Efficient DNA-Free Protoplast Gene Editing of Elite Winegrape Cultivars for the Generation of Clones With Reduced Downy Mildew Susceptibility
Abstract
Background and Aims: The grape and wine sector in Australia and around the world is under increasing pressure from climate change, disease threats and sustainability concerns, necessitating the development of fast and innovative solutions that can also preserve the sought-after quality traits of elite cultivars.
Methods and Results: A simplified and cost-effective method for DNA-free protoplast gene editing and plant regeneration was assessed for its broad applicability using the two most common red and white winegrape cultivars commercially grown in Australia. Plant regeneration and editing efficiencies were investigated with downy mildew disease and associated susceptibility genes, VviDMR6-1 and VviDMR6-2, as the trait and gene-editing targets. High rates of edited plant regenerants displaying normal growth and development were recorded for all four cultivars within 4–5 months, and flow cytometry assays were employed to assess the ploidy status of regenerants and filter out nondiploid plants. Fungal assays with glasshouse-grown, edited Chardonnay plants suggested a previously unreported role for VviDMR6-2 and jasmonic acid in grapevine susceptibility to downy mildew.
Conclusions: This study has demonstrated that a relatively simple and robust protoplast isolation, DNA-free protoplast transfection and plant regeneration workflow can be used to efficiently produce nontransgenic, diploid, edited clones with desired phenotypes of four elite winegrape cultivars, including the highly recalcitrant Cabernet Sauvignon.
Summary
- •
Significance of the Study
- ∘
The DNA-free editing workflow reported in this study is a major step towards addressing many of the challenges that the winegrape sector is facing by enabling fast and targeted genetic improvements of elite cultivars. Resulting improved clones could be a key component of typicity-preserving strategies to counteract major threats to the sector as demonstrated by the increased resistance of edited Chardonnay plants to downy mildew infection.
1. Introduction
Innovative approaches to alter a multitude of winegrape (Vitis vinifera L.) traits are urgently required to safeguard the grape and wine sector in Australia, and around the world, from climate change–related challenges affecting grape growing and wine making [1], and to enable the adoption of more sustainable practices, including reduced fungicide applications [2]. In addition to modified management practices, solutions to these major challenges will need to involve overcoming the limited genetic adaptation potential of winegrapes, common to all vegetatively propagated crops [3]. This includes conventional breeding strategies employed in many countries with significant winegrape production [4], but, due to high levels of heterozygosity and long generation times, fast and targeted trait improvements that allow for the preservation of typicity and marketability of established, commercially important elite cultivars require the use of genetic transformation approaches or gene-editing tools such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) [5]. Unlike genetic transformation, which introduces novel genetic materials typically derived from other organisms, and which is burdened by commercialisation-inhibiting regulations and social acceptability issues [6], gene-editing methods can modify the existing genetic material in a way that is extremely precise and indistinguishable from naturally occurring mutations [7]. As a result, an increasing number of jurisdictions around the globe, including Australia, have exempted products of mutagenic gene editing from genetically modified organism (GMO) regulations [8], which is also expected to aid with public acceptance of gene-edited crops and food products [6].
CRISPR/Cas9-based crop improvement technologies therefore offer the opportunity to develop sustainable, nontransgenic solutions to many of the challenges facing the grape and wine sector, provided that associated genetic factors can be identified and targeted for beneficial trait modification.
Protoplasts are attractive vesicles for the delivery of the CRISPR/Cas9 gene-editing machinery as the cell wall barrier is removed, allowing efficient transfection with plasmids or ribonucleoproteins (RNPs) [9]. In addition, the targeting of single cells reduces the occurrence of chimerism in regenerants [10] and enables the generation of edited clones of the plant from which the transfected protoplasts originate, making it the editing method of choice for vegetatively propagated crops [11]. However, grapevine has proven to be a recalcitrant plant with regard to protoplast generation [10, 12], induction of embryogenesis, and plant regeneration [13], and hence only limited grapevine cultivars, namely Chardonnay [14, 15], Crimson Seedless and Sugraone [16, 17], Colombard and Merlot [18] as well as Thompson Seedless [18, 19] and Nebbiolo [20], have successfully undergone protoplast RNP transfections, with even fewer reports of edited table grape [16, 17, 19] and winegrape [18, 20] regenerants. Common to all these reported studies on protoplast-based, DNA-free editing of grapevine is their limited practical applicability for the genetic improvement of grapevine cultivars, either due to low recovery rates of regenerated mutant plants [16, 17, 19, 20], or the use of complex regeneration techniques involving feeder cultures [18].
The aim of this study was to establish a feasible pathway for the production of improved clones of elite winegrape cultivars by testing a simplified and cost-effective method for DNA-free protoplast gene editing and plant regeneration on the two most common red (Vitis vinifera cv. Shiraz and Cabernet Sauvignon) and white (V. vinifera cv. Chardonnay and Sauvignon blanc) grape cultivars commercially grown in Australia [21].
Downy mildew (DM; Plasmopara viticola ((Berk. & M.A. Curtis) Berl. & De Toni)) infection/disease was chosen as the target trait to modify, as it is a perfect example of an economically important trait for which currently no sustainable management strategy exists [22], but for which suitable gene-editing target genes of the downy mildew resistant 6 (DMR6) family have been identified and inactivated to produce mutants of several plant species, such as tomato [23], potato [24], banana [25], sweet basil [26] and grapefruit [27], with reduced susceptibility to DM and a range of other pathogens. Grapevine contains a family of five DMR6/DMR6-like genes [28] with overexpression and complementation studies in Arabidopsis thaliana L. as well as gene expression and network analyses [29] initially suggesting VviDMR6-1 as the most promising candidate gene for use in gene-editing approaches to obtain grapevine plants with increased resistance to pathogen infection. Since then, a couple of gene-editing studies on table grapes using transgenic or RNP-based gene-editing approaches have produced contradictory results suggesting that either the inactivation of one VviDMR6 gene (VviDMR6-1 or its homologue, VviDMR6-2) is sufficient for producing grape clones with reduced DM susceptibility [30], or that both VviDMR6 genes need to be edited for increased DM resistance to manifest [31]. In this work, editing of the DM susceptibility genes VviDMR6-1 and VviDMR6-2 was used as a case study to assess the cross-cultivar performance of the adapted winegrape clone production workflow. New findings regarding the contribution of the two target genes to DM susceptibility and changes to plant defence hormone abundances are presented for VviDMR6-edited, glasshouse-grown Chardonnay mutants.
2. Materials and Methods
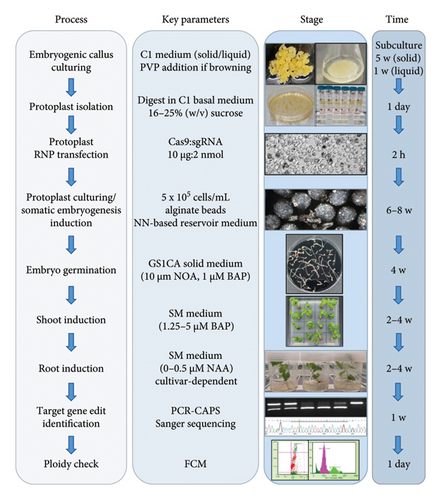
2.1. Initiation and Solid Media Culturing of Embryogenic Callus
Immature grapevine flowers were collected in the spring (mid-September to November) of 2021 (V. vinifera cv. Chardonnay clone V1011, Shiraz clone BVRC12) or 2022 (Sauvignon blanc clone F4V6) from a local research vineyard (−34°.97, 138°.63, elevation 100 m) or from glasshouse-grown canes in July-August 2022 (V. vinifera cv Cabernet Sauvignon clone CW44). Flowers were surface-sterilised for 40 s in 7% (w/v) calcium hypochlorite with Tween 20 (1-2 drops/100 mL) and then rinsed thoroughly with sterile water. Embryogenic callus formation was initiated from anthers on either PIV medium [32] with 4.5 μM 2,4-dichlorophenoxyaceticacid (2,4-D) and 8.9 μM benzylaminopurine (BAP) or HT medium (Chardonnay) [33] with 10 μM 2,4-D and 5 μM thidiazuron (TDZ).
Both media contained the salts and vitamins as listed for PIV, with sucrose reduced to 3% (w/v) in HT medium, and both were solidified with gellan gum (CultureGel—Type 1; PhytoTechnology Laboratories, Shawnee Mission, KS, USA).
After 67–82 days of culturing in the dark at 27°C on initiation medium, emerging embryogenic callus was transferred to C1 plates (1.65 g/L NH4NO3, 440 mg/L CaCl2·2H2O, 370 mg/L MgSO4·7H2O, 1.97 g/L KNO3, 170 mg/L KH2PO4; 37.2 mg/L Na2EDTA·2H2O, 9.3 mg/L FeSO4·7H2O, 6.2 mg/L H3BO3, 0.025 mg/L CoCl2·6H2O, 0.025 mg/L CuSO4·5H2O, 22.3 mg/L MnSO4·4H2O, 0.25 mg/L Na2MoO4·2H2O, 0.83 mg/L KI, 8.6 mg/L ZnSO4·2H2O; 100 mg/L myo-inositol, 10 mg/L thiamine HCl, 10 mg/L nicotinic acid, 1 mg/L pyridoxine HCl, 1 mg/L D-pantothenic acid, 0.01 mg/L biotin; 100 mg/L L-glutamic acid, 10 mg/L L-phenylalanine, 2 mg/L glycine, 5 μM 2,4-D, 1 μM BAP, 0.1% (w/v) casein enzymatic hydrolysate, 3% (w/v) sucrose, pH 5.8, 0.5% (w/v) gellan gum (CultureGel—Type 1; PhytoTechnology Laboratories, Shawnee Mission, KS, USA)) for proliferation followed by 5 weekly cycles of subculturing onto fresh C1 plates. For Sauvignon blanc cultures, C1 plates were supplemented with 0.5% (w/v) polyvinyl pyrrolidone 40 (PVP) to prevent browning.
2.2. Culturing of Embryogenic Callus in Suspension
Embryogenic suspension cultures (SCs) were established and maintained as described in [34]. In brief, about 200 mg of callus was harvested from C1 plates into 15 mL of C1 liquid medium (as above, without gellan gum), grown in the dark at 27°C with continuous shaking at 120 rpm, and the culture volume gradually increased to 120 mL by weekly doubling of the suspension volume. Healthy cultures were maintained for a maximum of 6 months by exchanging half the media volume weekly and splitting or sieving the cultures if required. For Sauvignon blanc cultures, all C1 media contained 1% (w/v) PVP to prevent browning.
2.3. Protoplast Isolation From Embryogenic Callus Material
For the isolation of protoplasts from embryogenic callus of Chardonnay, Shiraz, Cabernet Sauvignon and Sauvignon blanc, callus growing on solid medium at 4–5 weeks postsubculture was harvested into empty Petri dishes. Where SCs were used, callus in a liquid medium that had received 50% (v/v) fresh C1 medium 24 h prior was harvested by first decanting off as much medium as possible, removing the excess by absorption onto a stack of filter paper and then transferring callus to Petri dishes. A cell wall digesting solution was added at 10 mL/g callus, and Petri dishes were placed in the dark at 25°C on a platform shaker at 30 rpm for 16–20 h. The cell wall digesting solution comprised C1 liquid medium (as above, without plant growth regulators and casein hydrolysate), 0.5% (w/v) macerozyme R-10 (Yakult Pharmaceutical Ind. Co., Ltd. Kunitachi-Shi, Tokyo, Japan), 1% (w/v) cellulase R-10 (Yakult Pharmaceutical Ind. Co., Ltd. Kunitachi-Shi, Tokyo, Japan), 0.05% (w/v) pectolyase (Sigma-Aldrich, St. Louis, MO, USA), 5 mM MES (pH 5.7), 10 mM CaCl2 (total) and 0.5 M mannitol adjusted to pH 5.7. Callus from agar plates was manually disrupted immediately after the cell wall digesting solution was added or within the first hour of incubation by pipetting up and down with a P1000 with a 1 mL cut-tip and then with a 1 mL fine-tip. Undigested callus was removed by pouring the digest through a nylon mesh stack (Greiner Bio-One GmbH, Frickenhausen, Baden-Württemberg, Germany; 100 μm (top) and 70 μm (bottom)), prewetted with 3 mL MMG solution (4 mM MES, 0.5 M mannitol, 15 mM MgCl2, pH 5.7) and rinsed with 15 mL MMG solution. The digest was centrifuged at 120 × g for 3 min, then washed twice more with 20 mL MMG solution. The final pellet was resuspended in MMG solution and 2 mL aliquots were layered on 8 mL of a sucrose solution (16% (w/v) for Chardonnay, Shiraz and Sauvignon blanc, 25% (w/v) for Cabernet Sauvignon). After centrifugation (90 × g, 4 min, no brake), purified protoplasts were harvested as a band from the interface and washed twice with MMG solution (centrifugation at 100 × g, 3 min, no brake). Protoplasts were resuspended in a final volume of 1–2.5 mL MMG solution and protoplast concentration, and viability was determined using a haemocytometer after trypan blue staining. For the staining, trypan blue (Sigma-Aldrich, St. Louis, MO, USA) was prepared as a 0.4% (w/v) solution in PBS (pH 7.4), filter-sterilised, and used in a 1:1 (v/v) ratio to stain an appropriate dilution of protoplasts.
2.4. Transfection of Protoplasts With DM2c/Cas9-RNP
Protoplasts of all four target cultivars were transfected with an RNP comprised of the DM2c [17, 30] guide RNA (Alt-R CRISPR-Cas9 crRNA; Integrated DNA Technologies, Coralville, IA, USA), targeting the DMR6-2 gene, with known DMR6-1 off-target activity [31], duplexed with a universal scaffold RNA (Alt-R CRISPR-Cas tracrRNA; Integrated DNA Technologies, Coralville, IA, USA) as per the manufacturer’s instructions, and Cas9 protein (Alt-R S.p. Cas9; Integrated DNA Technologies, Coralville, IA, USA). For RNP formation with Cas9, 2 nmol of the crRNA/tracrRNA duplex (sgRNA) and 10 μg of Cas9 (diluted to 1 μg/μL in PBS buffer, pH 7.4) were combined in a 0.2-mL PCR tube, incubated at room temperature for 10 min and stored at −20°C for less than a month prior to protoplast transfection.
Purified protoplasts were diluted in MMG solution to 1 × 106 cell/mL, and 12 μL of DM2c/Cas9-RNP and 210 μL of 40% polyethylene glycol (PEG) solution (40% (w/v) PEG 4000, 0.2 M mannitol, 0.1 M CaCl2) were added to 200 μL (2 × 105 cells) cell aliquots (3–10) in 2-mL Eppendorf tubes. After incubation at 25°C on a platform shaker at 30 rpm for 30 min in the dark, 950 μL W5 solution (2 mM MES, 5 mM glucose, 154 mM NaCl, 125 mM CaCl2, 5 mM KCl, pH 5.7) was carefully added to each tube, followed by centrifugation for 3 min at 100 × g (no brake). The supernatant was removed, 500 μL W5 was added to each pellet, and the centrifugation was repeated, followed by removal of the supernatant and careful resuspension in 1 mL W1 solution (4 mM MES, 0.5 mM mannitol, 20 mM KCl, pH 5.7). After incubation at 25°C on a platform shaker at 30 rpm for 1 h in the dark, protoplasts were pelleted as above, resuspended in 500 μL W1 solution and pooled, followed by cell count and viability assays as described above.
2.5. Embedding and Culturing of Transfected Protoplasts in Alginate Beads
To embed transfected protoplasts in alginate beads, cell concentrations were adjusted to 1 × 106 cell/mL before mixing with an equal volume of 3.2% (w/v) sodium alginate solution in 0.4 M mannitol. The protoplast suspension was loaded into a 3-mL syringe and expelled as drops from a 27-G needle into Petri dishes containing a 50 mM CaCl2, 0.4 M mannitol solution. Beads were left to set for 30 min before straining in a stainless steel sieve and washing with 50 mL protoplast culture medium (PCM, reservoir medium described in [35] without charcoal; NN basal medium with NN vitamins, 10.75 μM 1-naphthaleneacetic acid (NAA), 2.25 μM BAP, 3.08% (w/v) sucrose and 5.4% (w/v) glucose). Beads were then placed into 35-mm (up to 150 beads) or 55-mm (150–280 beads) Petri dishes with 2.5–4 mL of reservoir liquid (PCM_5.4% glucose with 0.3% (w/v) activated charcoal), then dishes were sealed with parafilm and incubated in the dark at 27°C.
2.6. Plant Regeneration From Transfected Protoplasts
Two weeks after alginate bead plating, the level of glucose in the reservoir medium was reduced to 0.15 M, and from 1 month after alginate bead plating onwards, glucose was eliminated from the reservoir medium, which was refreshed every 2 weeks. Once embryos started to emerge (6–7 weeks after transfection), reservoir medium that had been removed as part of the fortnightly exchange was spread onto GS1CA plates ([32] without indole-3-acetic acid (IAA); NN macronutrients, MS micronutrients with B5 vitamins, 10 μM 1-naphthoxyacetic acid (NOA), 1 μM BAP, 6% (w/v) sucrose and 0.25% (w/v) activated charcoal, 1% (w/v) Difco (Bacto) agar (Becton, Dickinson and Co., Franklin Lakes, NJ, USA), pH 6.2). Embryos were shown to be present in this liquid and to continue to develop on these plates. Larger more-developed free-floating embryos were picked from around the beads and placed directly onto GS1CA plates. Embryos were incubated for a further 2 weeks in the dark at 27°C and then transferred to ambient light conditions (16-h photoperiod) until sufficiently developed to harvest cotyledonary explants for planting into shooting medium. Shooting medium comprised SM medium ([33]; SM macronutrients, MS micronutrients with B5 vitamins, 1.5% (w/v) sucrose and 1% (w/v) Difco (Bacto) agar (Becton, Dickinson and Co., Franklin Lakes, NJ, USA), pH 5.7, supplemented with 2.5–5 μM BAP. From there, developing shoots were transferred to a rooting medium (SM supplemented with 0.5 μM NAA), and once strong root systems had developed, plants were acclimatised to free-draining potting mix in a growth chamber (low light; 16-h photoperiod) for 4 weeks, before being transferred to the glasshouse.
2.7. Verification and Characterisation of Gene-Editing Events in Regenerated Plants
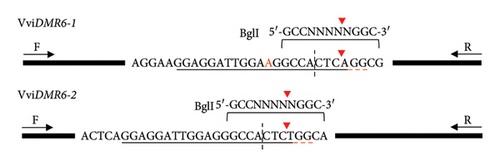
DNA was extracted from root or leaf tissue of regenerated plantlets (AGRF, Adelaide, SA, Australia) and screened by polymerase chain reaction-cleaved amplified polymorphic sequences (PCR-CAPS) with VviDMR6-1-specific (5′-TGACTCCATGGATGAACTGATT-3′, 5′-CCTCATCCTACTTTCAATGGTAG-3′) and VviDMR6-2-specific (5′-TCTTCCTCTAGAATGGCACAAG-3′, 5′-GGAGGACATCCGTATACTCATA-3′) primers flanking the region targeted by the DM2c guide followed by amplicon digest with the restriction enzyme BglI (NEB, Ipswich, MA, USA). This allowed for the identification of plants with mono- and biallelic edits in both DMR6 genes by restriction fragment lengths due to the abolishment of the BglI recognition site at the Cas9 cleavage site by single nucleotide polymorphisms (SNPs) or insertions/deletions (INDELs). Sanger sequencing (AGRF, Adelaide, SA, Australia) of the amplicons was then performed for plants of interest, and chromatograms were analysed using the ‘Inference of CRISPR Edits (ICE) v2′ tool (Synthego, Redwood City, CA, USA). Sequence alignments were performed using the Geneious Prime (Build 2023-07-20; GraphPad Software, Boston, MA, USA) alignment default settings.
2.8. Flow Cytometry (FCM) Ploidy Assays
Determination of plant ploidy was conducted using FCM using essentially the nuclei preparation method reported in [36] except that the nuclei suspension was only filtered once through a 40-μm filter. The staining of the isolated nuclei with propidium iodide was also conducted as described in [36]. Subsequent data acquisition and analysis were conducted using a Guava easyCyte HT (Cytek Biosciences, Fremont, CA, USA) flow cytometer where 300 μL aliquots of the stained nuclei were loaded into the instrument in a 96-well plate (Corning, Glendale, AZ, USA). The Cell Cycle software module was used to acquire fluorescent signals in the red range (690/50-nm bandpass filter) with the nuclei having been excited using a blue laser (488 nm). The flow cytometer settings were adjusted to optimise data collection and to ensure that the most distinct peak of nuclei from a control diploid plant was detected at a fluorescence intensity of approximately 1024 arbitrary units. Under the conditions used in this method, the flow rate was always set to 0.24 μL/s and the forward scatter gain was in the range of 553–557 V. The data collection was also ‘gated’ to eliminate the cell debris that had fluorescence intensities of less than 300 arbitrary units. Five thousand ‘events’ were then acquired from each sample, and the position of the peak of the histogram of propidium iodide staining intensity (i.e., red fluorescence) was compared to that of the diploid control. Those samples where the highest peak was close to 1024 were regarded as diploid, and tetraploids were characterised by having a peak on the fluorescence histogram with a mean at approximately 2048 arbitrary units. Data were acquired and analysed using guavaSoft 2.5 (Millipore, Billerica, MA, USA).
2.9. DM Leaf Disc Assay
Glasshouse-grown, healthy grapevines with biallelic edits in either or both VviDMR6 genes (Vvidmr6-1, Vvidmr6-2) were assessed for DM susceptibility using leaf disc assays as described by [37] with the following modifications: to account for ontogenic differences in susceptibility, leaves were selected based on developmental stage and restricted to node 5–7 leaves. Six discs/leaf were used, which were each inoculated with 20 μL of a 5 × 104 sporangia/mL DM inoculum solution. After 7 days of incubation, all six discs were placed into a 50-mL falcon tube containing 10 mL sterile water and vortexed for 20 s to dislodge sporangia for quantification using a haemocytometer. Each plant was tested in three independent experiments and compared to nonedited control plants originating from the same transfection experiment that produced the Vvidmr6 mutants.
2.10. Quantification of Plant Defence Hormones by LC-MS/MS
The measurement of jasmonic acid (JA) and salicylic acid (SA) in 50 mg of leaf material frozen and ground in liquid nitrogen after removal of leaf discs for DM sporulation assays was performed as described by Clayton-Cuch et al. [38] with the following modifications: two deuterated standards, 250 pmol of JA-d5 (OlChemim Ltd., Olomouc, Olomouc, Czech Republic) and 100 pmol of SA-d4 (CDN Isotopes, Montreal, Quebec, Canada), were added at the start of the extraction process.
For the LC-MS/MS analysis on an Agilent 1260 Infinity II HPLC (Agilent, Santa Clara, CA, USA) with an Agilent 6470 Triple Quad mass spectrometer, the HPLC sample effluent was introduced into the ionisation source (nebuliser pressure 60 psi) with a desolvation gas temperature of 300°C at a flow of 8 L·min−1, with the capillary voltage set to 4 kV for SA and 5.5 kV for JA. The detection of both hormones was performed by multiple reaction monitoring in negative ion mode. The optimisation of fragmentation was done with JA and SA (Sigma-Aldrich, St. Louis, MO, USA) as well as their labelled standards using the Agilent MassHunter Optimizer software (Version B03.01). The following main transitions were used for quantitation: JA-d5 214.2 > 62.1, JA 209.1 > 59.1, SA-d4 141.1 > 97.0, SA 137.0 > 93.0. In addition, the following qualifier ion transition was included: JA 209.1 > 165.1, SA-d4 141.1 > 69.1, SA 137.0 > 65.0. Analysis sensitivity was heightened by monitoring SA-d4 and SA in a different retention window (5–18.2 min) to JA-d5 and JA (18.3–21 min). The concentrations of SA and JA in the extracts were quantified in relation to their respective internal standards using calibration curves with 7 calibrator points (0.02–10 μM) and the Agilent Quantification software (Version 10.0).
2.11. Statistical Analysis
The significance of the effect of VviDMR6 gene edits on DM susceptibility and leaf plant defence hormone concentrations was assessed using the Kruskal–Wallis test followed by Dunn’s pairwise multiple comparisons (SigmaPlot; Version 15.1; Synstat Software, San Jose, CA, USA).
3. Results and Discussion
3.1. Efficient DNA-Free Protoplast Gene Editing and Plant Regeneration of Elite Winegrape Cultivars
Since the first reported regeneration of grapevine plants from RNP-transfected, edited protoplasts [17], additional research teams have demonstrated the same capability using modified transfection and regeneration protocols [18–20]. However, until now V. vinifera cv. Colombard, Merlot and Nebbiolo are the only winegrape cultivars for which the successful regeneration of edited plants from protoplasts has been documented [18, 20], albeit with seemingly low regeneration rates for Merlot and Nebbiolo and either low (Nebbiolo [20]) or undocumented editing efficiencies for all three cultivars. In this study, embryogenic callus culturing, protoplast isolation, RNP transfection, transfected cell culturing and embryo and plant regeneration protocols published previously for either table grape cultivars [17, 39], winegrape cultivars [15, 35] or both [40] were adapted to enable the efficient generation of nontransgenic, gene-edited clones of commercially important winegrape cultivars (Figure 1, Tables 1 and 2). The four elite winegrape cultivars used in this study, which encompass cultivars amenable to in vitro culturing and regeneration (Chardonnay [42] and Sauvignon blanc [43]) as well as those classed as moderately (Shiraz [42]) or highly recalcitrant (Cabernet Sauvignon [42, 44]), were used to test the wide-ranging applicability of the developed protocol.
| Affected material/process | [16, 17] | [19] | [18] | [20] | This work |
|---|---|---|---|---|---|
|
Embryogenic callus from agar plates | Embryogenic callus from agar plates C1 medium | Embryogenic callus from agar plates Pic/TDZ medium | Embryogenic callus from agar plates | Embryogenic callus from agar plates or suspension culture C1 medium (PVP addition if required) |
|
|
|
|
|
|
|
16 h | 5–6 h | 16–24 h | 2.5 h | 16–18 h |
|
MMG |
|
FW | WB | MMG |
|
16% (w/v) sucrose solution | None | 13% (w/v) dextran solution | 20% (w/v) sucrose solution | 16% (w/v) or 25% (w/v) sucrose solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
NN basal medium with NN vitamins, 88 mM sucrose, 300 mM glucose, 1 g/L charcoal, 0.93 μM kinetin, 2.22 μM BAP and 10.7 µΜ NAA. After 2 weeks, glucose decreases weekly by 25% until absent after 4 weeks. | NN basal medium with NN vitamins, 0.09 M sucrose, 0.3 M glucose, 0.5 mg/L BAP and 2 mg/L NAA. 2 g/L gellan gum for solid and 0.3% (w/v) activated charcoal for liquid. | 0.4 M Pic/TDZ medium supplemented with 1 mM putrescine, 0.1 mM spermidine, 1 mM spermine, 40 mM glycine, 5 mM L-arginine, 5 mM L-leucine, 5 mM L-lysine, 0.6 mM ascorbic acid, 0.7 mM citric acid, 0.1 mM reduced glutathione, 0.8 mM L-cysteine and 0.5 mL of a 7-day-old 1103P suspension culture conditioned to grow in 0.4 M Pic/TDZ (feeder suspension). Mannitol decreases fortnightly over 4 weeks (0.2 M, 0.1 M). | NN basal medium with NN vitamins, 0.09 M sucrose, 0.3 M glucose, 0.5 mg/L BAP and 2 mg/L NAA. 2 g/L gellan gum for solid and 0.3% (w/v) activated charcoal for liquid. Glucose abolished after 2 weeks; medium was replaced with liquid GSICA after 1 month. | NN basal medium with NN vitamins 10.75 μM NAA and 2.25 μM BAP, 0.3% (w/v) activated charcoal (added to medium 1–2 days before use) and glucose. Glucose decreasing fortnightly over 6 weeks until absent (0.3 M, 0.15 M, 0 M). |
|
|
|
|
|
|
| Shoot formation |
|
Four different media (C2D and MG1 based). | WPM solid medium supplemented with 20 g/L sucrose, 1 g/L casein, 1 mM MES, 500 mg/L activated charcoal and 0.05 μM indolebutyric acid (IBA). |
|
|
| Plant development |
|
MSN or RIM media. | WPM solid medium supplemented with 20 g/L sucrose, 1 g/L casein, 1 mM MES, 500 mg/L activated charcoal and 0.05 μM indolebutyric acid (IBA). |
|
|
| Measured parameter | Chardonnay (EC) | Chardonnay (SC) | Cabernet sauvignon (EC) | Shiraz (EC) | Sauvignon blanc (SC) |
|---|---|---|---|---|---|
| Callus mass (g) | 1 | 6 | 1.5 | 2 | 2.5 |
| Protoplast yield (total cell number) | ∼17 × 106 | ∼50 × 106 | ∼74 × 106 | ∼6 × 106 | ∼10 × 106 |
| Viability (%) | 86 | 90 | 87 | 88 | 94 |
| Embedded protoplasts (total cell number) | ∼9.88 × 106 | ∼4.75 × 105 | ∼8.50 × 105 | ∼1.11 × 106 | ∼6.91 × 105 |
| Alginate beads | 840 | 48 | 113 | 245 | 178 |
| Protoplasts/bead | ∼11,800 | ∼9900 | ∼7500 | ∼4500 | ∼3900 |
| Transfection to embryo formation (days)† | ∼49 | ∼42 | ∼45 | ∼45 | ∼44 |
| Germinated embryos | 2970‡ | 881 | 131 | 245 | 572‡ |
| Germinated embryo formation rate (%) | 0.030‡ | 0.185 | 0.015 | 0.022 | 0.083‡ |
| Plants checked for DMR6 edits | 443 | 126 | 47 | 79 | 63 |
| Edited plants (%)§ | 46 | 73 | 89 | 76 | 87 |
- †Mix of globular, heart-shaped and cotyledonary embryos.
- ‡This number is an underestimation as prolific embryo germination continued but was not further captured.
- §Includes all edits of VviDMR6-2 and/or VviDMR6-1 as determined by PCR-CAPS analysis.
To assess the editing component of the process and address a high-value trait for grapevine improvement, the highly efficient DM2c guide RNA targeting the DM susceptibility gene VviDMR6-2 [17], with VviDMR6-1 as a potential off-target [31], was used for RNP transfections throughout, with the aim to simultaneously edit both VviDMR6 genes. As detailed in Table 1, there is some overlap and a high degree of similarity between most of the steps of the protoplast transfection and plant regeneration protocol presented in this work (Materials and methods section, Figure 1) and protocols published previously by others. However, differences in the makeup of the digestion solution, small, cultivar-dependent modifications to callus culturing and protoplast purification, as well as differences in transfection, embedding and protoplast and plant culturing conditions may have contributed to the relatively high numbers of germinated embryos and edited regenerants (Table 2), even for the highly recalcitrant Cabernet Sauvignon cultivar, when compared to the related grapevine studies [16–20] referenced in Table 1. In our hands, the adequate purification of Cabernet Sauvignon protoplasts required an increase in sucrose solution concentration to 25% (w/v), due to the sedimentation of protoplasts in the 16% (w/v) solution used for the other three target cultivars, and the addition of PVP to the culturing media for Sauvignon blanc callus prevented the prevalent callus browning observed for this cultivar and enabled the isolation of protoplasts with the highest viability of all tested cultivars (Figure 1, Tables 1 and 2). Protoplast viability is a key parameter for any transfection and regeneration protocol, and in accordance with studies in other plant species [45], centrifugation through a sucrose solution yielded protoplast preparations with high numbers of viable cells (86%–94%). The highest viability rates were recorded for protoplasts isolated from embryogenic cell SC (Chardonnay and Sauvignon blanc; Table 2), and a comparison between Chardonnay transfections with embryogenic starting material grown either on agar plates (EC) or in liquid medium (SC) revealed the additional improvements of reduced time to form embryos (42 days vs. 49 days), a potentially higher embryo germination rate, and a 27% increase in editing efficiency when using SC-derived protoplasts (Table 2). It is, however, possible that a reduction in protoplast plating density was an additional contributing factor to the observed improvements. The up to threefold variances in numbers of embedded protoplasts per bead between the transfections (Table 2), despite a common embedding concentration of 5 × 105 cells/mL (Table 1) and a common alginate bead production process, suggested cell count inaccuracies rather than large bead size variations as the main reason for the observed inconsistencies, which likely resulted in different protoplast plating densities. This process parameter was higher for the Chardonnay EC (∼11,800 cells/bead) than the SC transfection (∼9900 cells/bead), and lower plating densities (∼3900–7500 cells/bead) appeared to be associated with higher editing rates in the other three tested cultivars, independent of the culturing method for the embryogenic starting material (Table 2). The observed neutral or positive effects of SC on several key parameters of the protoplast transfection and regeneration protocol, as well as considerable time- and labour savings related to subculturing combined with ease of upscaling, make SC an attractive culturing method for embryogenic cells used for the DNA-free gene editing of winegrape cultivars.
The large proportion of healthy protoplasts used for RNP transfections in this work likely contributed to enhanced transfection efficiencies [45], resulting in editing rates of 46%–89% (Table 2), which represents a significant improvement on the ∼10%–20% editing rates reported previously for winegrape cultivars [19, 20]. Another contributor to these high editing efficiencies may have been the RNP composition of 10 μg Cas9 and 2 nmol (∼60 μg) DM2c sgRNA (Figure 1, Table 1), combining a relatively low amount of Cas9 with a sixfold higher amount of sgRNA intended to both minimise potential off-target cytotoxic effects caused by excessive Cas9 activity [46] and increase the likelihood of editing events in both VviDMR6-2 and the 1 bp off-target VviDMR6-1 by elevating the sgRNA concentration [47]. Other factors that likely assisted with the recovery of comparatively large numbers of edited regenerants were the high cell concentration of 5 × 105 cells/mL at the point of embedding and the large overall numbers of embedded protoplasts, achieved by pooling up to 13 separate transfections (Tables 1 and 2). In addition, the combination of alginate beads with a simple reservoir medium is unique to the presented protocol (Figure 1, Table 1) and represents an easy-to-upscale, cost- and labour-effective method adaption resulting in the regeneration of high numbers of plants, from 131 plants (0.015% of embedded protoplasts) for the most recalcitrant Cabernet Sauvignon cultivar to many hundreds-thousands (up to 0.185% of embedded protoplasts) for Chardonnay and Sauvignon blanc (Table 2). Plant survival rates once cultured in NAA-supplemented medium for root development were > 95% for Chardonnay and Cabernet Sauvignon, and > 70% for Shiraz and Sauvignon blanc. For the latter two cultivars, experimentation with different NAA concentrations and other auxinic compounds is currently ongoing to improve recovery rates.
The protocol is amongst the fastest published to date, with regenerated, edited plants ready for soil acclimatisation after 4–5 months, independent of the cultivar.
Target gene editing in 63 (Sauvignon blanc), 47 (Cabernet Sauvignon), 79 (Shiraz), 126 (Chardonnay SC) and 443 (Chardonnay EC) regenerants was initially assessed by extracting DNA from root or leaf tissue and screening for target gene editing via restriction enzyme digest. As there were no sequence differences in the vicinity of the VviDMR6-1 and VviDMR6-2 target regions between the tested cultivars (Supporting Figure 1), the schematic illustration of the conducted PCR-CAPS assay (Figure 2(a)) applies to all four target cultivars, with an example shown for a selection of Shiraz plants in Figure 2(b).
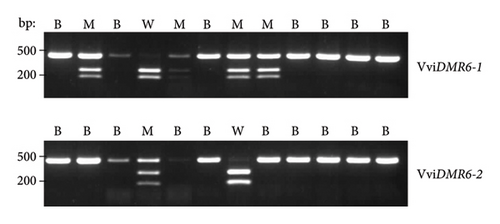
Out of the 443 regenerants tested for the Chardonnay EC transfection, 204 (46%, Table 2) were confirmed as carrying an edit in at least one allele of at least one VviDMR6 gene, with all possible combinations of mono- and biallelic edits detected, including 63 plants with biallelic edits in both VviDMR6-1 and VviDMR6-2 (Table 3). Most allelic genotypes were also identified for Cabernet Sauvignon, Chardonnay SC, Shiraz and Sauvignon blanc regenerants (Table 3), and recovery of all possible genotypes can be expected with higher rates of regenerant testing. The abundance differences between regenerated genotypes (Table 3) clearly illustrate the higher DM2c editing rates for the perfect match VviDMR6-2 gene compared with the 1-bp mismatch VviDMR6-1 gene, with Vvidmr6-1/Vvidmr6-1/VviDMR6-2/VviDMR6-2 as the rarest recovered genotype represented by only two Chardonnay and one Shiraz line.
| Genotype | Cas9/DM2c-RNP transfection (genotype regenerants/total number of plants tested) | ||||
|---|---|---|---|---|---|
| Chardonnay (EC) | Chardonnay (SC) | Cabernet sauvignon (EC) | Shiraz (EC) | Sauvignon blanc (SC) | |
| WT/WT (DMR6-1/DMR6-1/DMR6-2/DMR6-2) | 239/443 | 35/126 | 5/47 | 19/79 | 8/63 |
| Monoallelic/WT (DMR6-1/dmr6-1/DMR6-2/DMR6-2) | 20/443 | 1/126 | 0/47 | 7/79 | 1/63 |
| WT/Monoallelic (DMR6-1/DMR6-1/DMR6-2/dmr6-2) | 46/443 | 14/126 | 8/47 | 3/79 | 0/63 |
| Monoallelic/Monoallelic (DMR6-1/dmr6-1/DMR6-2/dmr6-2) | 25/443 | 7/126 | 5/47 | 4/79 | 1/63 |
| Biallelic/WT (dmr6-1/dmr6-1/DMR6-2/DMR6-2) | 2/443 | 0/126 | 1/47 | 0/79 | 0/63 |
| WT/Biallelic (DMR6-1/DMR6-1/dmr6-2/dmr6-2) | 17/443 | 13/126 | 5/47 | 10/79 | 13/63 |
| Monoallelic/Biallelic (DMR6-1/dmr6-1/dmr6-2/dmr6-2) | 27/443 | 27/126 | 6/47 | 19/79 | 19/63 |
| Biallelic/Monoallelic (dmr6-1/dmr6-1/DMR6-2/dmr6-2) | 4/443 | 1/126 | 4/47 | 0/79 | 2/63 |
| Biallelic/Biallelic (dmr6-1/dmr6-1/dmr6-2/dmr6-2) | 63/443 | 28/126 | 13/47 | 17/79 | 19/63 |
Lines for each genotype recovered for the four different cultivars have been regenerated in tissue culture, with no obvious phenotypic differences between the origin cultivars, WT lines and edited plants, including those with biallelic edits in both VviDMR6 genes (Figure 3).




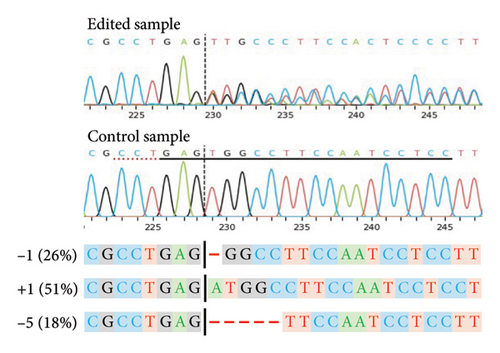
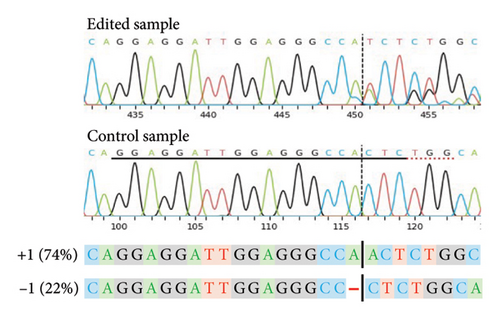
For a selection of 39 (Sauvignon blanc), 41 (Chardonnay SC), 27 (Cabernet Sauvignon), 39 (Shiraz) and 148 (Chardonnay EC) plants, the PCR-CAPS analysis results shown in Table 3 were confirmed by Sanger sequencing (data not shown). Independent of the cultivar, the most common editing events (> 70%) identified for both VviDMR6 genes by the sequencing analysis were 1-bp deletions (−1) and insertions (+1), with an even distribution of −1/+1 INDELs recorded for VviDMR6-2, and 6-10-fold higher rates of +1 events compared with −1 events detected for VviDMR6-1. Accordingly, regenerants with biallelic edits in one or both target genes were mainly characterised by allelic profiles of homozygous +1 for VviDMR6-1 (50%–70%) or heterozygous −1/+1 for VviDMR6-2 (50%). The notable exception to this was the Shiraz cultivar, where 60% of biallelic VviDMR6-2 regenerants were found to carry homozygous −1 edits and no heterozygous −1/+1 plants were identified. This differing mutation type frequency may have been related to cultivar-specific variations in the sequence environment close to the target site or could have been the result of a different prevalent cell cycle stage at the time of editing for the Shiraz protoplasts compared with the other cultivars [48].
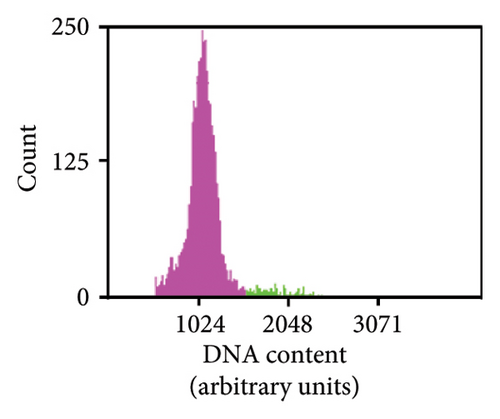
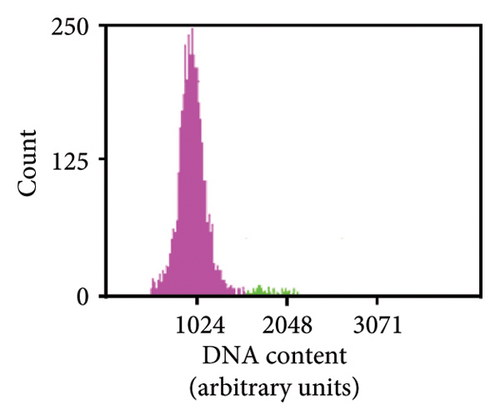
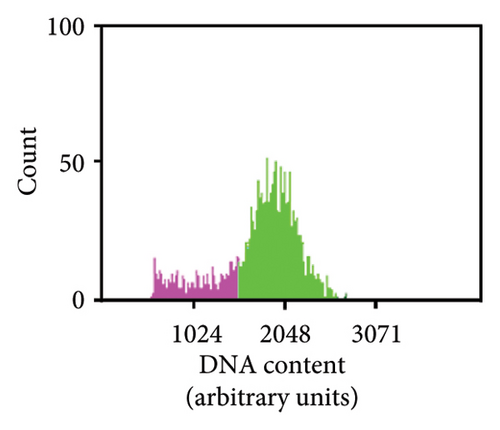
As previously noted by Scintilla et al. [16] for the table grape cultivar Sugraone, up to a quarter of protoplast regenerants were characterised by allele frequencies diverting from the single allele or 1:1 allele ratios expected for a diploid plant, instead displaying approximated 3:1, or triple allele 2:1:1 ratios. These types of allele frequencies were indicative of an increase in ploidy level caused by PEG-mediated cell fusion [49] rather than chimerism. As the PEG concentration range and exposure time for efficient protoplast fusion and RNP transfection are highly similar [45, 50, 51], changes to these parameters are unlikely to significantly reduce cell fusion whilst maintaining the desired high editing efficiencies. Again, the Shiraz cultivar differed from the other three cultivars, with no polyploidy indicating allele frequencies identified in 39 sequenced regenerants, further supporting the above-mentioned possibility that protoplast cell cycle stage at the time of transfection differed, which may have reduced fusion rates. An example of extracted Sanger sequencing traces indicating polyploidy is shown for Chardonnay EC in Figures 4(a) and 4(b). As cell fusion and associated polyploidy are not a desirable outcome for the production of true-to-type clones of elite winegrape cultivars [52], and as, contrary to the stunted growth described by Scintilla et al. [16], suspected polyploid plants did not display any obvious distinguishing growth abnormalities in tissue culture, an efficient FCM-based method for ploidy determination of regenerants was used to screen for polyploid plants and exclude them from any further analyses (Figures 4(c), 4(d), 4(e), 4(f), 4(g)). FCM-based ploidy determination was of particular importance for WT lines and edited plants with apparent diploid allelic profiles (Table 4).
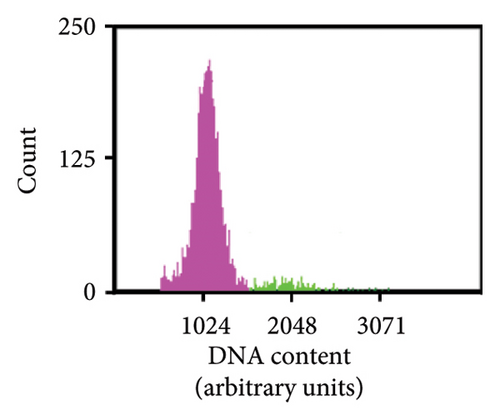
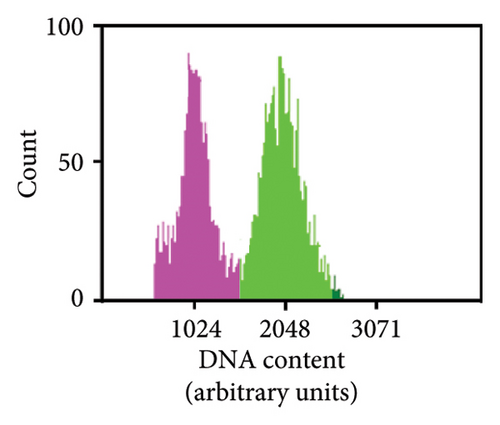
| Tested plant | Cas9/DM2c-RNP transfection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chardonnay EC | Chardonnay SC | Cabernet sauvignon EC | Shiraz EC | Sauvignon blanc SC | |||||||||||
| Allelic profile | FCM | Allelic profile | FCM | Allelic profile | FCM | Allelic profile | FCM | Allelic profile | FCM | ||||||
| DMR6-1 | DMR6-2 | DMR6-1 | DMR6-2 | DMR6-1 | DMR6-2 | DMR6-1 | DMR6-2 | DMR6-1 | DMR6-2 | ||||||
| 1 | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di |
| 2 | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di |
| 3 | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di | WT/WT | WT/WT | di |
| 4 | WT/WT | WT/WT | tetra | WT/WT | WT/WT | di | WT/WT | WT/WT | di | +1/+1 | +1/+1 | di | +1/+1 | −1/−1 | di |
| 5 | +1/+1 | +1/+1 | di | +1/+1 | +1/+1 | tetra | +1/+1 | +1/−1 | di | +1/+1 | +1/+1 | di | WT/WT | +1/−1 | di |
| 6 | +1/+1 | +1/+1 | di | +1/+1 | +1/−1 | di | +1/+1 | +1/−1 | di | +1/+1 | +1/+1 | di | WT/−2 | −1/−1 | di |
| 7 | +1/+1 | +1/−1 | di | WT/WT | +1/−5 | tetra | +1/−1 | +1/−1 | di | +1/+1 | +1/−1 | di | +1/+1 | +4/+4 | di |
| 8 | +1/+1 | +1/+1 | di | +1/+1 | −1/−4 | di | WT/−18 | +1/−1 | di | −1/−3 | +1/−1 | di | WT/−1 | −1/−1 | di |
| 9 | +1/−5 | +1/−1 | di | +1/+1 | +1/−1 | di | +1/+1 | WT/−1 | di | +1/+1 | +1/+2 | di | +1/+1 | WT/+4 | di |
| 10 | +1/+1 | −1/−1 | di | WT/+1 | +1/+1 | tetra | +1/+1 | +1/−1 | di | +1/+1 | −1/−1 | di | −1/−1 | −1/−1 | di |
- Note: di, diploid; EC, protoplasts derived from agar-plate-grown embryogenic callus; FCM, flow cytometry; SC, protoplasts derived from embryogenic callus suspension cultures; tetra, tetraploid; WT, wild-type amplicon sequence.
Out of 10 lines per transfection, which were a mixture of WT plants and edited lines with apparent diploid allelic profiles by Sanger sequencing, one Chardonnay EC transfection WT plant and three Chardonnay SC transfection edited regenerants were found to be tetraploid by FCM analysis (Table 4). These results suggest that allelic profile-based screening was able to reduce the frequency of polyploid regenerants to 0%–10% for most transfections and should therefore be employed as a useful preselection tool, but it cannot be solely relied upon for the detection of WT and edited plants with altered ploidy levels.
3.2. VviDMR6-Edited Chardonnay Clones With Reduced DM Susceptibility
A recent study [31] describing the Agrobacterium tumefaciens (Smith & Townsend) Conn-mediated editing of both VviDMR6 genes in two table grape cultivars (V. vinifera cv. Crimson seedless and Sugraone) revealed cultivar-dependent differences in the effect of the gene edits on DM susceptibility, with double mutants of the Crimson seedless cultivar being more resistant than Sugraone mutants. Furthermore, there is ongoing uncertainty regarding the relative contributions of the two VviDMR6 genes to grapevine DM susceptibility [29–31].
It is therefore essential to assess the effects of VviDMR6 mutations for the winegrape cultivar clones produced in this work, which has been completed for Chardonnay EC regenerants with biallelic edits in one or both target genes (Figures 5 and 6). Mutants and nonedited WT plants derived from the same transfection and displaying apparent diploid allelic profiles by Sanger sequencing (Supporting Table 1) were transferred to potting mix and cultivated in the glasshouse for a year, where all plants developed without any noticeable phenotypic abnormalities (Figure 5), confirming similar previous observations for table grape Vvidmr6 mutants [30, 31].

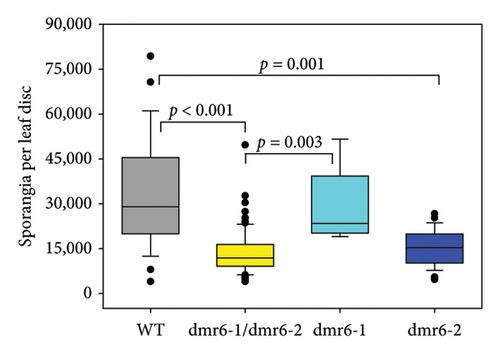
Changes to DM susceptibility conferred by edits in Chardonnay VviDMR6 genes were assessed by detached leaf disc assays, involving the quantification of DM sporangia produced within 7 days of inoculation. A significant reduction in sporangia counts of about 50% was observed for the double-mutant lines as well as for Vvidmr6-2 single mutants, whereas Vvidmr6-1 single mutants displayed WT infection severity (Figure 6(a)).
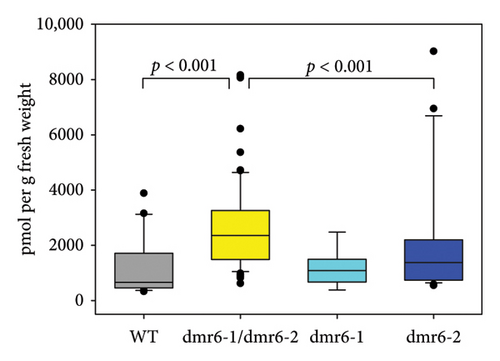
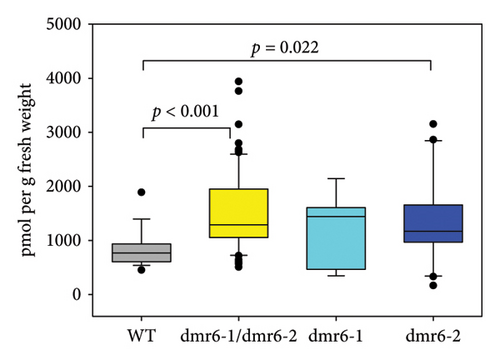
The DM—and broader—resistance phenotype often observed for dmr6 mutants is based on the hydroxylase activity of functional DMR6 enzymes, which leads to the inactivation of the plant hormone SA ([53, 54]), thereby reducing the concentration of this essential signalling component in defence pathways against biotrophic pathogens [55]. Consequentially, increased SA levels are typically observed in leaves of dmr6 mutants with reduced DM/pathogen susceptibility [23, 56]. However, the expected elevated concentration of SA in Vvidmr6 Chardonnay plants with decreased DM sporangia counts was only detected in double-mutant leaves (Figure 6(b)), reflecting the findings by Giacomelli et al. [31] in two table grape cultivars. Except for three outlier data points stemming from one of the nine analysed Vvidmr6-2 mutant lines, leaf SA concentrations of Vvidmr6-2 plants were comparable to WT and Vvidmr6-1 lines (Figure 6(b)).
Whilst SA is the predominant plant hormone associated with defence responses against biotrophic pathogens [31], a second hormone, JA, has also been linked to the grapevine DM response in previous studies [57, 58]. In their role as defence hormones, SA and JA are most commonly known for their antagonistic interactions in response to biotrophic and necrotrophic pathogens [59], but extensive cooperative gene regulation in Arabidopsis [60], including the co-induction of hundreds of genes linked to defensive responses against both types of pathogens [61], is suggestive of a potential synergistic relationship between the two hormones in the grapevine response to DM. Measurements of leaf JA content in Chardonnay WT and Vvidmr6 mutants revealed increased concentrations of this defence hormone in both Vvidmr6-1/Vvidmr6-2 double mutants as well as Vvidmr6-2 single mutants (Figure 6(c)), thereby providing a possible causal link to the observed reduced DM susceptibility of Vvidmr6-2 lines (Figure 6(a)).
Editing of unintended targets with homology to the DM2c guide sequence provides one possible explanation for these elevated JA levels, as they may have been caused by mutations in genes negatively linked to JA accumulation. However, a comprehensive meta-analysis of such off-target effects in plant gene editing experiments found that for over 5500 sequences that differed by four or more nucleotides from a guide sequence, only five (0.09%) off-target editing events were recorded [62]. For the DM2c guide used in this study, there are no predicted off-targets in exons with less than 4 mismatches to the guide sequence (CRISPOR [63] analysis). In addition, transient delivery mechanisms, such as the RNP method used to produce the Chardonnay Vvidmr6 mutants, appear to have a lower off-target rate than stable transformation methods [62], further reducing the likelihood of unintended mutations being the cause for the observed changes to JA concentrations.
Nevertheless, a definitive determination of the possible contributions of off-target editing and other somaclonal mutations to the observed reduction in DM susceptibility will require the collection and analysis of whole-genome sequencing data in future studies.
Another possible explanation for the elevated JA levels in the double and Vvidmr6-2 single mutants is the unknown substrate range of the VviDMR6-2 enzyme, which may include compounds other than SA. JA itself appears to be an unlikely substrate for this grapevine enzyme considering that a closely related Arabidopsis enzyme was shown to be SA-specific and did not accept even structurally similar phenolic compounds as substrates [64]. However, a recent study on the maize (Zea mays L.) enzyme ZmS5H [65] with high sequence similarity to SA hydroxylases from rice (Oryza sativa L.) and Arabidopsis [53, 64] found that, in addition to converting SA to 2,5-dihydroxybenzoic aid (2,5-DHBA), this enzyme can convert the flavonoid dihydroquercetin to quercetin in vitro. Similar to what was observed for the Chardonnay Vvidmr6-2 mutants (Figure 6), the inactivation of the corresponding maize gene led to increased pathogen resistance with no changes to SA concentrations in the mutant plants. This is an indication that the catalytic properties and function of SA hydroxylases in different plant species have evolved and may include the regulation of active forms of flavonols or other phenolic compounds with a role in plant defence. It is therefore feasible that the elevated JA concentrations in Vvidmr6 mutants (Figure 6(c)) were either the result of increased concentrations of phenolic compounds caused by the loss of activity of VviDMR6-2, which may have elicited a defence response involving JA, or the less likely diminished VviDMR6-2 activity on JA itself. The enzymatic characterisation of grape DMR6 proteins combined with metabolomic analyses of the mutants produced in this study may provide insights into DMR6 substrates beyond SA and their role in grapevine defence against DM and other pathogens.
4. Conclusions
This study has demonstrated that a relatively simple and robust protoplast isolation, DNA-free protoplast transfection and plant regeneration workflow can be used to efficiently produce nontransgenic, edited clones of four elite winegrape cultivars. The presented method was shown to allow for inevitable variations in starting material, protoplast yields, plating density in alginate beads and embryo germination rates, and hundreds of plants with editing efficiencies of 46%–89% were successfully regenerated, including from protoplasts of the highly recalcitrant Cabernet Sauvignon cultivar. Ploidy analyses of regenerants demonstrated the need to test all grapevine plants derived from PEG-transfected protoplasts for potential polyploid genotypes as these cannot unambiguously be inferred from sequencing data alone. Resulting glasshouse-grown Chardonnay plants were shown to be significantly less susceptible to DM infection when carrying edits in either VviDMR6-2 alone, or in combination with VviDMR6-1, which represents a novel finding regarding cultivar-specific contributions of VviDMR6 genes to DM susceptibility. Leaf hormone measurements in the mutants confirmed the known link of SA with DM susceptibility reduction, but also uncovered a potential, VviDMR6-2-associated, role for JA in grapevine defence against this pathogen.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research project was partly funded by Australia’s grape growers and winemakers through their investment body Wine Australia, with additional funds provided by CSIRO (CSA 2301-01, CSA 2301-02). Wine Australia and CSIRO have approved the publication of the presented research.
Acknowledgements
We want to acknowledge the foundational work by Ian Dry and Amanda Walker who established the plant transformation and regeneration capabilities in our research facility. We also thank Tanya Kovalenko, Iryna Mazonka, Crista Burbidge, Keshala Kuruwita Bandaralage, Inna Mazonka and Emily Nicholson for their invaluable contributions to the experimental work presented in this manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




