteins was carried out using the procedure established by Yadav et al. [38]. ChemiDoc (Analytik Jena) was utilized to detect the signals, and densitometry was performed using AlphaEaseFC. The antibodies utilized in this investigation are listed in Table S1, along with their dilution.
2.14. Statistical Analyses
GraphPad Prism (version 9.0) software was employed to analyze data in the form of mean ± SEM by one-way and two-way ANOVA using Tukey post hoc tests for intergroup comparison. At p < 0.05, the changes were found to be significant.
3. Results
3.1. BW and Testis Weight
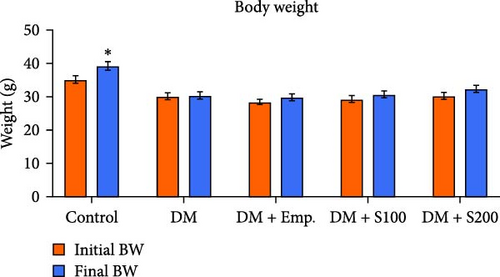
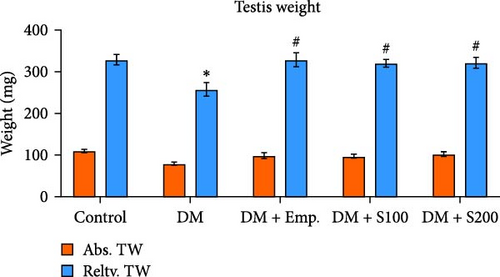
Final BW was found to be increased significantly in controls compared to their initial BW. However, there was no significant change in the initial and final BW of diabetic mice or diabetic mice treated with Empagliflozin and Shilajit (Figure 1A). Additionally, the relative testicular weight of diabetic mice was substantially lower when compared to controls; however, the relative testicular weight of diabetic mice administered with Shilajit was significantly higher compared to diabetic mice (Figure 1B).
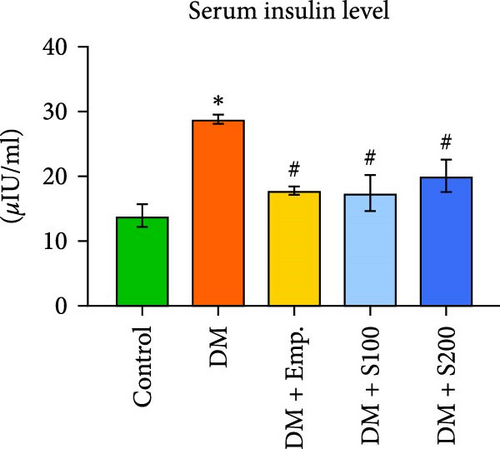
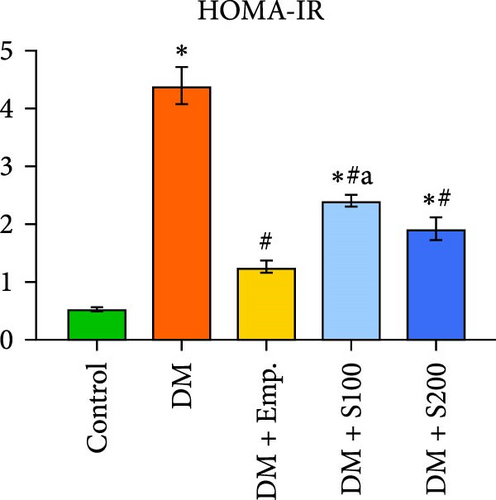
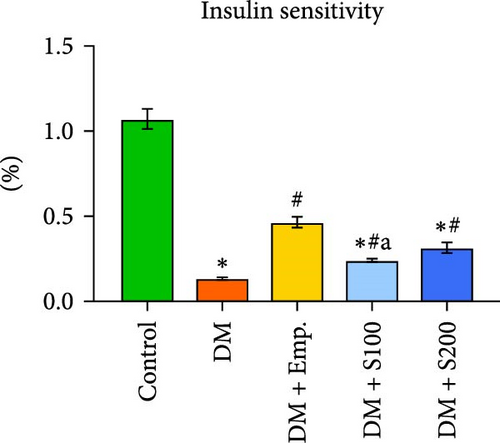
3.2. Blood Glucose, Serum Insulin Levels, HOMA-IR, and Insulin Sensitivity
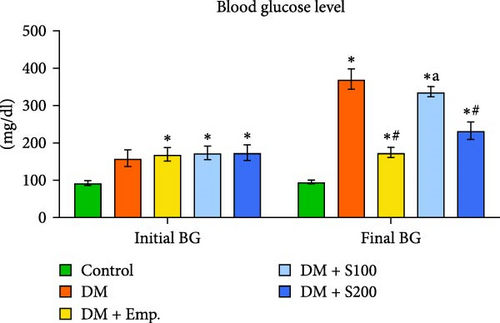
Compared to controls, diabetic mice exhibited significantly higher blood insulin and glucose levels, as well as HOMA-IR score, and significantly decreased insulin sensitivity (Figure 1C–F). However, in diabetic mice, treatment with Shilajit resulted in significantly lower blood glucose and insulin levels, as well as HOMA-IR score and a significantly higher insulin sensitivity (Figure 1C–F). Furthermore, at higher dose of Shilajit treatment, the decrease in final blood glucose, insulin levels, HOMA-IR, and increase in insulin sensitivity was comparable to Empagliflozin-treated mice (Figure 1C–F).
3.3. Sperm Parameters and DSP
Diabetic mice showed adverse effects on the morphology, count, viability, and motility of spermatozoa in cauda epididymis (Figure 2A,D). The most frequent morphological abnormalities in sperm were abnormal tails, followed by head abnormalities (Figure 2C,D). In contrast, Shilajit treatment in diabetic animals significantly improved the count, motility, viability, and morphology of spermatozoa in the cauda epididymis (Figure 2A,C,D).
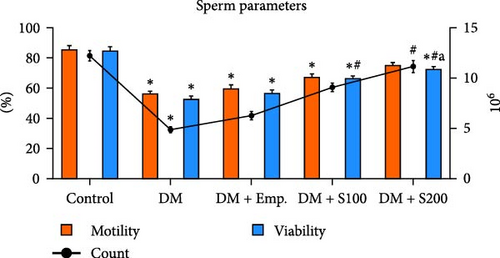
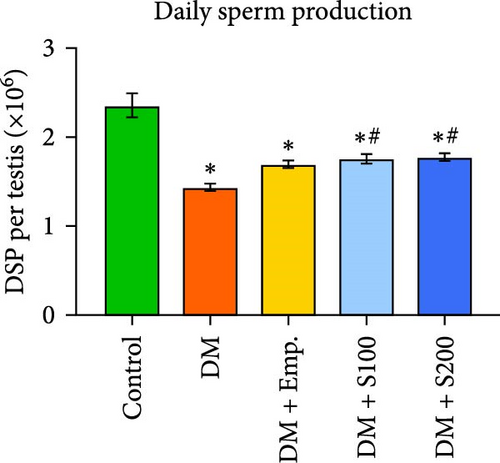
In comparison to controls, DSP was significantly decreased in diabetic mice. However, Shilajit administration significantly raised DSP in diabetic mice (Figure 2B).
3.4. Analysis of Germ Cell Dynamics
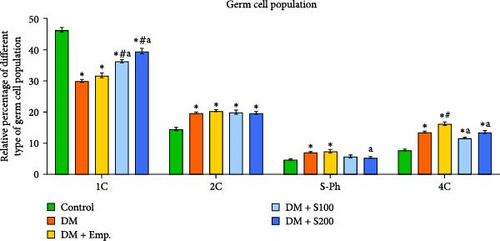
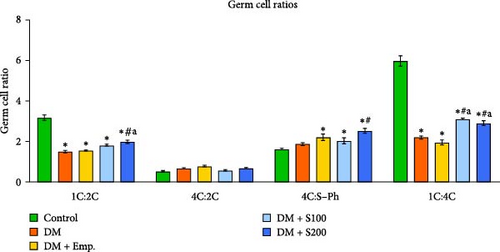
Flow cytometric data revealed that there was a significant decrease in the numbers of 1C germ cell populations, 1C:2C and 1C:4C germ cell ratios, and a significant increase in the numbers of 2C, S-Ph, and 4C populations in diabetic mice when compared to controls (Figure 3A–C). However, treatment with Shilajit markedly increased 1C populations along with an increase in 1C:2C, 4C: S-Ph, and 1C:4C germ cell ratios in diabetic mice (Figure 3A–C).
3.5. Histopathology of Testis
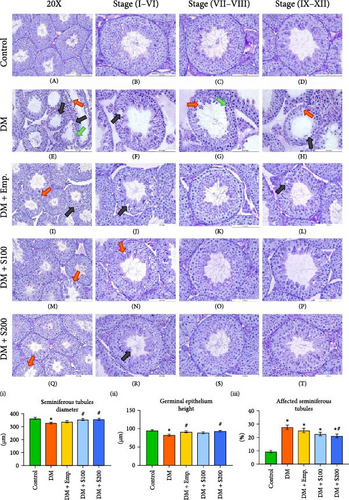
The testes of control mice showed normal ST histoarchitecture upon histological analysis (Figure 4A–D). Diabetic mice, on the other hand, displayed distinct histopathological alterations in the ST, including a reduction in germ cell number, vacuolization, and loosening of the seminiferous epithelium. All stages (I-XII) of the spermatogenic cycle showed these adverse changes (Figure 4E–H). However, treatment of diabetic mice with low and high doses of Shilajit (Figure 4M–P; Figure 4Q–T, respectively) resulted in marked improvement in the histoarchitecture of ST except for very few vacuoles and a little bit of loosening of germinal epithelium. Additionally, Shilajit treatment significantly improved GEH, decreased the affected ST, and increased STD when compared to diabetic mice, according to quantitative analysis of histopathology (Figure 4(I)–(III)).
3.6. Histopathology of Epididymis
The histology of epididymis in controls was normal in all segments (I–V) (Figure 5A–E). On the other hand, epididymis of diabetic mice showed noticeable histological alterations, such as intraepithelial vacuoles in segments II and a relatively lesser number of spermatozoa in segments IV and V (Figure 5F–J) as compared to controls. Treatment with Shilajit (Figure 5P–T, low dose; 5U-Y, high dose) restored normal histology in nearly all segments (I–V) and increased the number of spermatozoa in segment V of the epididymal lumen in diabetic mice.

3.7. Analysis of PCNA
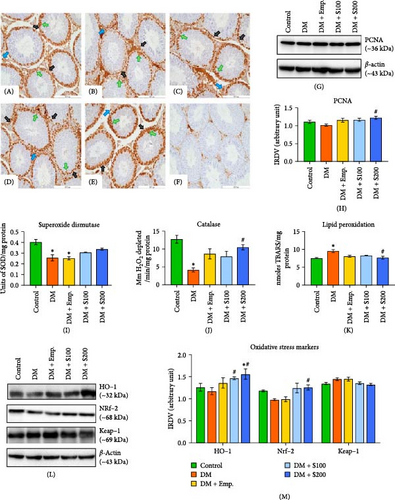
PCNA immunohistochemistry in testicular tissue demonstrated the presence of this protein in both spermatogonia and early spermatocytes in controls (Figure 6A). However, diabetic mice (Figure 6B) showed relatively less PCNA-immunopositive signals compared to control mice. Shilajit treatment, on the other hand, comparatively increases PCNA-immunopositive signals in diabetic mice (Figure 6D,E). However, western blot examination of PCNA showed no significant difference in expression between control and diabetic mice. In contrast, supplementation with a high dosage of Shilajit markedly improved the expression of PCNA in diabetic mice (Figure 6G,H).
3.8. Analysis of SOD, Catalase, and LPO
LPO level was significantly higher in the testis of diabetic mice compared to controls (Figure 6K); however, SOD (Figure 6I) and catalase (Figure 6J) activity were found to be significantly decreased. Shilajit treatment at a higher dose significantly reduced LPO levels increased by diabetic induction in the testis, while no change was observed in SOD activity compared to diabetic mice. Catalase activity was increased significantly at a higher dose of Shilajit compared to diabetic mice.
3.9. Analysis of Heme Oxygenase 1 (HO-1), Nrf-2, and Keap-1
Western blot analysis showed no statistically significant difference in Keap-1 expression in diabetic mice compared to controls. Shilajit at a higher dose decreases Keap-1 expression compared to diabetic mice (Figure 6L–M). Further, diabetic mice showed a notable decrease in expression of Nrf-2 and HO-1 compared to controls. In contrast, Shilajit treatment significantly elevated the expression of HO-1 and Nrf-2 in diabetic mice (Figure 6L–M).
3.10. Serum Testosterone and Estradiol Level
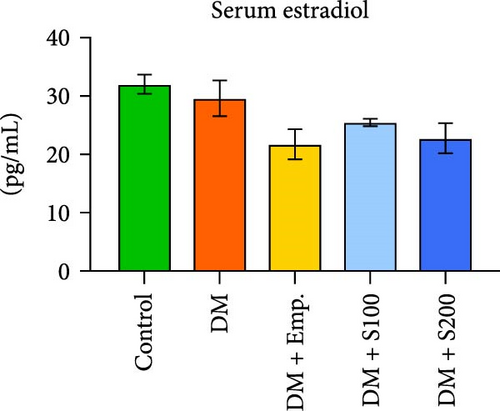
Diabetic mice had significantly lower testosterone levels than the controls. However, Empagliflozin and a high dose of Shilajit treatment resulted in a significant increase in serum testosterone levels in diabetic mice (Figure 7A). In contrast, there was no significant difference in estradiol levels in any of the treatment groups (Figure 7B).

3.11. Analysis of Steroidogenic Markers
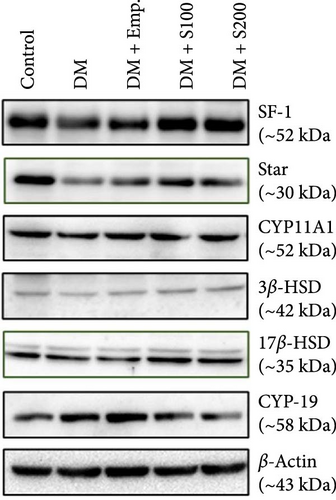
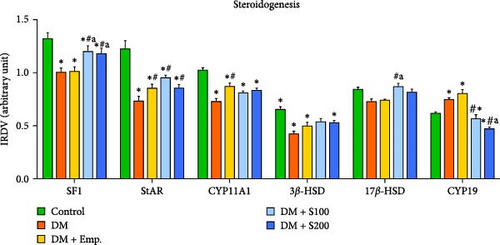
Western blot analyses revealed that there was a significant decrease in expression of SF-1, StAR, CYP11A1, and 3β-HSD in diabetic mice when compared to control mice. However, diabetic mice, when treated with Shilajit, showed significantly increased expression of SF-1, StAR, and 17β-HSD, along with a nonsignificant increase in expression of CYP11A1 and 3β-HSD. CYP19 expression was considerably increased in diabetic mice when compared with controls. On the other hand, diabetic mice treated with Shilajit showed significantly decreased expression of CYP-19 compared to diabetic mice (Figure 7C,D).
3.12. Analysis of Apoptotic Markers
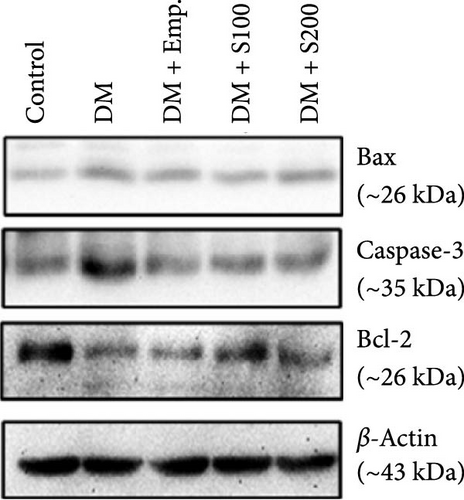
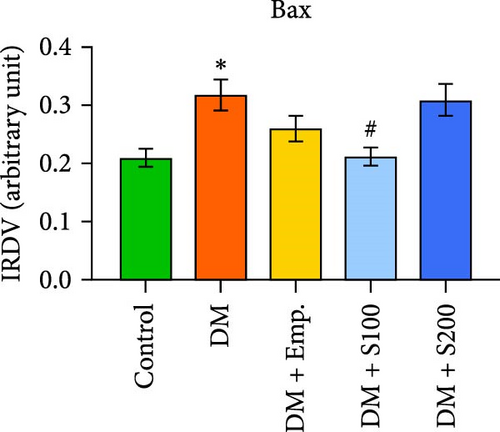
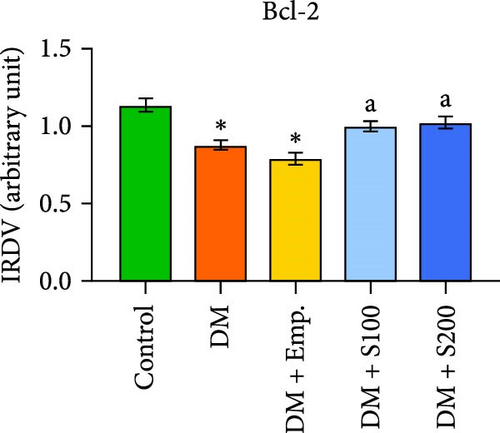
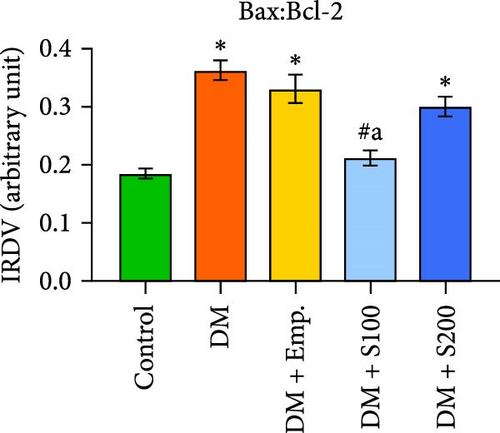
Western blot analyses revealed that in diabetic mice, Bax expression was significantly enhanced, but Bcl-2 expression was significantly lowered compared to controls. Nevertheless, following the administration of Shilajit to the diabetic mice, the expression of Bax was significantly decreased at a lower dose of Shilajit, and the expression of Bcl-2 was increased, although the increase was not statistically significant. The expression of caspase-3 and the Bax: Bcl-2 ratio were significantly higher in diabetic mice compared to control mice. Shilajit supplementation in these diabetic mice, however, markedly decreased the Bax: Bcl-2 ratio and Caspase-3 expression (Figure 8A–E).

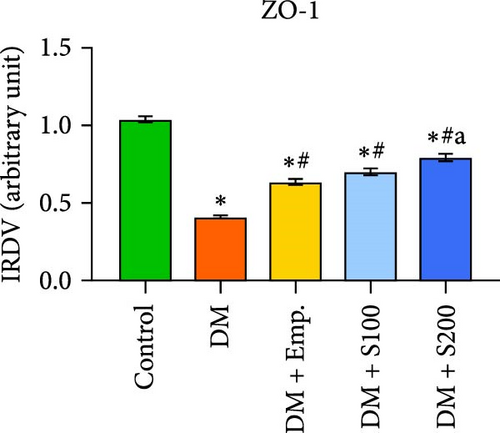
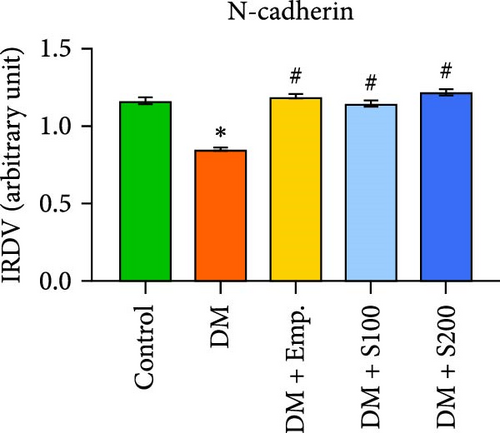
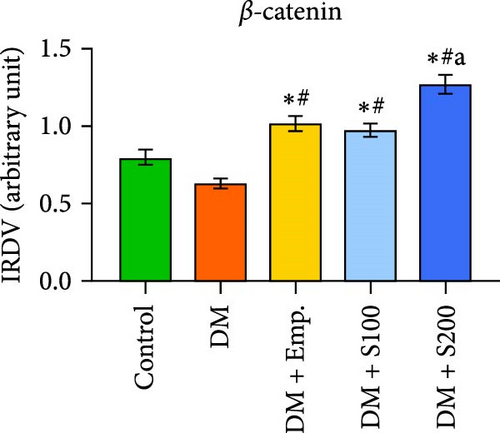
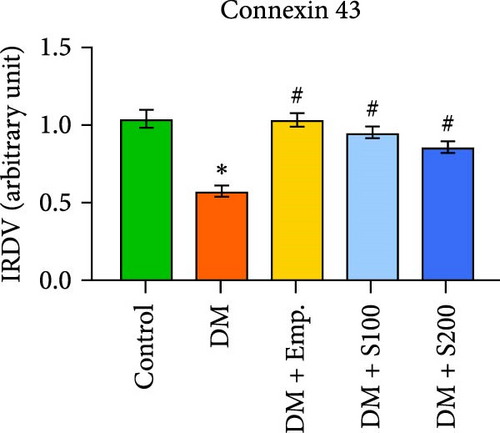
3.13. Analysis of BTB
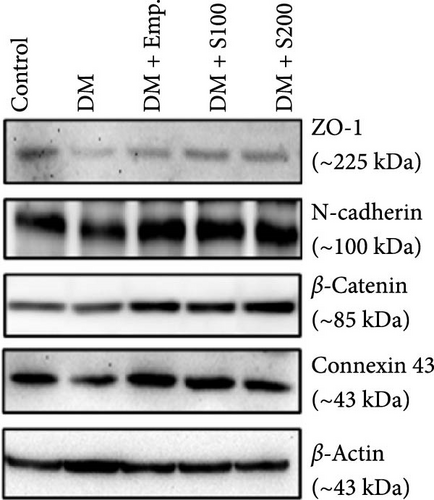
Western blot analyses revealed that the expression of ZO-1, Connexin-43, and N-Cadherin significantly declined in diabetic mice compared to controls. However, Shilajit treatment significantly increased ZO-1, N-cadherin, and Connexin-43 expression in diabetic mice (Figure 8F–J). Further, there was no significant change in the expression of β-catenin in controls and diabetic mice. However, Shilajit treatment significantly increased β-catenin expression in diabetic mice (Figure 8F–J).
4. Discussion
Diabetes-induced dysfunction in spermatogenesis and steroidogenesis underscores the necessity for supplements that protect the testes from the detrimental effects of hyperglycemia on male reproduction [39]. Conventional antidiabetic medications exhibit limited efficacy and substantial side effects [40]. Therefore, there is an urgent need for safe and natural medicines that protect male reproductive health under diabetic conditions. Therefore, this investigation aims to examine the antioxidant, antidiabetic, and spermatogenic properties of Shilajit in the context of diabetes-induced reproductive dysfunction.
Hyperglycemia induced by STZ is commonly used to investigate the pathophysiology of diabetes [41]. We also determined HOMA-IR using fasting blood glucose and insulin levels. HOMA-IR is a simplified measure for evaluating insulin resistance and is widely used in clinical and animal investigations [42]. In the present study, STZ-treated diabetic mice exhibited hyperglycemia with insulin resistance, as evidenced by increased HOMA-IR, resulting in hyperinsulinemia and decreased insulin sensitivity. Shilajit administration significantly lowered fasting blood glucose in diabetic mice in a dose-dependent way. The decrease in serum insulin level indicates that the body requires less insulin, a typical sign of improved insulin sensitivity observed after Shilajit treatment. Notably, the beneficial effects of Shilajit at higher dose were comparable to those of the conventional antidiabetic drug Empagliflozin. Furthermore, the decrease in HOMA-IR supports the notion of improved insulin sensitivity following Shilajit treatment under hyperglycemic conditions.
Hyperglycemia induced by DM reduced absolute and relative testicular weights, highlighting its specific impact on the male reproductive system. Further, diabetes increases gluconeogenesis, glycogenolysis, and lipolysis, contributing to tissue protein loss, which negatively affects the BW and weight of reproductive organs [43]. Shilajit treatment increased testicular weight, indicating a restorative effect on testicular function compromised by diabetes.
Long-term diabetes disturbs the homeostasis of metabolic processes within the testes due to hyperglycemia and hyperinsulinemia, ultimately leading to oxidative stress [44, 45]. Germ cells are highly susceptible to oxidative stress, leading to testicular germ cell apoptosis [30, 46]. In the present investigation, the level of LPO in testicular tissue significantly increased while the activity of SOD and catalase was significantly decreased, suggesting oxidative stress in diabetic conditions. Shilajit improved the antioxidant status of the testis in diabetic mice by reducing LPO and raising SOD and catalase activity. Shilajit has been shown in several previous studies to reduce oxidative stress caused by mercury chloride and carboplatin in rat models [9, 17]. Further, Nrf-2, a transcription factor, is essential for reducing oxidative stress by regulating intracellular antioxidants (SOD, catalase) [47]. HO-1, an antioxidant and anti-inflammatory enzyme induced by Nrf-2, protects cells from oxidative stress and maintains cellular homeostasis [48]. Both HO-1 and Nrf-2 activate antioxidant genes in response to oxidative stress in the testes [49]. Additionally, the Nrf-2/HO-1 signaling pathway plays a crucial role in maintaining mitochondrial homeostasis, regulating Ca++ concentration, regulating ferroptosis, and regulating autophagy. Hyperglycemia downregulates HO-1 and Nrf-2 while upregulating Keap-1, an inhibitor of Nrf-2, in the testes [50, 51]. Furthermore, excessive reactive oxygen species (ROS) production in diabetes contributes to disturbances in mitochondrial homeostasis [52], an increase in calcium-sensing receptors [53], the induction of ferroptosis [54], and dysregulation of the autophagic process [55] in the testes of diabetic mice, likely through alterations in the Nrf-2/HO-1 signaling pathway. In the current study, Shilajit treatment upregulated the expression of Nrf-2 and HO-1 in the testes of diabetic mice, suggesting its protective role against oxidative stress under hyperglycemic conditions. The ameliorative effect of Shilajit on the Nrf-2/HO-1 signaling pathway in diabetic mice may be attributed to its modulation of mitochondrial homeostasis. Notably, a previous study demonstrated that Shilajit preserves mitochondrial function and integrity in rats with chronic fatigue syndrome by modulating mitochondrial bioenergetics [56].
DM induces apoptosis in the long term, resulting in the loss of Sertoli and spermatogonial cells [57]. In this study, the diabetic condition significantly increased the Bax (pro-apoptotic marker) and decreased the Bcl-2 (antiapoptotic marker) in testicular tissue. The ratio of Bax to Bcl-2 has been identified as an important factor that determines cell fate [16, 58]. Shilajit supplementation significantly reversed this trend by upregulating Bcl-2 expression and downregulating Bax in the testis of diabetic mice, indicating the potential of Shilajit to increase antiapoptotic properties, as evidenced by the decreased Bax/Bcl-2 ratio.
Oxidative stress, together with insulin resistance, downregulates LH receptors, potentially leading to decreased testosterone biosynthesis in diabetic mice [27, 59]. Reduced activity of key steroidogenic enzymes and proteins, as observed in the present investigation in diabetic mice, suggests hyperglycemia induces alteration in testosterone biosynthesis. Our findings showed that Shilajit facilitates the conversion of cholesterol by the CYP11A1 enzyme to pregnenolone and pregnenolone via the Δ5 3β-HSD and 17β-HSD enzymes to testosterone. Additionally, Shilajit lowers the activity of aromatase, which inhibits the conversion of testosterone to estradiol. Thus, Shilajit appears to stimulate steroidogenesis in diabetic mice, with a stronger stimulatory effect at higher dose.
Testosterone maintains the integrity and functioning of the BTB, Sertoli-germ cell adhesion, and the release of spermatozoa into the lumen, all were compromised by diabetes [57, 60]. Low levels of testosterone and disturbed glucose homeostasis in diabetic mice resulted in disturbances in spermatogenesis [61]. Sertoli cells provide essential structural support, form the BTB, and engage in dynamic interactions with germ cells, collectively contributing to the overall integrity and organization of the germinal epithelium. The key phases of spermatogenesis predominantly occur during stages I–VIII, wherein Sertoli cells regulate the internal environment of ST via the BTB and interact directly with germ cells. Any alteration in Sertoli cell function compromises the structural integrity of seminiferous epithelium [62, 63]. Disruption of the BTB, which is the initial step in testicular damage, results in meiotic arrest and adversely impacts spermatogenesis and male fertility [5, 39]. In this study, BTB-associated proteins (ZO-1, Connexin-43, N-Cadherin, and β-catenin) were downregulated post-diabetes induction, leading to severe degenerative changes in seminiferous epithelium such as intraepithelial vacuolization, loosening and thinning of the germinal epithelium. These degenerative changes were frequent in all stages (I–XII) of spermatogenesis. The severely damaged structure of seminiferous epithelium caused by disorganization in BTB structure following diabetes, as mentioned above, hinders spermiogenesis, spermiation, and total spermatogenesis, as proven by our flow cytometric data. Diabetes severely diminished 1C populations and 1C:2C and 1C:4C ratios in mice, showing suppression of spermatogonia transformation to spermatids. The marked decline in the 1C population after diabetes induction indicates the high sensitivity of postmeiotic cells to diabetes. These results were further confirmed by reduced DSP and PCNA expression, an established marker of cell proliferation associated with spermatocyte and spermatogonia proliferation [43]. Shilajit supplementation counteracted the detrimental effects of diabetes on testis histoarchitecture, BTB proteins, the transformation of spermatogonia to spermatids, and PCNA expression, and, thereby, overall spermatogenesis. Shilajit administration assures that adequate testosterone is available for normal spermatogenesis in diabetic mice by stimulating steroidogenesis and reducing aromatase activity.
Spermatozoa moving from the testis to the initial segment of the epididymis are functionally not mature and lack motility. The epididymis plays a crucial role in the functional maturation of spermatozoa [33, 64]. Being an androgen-dependent organ, the epididymis requires optimal levels of testosterone and a functional microenvironment for the maturation and development of progressive motility in spermatozoa [65, 66]. In our study, diabetic mice showed a significant decrease in sperm concentration, motility, viability, and morphology. The decrease in cauda epididymal sperm count might be due to the adverse effects of diabetes on spermatogenesis, as observed in our investigation. Furthermore, the negative effects of diabetes on motility, viability, and morphology may result from the detrimental impact of diabetes on the epididymal functional environment, as evidenced by altered histoarchitecture and decreased testosterone levels in our study. In contrast, Shilajit supplementation improved cauda epididymal sperm count by enhancing testicular germ cell dynamics in diabetic mice, as observed in our investigation. The improvement in motility, viability, and morphology following Shilajit supplementation appears to result from the restoration of the epididymal functional environment in diabetic mice. Notably, Shilajit supplementation has been shown to restore the functional environment of the epididymis by increasing sialic acid secretion and testosterone levels in heavy metal-exposed mice [15, 16].
5. Conclusion
In conclusion, Shilajit supplementation shows considerable promise in alleviating diabetes-induced reproductive dysfunction. It enhances insulin sensitivity, reduces oxidative stress, and promotes spermatogenesis and steroidogenesis. Shilajit effectively restores testicular function and the epididymal environment, mitigating the adverse impacts of diabetes on sperm concentration, motility, viability, and morphology. This study demonstrates the beneficial effects of Shilajit in reducing oxidative stress and enhancing the antioxidant system, primarily via activation of the Nrf-2/HO-1 signaling pathway. Notably, our investigation is the first to highlight the role of Shilajit in improving germ cell dynamics and Sertoli cell functions (maintaining the integrity of BTB) by modulating steroidogenesis and reducing apoptosis. These findings suggest that Shilajit could serve as a safe, natural therapeutic option for protecting male reproductive health under diabetic conditions, providing benefits comparable to those of conventional antidiabetic drugs.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Raghav Kumar Mishra: experimental plan, supervision, data interpretation, manuscript writing. Arti Rajpoot: animal experimentation and execution of the experimental plan, data interpretation, statistical analysis, the first draft of manuscript, figures, and tables. Ajai Kumar Pandey: data interpretation, review of manuscript. Vikas Kumar Roy: data interpretation, review of manuscript.
Funding
This investigation was funded by IoE (6031) from Banaras Hindu University to Raghav Kumar Mishra.
Acknowledgments
Arti Rajpoot is a recipient of JRF and SRF from CSIR, New Delhi (09/013(0899)/2019-EMR-I).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.