The Effect of Physicochemical Properties of Water Bodies on Schistosomiasis Intermediate Host Snails’ Distribution in Sub-Saharan Africa: A Systematic Review
Abstract
Background: Schistosomiasis, a disease affecting 800 million people in sub-Saharan Africa (SSA), is transmitted through direct contact with infected snail intermediate hosts, in freshwater water bodies. Environmental, climatic and physicochemical properties of the water bodies can interfere with parasite development, reproduction, snail growth and survival rate. This systematic review provides a summary of the effect of water physicochemical properties on schistosomiasis intermediate host snails’ distribution in SSA.
Methods: A systematic search was conducted in EBSCOhost (Medline, Academic Search Ultimate, Greenfile and Health Source), PubMed and Google Scholar. Inclusion criteria comprised peer-reviewed articles that were published in English between 2013 and 2023. Forty-one selected studies were critically appraised for relevance using the JBI critical appraisal tools, and 30 studies were included in the study. The protocol was registered on Prospective Register of Systematic Reviews (PROSPERO) database with the identifier CRD42023428704. A thorough quantitative descriptive analysis was performed, and the results were explained in a comprehensive narrative summary that was consistent with the review questions.
Results: For most studies (n = 12), temperature does not have any influence on snail abundance. Findings further show that pH (n = 12) and dissolved oxygen (DO) (n = 11) have a positive influence on snail abundance. However, findings suggests that snail abundance is not solely determined by a single physicochemical property but by a combination of these properties, with a varying impact on snails’ abundance in water bodies.
Conclusion: This systematic review identifies the physicochemical properties that have the greatest impact on intermediate host snails. This data may assist officials in disrupting the transmission cycle in regions with schistosomiasis. Physicochemical properties can also predict snail abundance and water suitability, which are key factors when searching for transmission sites.
1. Introduction
Neglected tropical diseases (NTDs) are a diverse group of tropical infections most common in areas with extreme poverty, especially among those economically disadvantaged living remotely. Among the NTDs, schistosomiasis, commonly known as bilharzia, is the second most frequent after malaria [1] and is a water-borne parasitic illness affecting humans and animals such as dogs, pigs, cattle, sheep and buffaloes [2]. Schistosomiasis is caused by blood flukes, which are trematode flatworms of the genus Schistosoma [3]. The disease is caused by five major species of blood flukes, Schistosoma mansoni, S. japonicum, S. mekongi and S. intercalatum, which cause intestinal schistosomiasis, and S. haematobium, which leads to urogenital schistosomiasis [3]. S. mansoni and S. haematobium are endemic to sub-Saharan Africa (SSA) [3], with freshwater snails from the genus Biomphalaria pfeifferi and Bulinus globosus serving as the primary vectors, respectively [4, 5]. The disease affects almost 800 million people currently, causing over 200,000 deaths per year, and is most prevalent in SSA [1]. About half of all cases of Schistosoma infection in the region are attributable to S. haematobium, which is responsible for about 112 million cases of urogenital schistosomiasis [6, 7]. Transmission occurs when a human makes direct contact with a water body inhabited by the parasite’s obligate intermediate host snails, which are infected [4]. Hence, susceptibility to the disease is increased by household chores such as doing laundry, bathing and fetching water and leisure activities such as fishing, playing and swimming in water infested by infected snails [8].
During the life cycle of the disease (Figure 1), Schistosoma eggs are expelled through faeces or urine, depending on the species. Under suitable conditions, the eggs hatch and release miracidia, which swim and enter particular snail intermediate hosts [9]. The snail goes through two sporocyst generations before producing cercariae. Following their discharge from the snail, infective cercariae swim, penetrate the epidermis of the human host and lose their forked tails, transforming into schistosomulae [9]. Schistosomulae travel to the lungs via venous circulation, then to the heart and lastly to the liver, where they mature and exit through the portal vein. Adult worms, both male and female, copulate and dwell in mesenteric venules, which differ by species. S. japonicum is mostly encountered in the superior mesenteric veins draining the small intestine, while S. mansoni is more frequently observed in the veins of the inferior mesenteric that drain the large intestine. Nevertheless, both species are capable of residing at either location or migrating between them [9]. S. intercalatum and S. guineensis similarly inhabit the inferior mesenteric plexus, although lower in the colon than S. mansoni. While prevalent in the vesicular and pelvic venous plexuses of the bladder, S. haematobium can also be identified in the rectal venules [9]. Females lay eggs in the tiny venules of the portal and perivesical systems. In the case of S. mansoni, S. japonicum, S. mekongi and S. intercalatum, the eggs are progressively conveyed to the intestinal lumen, where they are expelled by faeces. Meanwhile, S. haematobium eggs are conveyed to the bladder and ureters, where they are subsequently expelled by urine. When an infected person urinates or defecates in a water body, the cycle restarts again [9].
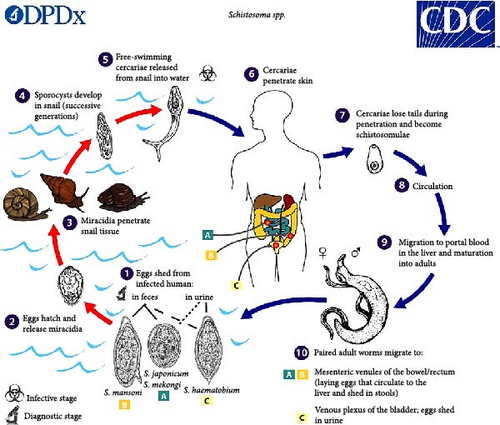
Intestinal schistosomiasis may cause diarrhoea, abdominal pain and blood in the stool. Liver enlargement is typical in advanced instances and is commonly linked with peritoneal fluid buildup and abdominal blood vessel hypertension, and in such instances, splenic enlargement is possible [3]. Haematuria is the typical symptom of urogenital schistosomiasis (blood in urine), with bladder cancer highly likely in the latter stages. Urogenital schistosomiasis can manifest as genital sores, pain during sexual activity, vaginal bleeding and nodules in the vulva in females. In males, it can lead to pathology in the prostate, seminal vesicles and other organs. This condition may have further permanent, long-term effects, including infertility [3]. Even though schistosomiasis is rarely fatal, it causes long-term illnesses such as anaemia induced by worm infestation and motility; iron shortage is also a cause of the disease due to nutritional impairments such as nutrient deficiency and digestive difficulties [10].
To facilitate the transmission of snail-borne illnesses, intermediate host snails and appropriate water bodies must be present. However, the evidence of disease transmission cannot be solely based on these two parameters, as the development, reproduction, growth and survival rate of the parasite in the snail may be influenced by underlying environmental, climatic and physicochemical properties [8]. There is evidence that the distribution and quantity of intermediate host snails for schistosomiasis are influenced by physicochemical water properties, including velocity, temperature, turbidity, salinity, electrical conductivity (EC), dissolved oxygen (DO) and pH [8]. It is notable that chemotherapy is effective in reducing schistosomiasis morbidity in schistosomiasis control; however, its widespread implementation is hindered by its high cost and logistical complications. Thus, snail control is recommended as a preventative measure in conjunction with chemotherapy, but a thorough understanding of snail distribution is first required [11]. This is why a comprehensive understanding of the ecological factors that regulate snail abundance is necessary to predict and understand snail distribution. For example, snail ecology, physiology and schistosome infection are significantly influenced by water chemistry and temperature [11]. Studies on schistosomiasis have mostly focused on the disease’s prevalence and magnitude in human populations, with very few attempting to locate the intermediate snail hosts in the areas where the disease is prevalent [8]. In addition, there are no previous systematic reviews that have sought to understand the correlation between the physicochemical parameters of water bodies and the distribution of schistosomiasis intermediate host snails in SSA. Therefore, this review will identify, analyse and consolidate research findings regarding the relationship between physicochemical properties and intermediate host snails in order to produce a comprehensive overview of current evidence that can be used to inform evidence-based practice and intervention strategies to mitigate the disease’s transmission.
1.1. Aim of the Review
The aim of this review was to identify, analyse and consolidate research findings regarding the relationship between water physicochemical properties and schistosomiasis intermediate host snails’ distribution in SSA.
1.2. Objective/s
The aim of this review was to determine the effect of physicochemical properties of water bodies on schistosomiasis intermediate host snails’ distribution in SSA.
1.3. Review Question
The Population, Intervention, Comparison and Outcome (PICO) format was used to answer the research question. What are the effects of physicochemical properties of water bodies on schistosomiasis intermediate host snails’ distribution in SSA?
2. Materials and Methods
2.1. Search Strategy
A systematic search of literature pertinent to the topic and review objective was implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The protocol for the current review was prospectively devised and registered on the online database Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023428704. A search was conducted from August to October 2023 using six electronic databases (EBSCOhost [Medline, Academic Search Ultimate, Greenfile and Health Source], PubMed and Google Scholar) to address the following review question: ‘What are the effects of physicochemical properties of water bodies on the distribution of schistosomiasis intermediate host snails in sub-Saharan Africa?’ This was followed by a manual search of two grey literature preprint savers (BioRxiv and MedRxiv) and a citation search to ensure a comprehensive search of eligible studies from January 2013 to October 2023. The identification of secondary sources was achieved by verifying the references of articles identified during the search and evaluating their suitability for inclusion in the review. The following search terms were used ‘Schistosomiasis, Intermediate host snails, Physicochemical properties, Effects, Environmental factors, sub-Saharan Africa’. Boolean operators (AND and OR) and truncations to narrow down the set of ‘free terms’ and ‘index terms’ were used in the search. These search terms included ‘Schistosomiasis OR Bilharzia OR Snail Fever’ AND ‘Intermediate host snails OR Freshwater snails’ AND ‘Physicochemical properties OR Environmental factors’ AND ‘Effects OR Impact’.
2.2. Eligibility Criteria
Peer-reviewed articles reporting on the effects of water physicochemical properties on schistosomiasis intermediate host snails’ distribution in SSA were included. The following criteria were met by the articles that were included: (i) published in a peer-reviewed journal, (ii) conducted in a sub-Saharan African country, (iii) between 2013 and 2023, (iv) longitudinal studies, repeated cross-sectional studies, (v) in English and (vi) reported on the relationship between the physicochemical properties of water bodies and the distribution of schistosomiasis intermediate host snails. Articles were excluded if they (i) did not exclusively report on the relationship between the physicochemical properties of water bodies and the distribution of schistosomiasis intermediate host snails, (ii) focused on zoonotic schistosomiasis or (iii) were reviews. In cases where multiple studies were conducted in the same area, the most recent study was included in the systematic review and not both. The systematic review was conducted in 2023; hence, we included only studies up to that year. The authors opt to include solely studies completed within the last decade (2013–2023) due to their contemporary relevance regarding methodology and technological advancements. This enables the authors to evaluate research that are somewhat analogous in assessing the specified parameters.
2.3. Selection Process
To retrieve articles from the selected databases, the Mendeley reference manager (version 2.110.2) was employed. Duplicates were eliminated, and the remaining articles were exported to an Excel spreadsheet. At first, the titles of the articles were screened, followed by the abstracts. The full text of the selected articles was then screened. Microsoft Excel spreadsheets were used throughout the article selection procedure. The first author (P.S.C.) conducted the selection, which was subsequently verified by the second, third and fourth authors (W.t.H.-B., J.B.A. and M.T.).
2.4. Critical Appraisal and Risk of Bias
The first author and two reviewers independently evaluated the quality of the relevant articles using the Joanna Briggs Institute (JBI) critical assessment tool for analytical cross-sectional studies [13]. The overall quality score for each study was calculated via the JBI critical appraisal checklist for analytical cross-sectional studies (Supporting Information 1: Checklist S1), with responses categorised as ‘No’, ‘Yes’, ‘Unclear’ or ‘N/A’ (not applicable). Total risk scores ranged from 0% to 100%, with 0%–54% indicating high risk, 55%–79% indicating moderate risk and 80%–100% indicating low risk. The overall quality scores of the articles examined in this review ranged from moderate to high across the studies. This review critically rated and included 30 studies in the analysis.
2.5. Data Extraction and Analysis
- a.
Study profile (authors, year of publication and country)
- b.
Study design
- c.
Sampling period
- d.
Sampling method and equipment
- e.
Sample size and snail specie
- f.
Measured physicochemical properties
- g.
Summary of main findings
Data from the results of the included studies were compiled into Excel spreadsheets, and a comprehensive quantitative descriptive analysis was performed to organise and display the data in tables and bar graphs. A detailed narrative summary was developed to provide a comprehensive explanation of the graphs and tabulated data within the constraints of the review objective. The Mendeley reference manager was also employed to store literature and generate bibliographies, citations and references. In order to enhance the transparency of the study, the PRISMA checklist for abstracts (Supporting Information 2: Table S1) and the PRISMA 2020 checklist (Supporting Information 3: Table S2) were adopted.
To eliminate bias, interpretations in this systematic review were based only on empirical estimates rather than the authors’ subjective interpretation of their results. Also, the studies were not focusing on or reporting on the same physicochemical properties; therefore, the authors only interpreted the results from each selected article and then collated them. Reported physicochemical properties’ effects on snail abundance were identified and classified into one of three broad categories: positive influence, zero influence or negative influence. Positive influence indicates that the parameter is positively correlated with snails. When the parameter’s value increases, so does the snail population, indicating that snails flourish when the parameter’s value is high. Zero influence indicates that there is no correlation between the parameter and snails. This means that the parameter’s value has no effect on snail abundance, whether it is high or low. Negative influence indicates that the parameter is negatively correlated with snails. When the parameter’s value grows, snail abundance drops; conversely, when the parameter’s value decreases, snail abundance tends to increase. The data herein were obtained from the studies’ reported correlation between snails abundance and physicochemical properties.
3. Results
3.1. Search and Selection Results
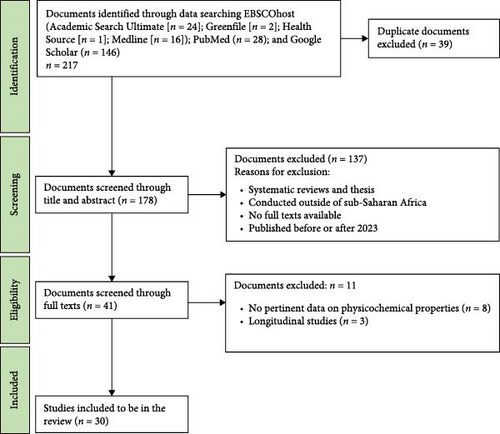
The current systematic review consolidated studies with a critical focus on the effects of physicochemical properties of water bodies on the distribution of schistosomiasis intermediate host snails in SSA. In total, 217 articles were identified from six electronic databases. Thirty-nine articles were screened by title, and duplicates were eliminated, resulting in 178 studies that were eligible for abstract screening. The screening process resulted in the exclusion of 137 irrelevant articles, and 41 articles were assessed for full-text review. In the end, 30 articles were considered eligible for the review, as 11 were excluded due to their failure to satisfy the study inclusion criteria. The studies were primarily excluded due to the absence of germane data on physicochemical properties and their effects on the snails (n = 8), longitudinal studies (n = 3) and non-cross-sectional studies. The PRISMA flowchart for the study’s approach of identifying, filtering and including studies is illustrated in Figure 2.
3.2. Study Characteristics
Table 1 provides a comprehensive summary of all the studies that were evaluated in this systematic review. The final analysis comprised 30 articles from SSA. The studies were conducted in the following 11 countries: Nigeria (n = 13), Ethiopia (n = 5) and Tanzania (n = 3) were the countries from which most of the studies were conducted. South Africa and Kenya each conducted two investigations, while Benin, Cameroon, Chad, Ghana, Cote d’Ivoire and Uganda each conducted one study. In terms of the sampling period, six studies collected data for a period of 12 months, another six studies collected data for a period of 3 months, and four studies collected data for a period of 2 months. Data were collected over a period of 8 months in two studies, 4 months in another two studies and 1 month in another two studies. Moreover, data had been collected for periods of 6 months, 9 months, 13 months, 24 months and 29 months in other studies. The sampling method and equipment used by the numerous studies for snail collection were as follows: The snails were collected in 23 studies using long-handed scoops or dip nets, while four studies used metal sieves or strains. One study employed the Ekman’s grasp, another employed a long plastic spoon, and a third employed metal scoops to collect the snails. In addition to the apparatus employed during the collection process, 10 studies also mentioned the use of the hand-picking technique to collect snails that were visually visible, and five studies mentioned the use of forceps. Concerning the instruments used to measure the water physicochemical properties, eight studies used the HANNA portable multimeter, seven studies used the Hach Lange multimeter, and four studies used the YSI multiparameter meters. Other instruments that were used or mentioned in the studies include the Extech multipurpose meter, Lovibond comparactor pH, centigrade thermometer, Winkler’s method, APHA method, refractometer, pH meters, conductivity meters, handheld fluorometer/turbidimeter, mercury-in-glass thermometer, PCSTestr 35, calibrated Secchi disc, Tracer pocket tester and other multiparameter meters.
| No. | Author/year of study | Country | Sample size and snail species | Study design | Sampling period | Physicochemical properties measured | Sampling method and equipment | Summary of main findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Alhassan et al., 2020 [15] | Nigeria | Out of the total snails collected in the study, 172 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (127) and Bulinus (45) | Cross-sectional | 4 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen, alkalinity | Snails: collected by collecting bottom sediment using Ekman’s grab model (number 923), measuring 19 cm × 14 cm with an area of 0.0266 m2. Snails found at the river bank were also handpicked. Physicochemical properties: measured using HANNA portable combined waterproof tester (model HI 98129) | Findings revealed that the presence, abundance and variety of snails in the two reservoirs were influenced by pH, electrical conductivity (EC), total dissolved solids (TDS), alkalinity and temperature, as evidenced by a positive association between the physicochemical factors stated and snail composition. Snails, on the other hand, correlated negatively with other physicochemical factors such as dissolved oxygen (DO) and hardness |
| 2 | Krauth et al., 2016 | Côte d’Ivoire | Out of the total snails collected in the study, 119 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (43) and Bulinus (76) | Cross-sectional | 3 months | Temperature, pH, electrical, conductivity | Snails: collected using a long-handed kitchen scoop (1.5 m pole; 2 mm mesh size) Physicochemical properties: measured using a portable multimeter (Hach Lange, HQ30D) | Biomphalaria snails were mostly found in turbid environments. Bulinus snails were found to be highly associated with EC. Bulinus snails preferred areas with higher EC (about 127 S/cm) when compared to Biomphalaria (89 S/cm). Temperature (29.8°C), pH (7.1) and turbidity were typical of Bulinus habitats, which fell between Lymnaea and Biomphalaria and matched to an average water site in the region |
| 3 | Usman et al., 2017 [16] | Nigeria | A total of 1605 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (24) and Bulinus (1581) | Cross-sectional | 12 months | Temperature, pH, dissolved oxygen | Snails: collected using a long-handed dip net while hand picking of snails from vegetation around the selected water bodies was done concurrently Physicochemical properties: measured using portable HANNA instruments and Wicklers method (2011) | In their natural environments, most of the snails recorded in this study survived temperatures ranging from 22.3 to 31.9°C. Snail populations were found to be low or absent in water bodies with little DO and high temperatures. Also, the pH values assessed during the study ranged from 7.0 to 8.5, and most of the snails were identified with pH values less than 8 |
| 4 | Manyangadze et al., 2021 [4] | South Africa | A total of 1846 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (985) and Bulinus (861) | Cross-sectional | 13 months | Temperature, pH, salinity and conductivity | Snails: collected using scoops made from a kitchen sieve mounted on a broom stick and handpicking live visible snails Physicochemical properties: measured using a portable water meter (HANNA Instruments) | In comparison to B. globosus, B. pfeifferi was very sensitive to alterations in pH and DO. The monthly abundance of B. pfeifferi revealed a substantial negative relationship with the minimum land surface temperature. Only DO exhibited a statistically significant positive relationship with B. pfeifferi abundance (p 0.05) |
| 5 | Joseph et al., 2023 [17] | Nigeria | Out of the total snails collected in the study, 453 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (263) and Bulinus (190) | Cross-sectional | 12 months | Temperature, pH, electrical, conductivity, dissolved oxygen, alkalinity, hardness | Snails: collected using a snail scoop net Physicochemical properties: missing | The presence of B. truncatus in sampled sites was positively linked with water temperature. All snail species reported at all locations had a positive correlation with alkalinity. There was no statistically significant association between pH and various bullinids (B. truncatus and B. globosus). In this study, there was no significant association between DO and snail abundance |
| 6 | Abdulkadir et al., 2013 [18] | Nigeria | Out of the total snails collected in the study, 8394 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria | Cross-sectional | 12 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen | Snails: collected using a long handled scoop net and those observed visually were collected with forceps Physicochemical properties: measured using a HANNA Instrument, a pocket size pH meter, COMBO meter (model COM 100) and the APHA method | The findings suggested that DO, pH and TDS were the key parameters determining snail distribution in the water body, whereas temperature and conductivity had little effect. They also discovered that alkaline pH was better for snail growth |
| 7 | Angelo et al., 2014 | Tanzania | A total of 1809 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (1470) and Bulinus (339) | Cross-sectional | Missing | Temperature, pH | Snails: collected using handheld scoops and dredges Physicochemical properties: missing | Snail abundance was shown to be highly related to water temperature. Temperatures varied between sampling sites, ranging from 21.9 to 30°C, indicating that the conditions were favourable for the B. sudanica to reproduce, resulting in an increase in snail population density. Furthermore, the pH range was negatively associated to snail abundance, ranging from 7.2 to 7.5. The lack of a relationship between EC and snail abundance suggests that EC had no effect on snail abundance in the research area |
| 8 | Deribew et al., 2022 [19] | Ethiopia | A total of 1125 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Bulinus | Cross-sectional | 9 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen, salinity | Snails: collected using a metal scoop net and occasionally by handpicking Physicochemical properties: measured using Tracer pocket tester (LaMotte 1749) and HQ40d multimeter | EC and DO levels were similar across all sample sites. All the physicochemical variables measured (temperature, pH, TDS, salinity, EC and DO) had no correlation with Bulinus snail abundance |
| 9 | Kamwa Ngassam et al., 2014 [20] | Cameroon | A total of 658 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Bulinus | Cross-sectional | 1 month | Temperature, pH, electrical, conductivity, total dissolved solids, Salinity | Snails: collected using a standard kitchen sieve mounted on a 2-m wooden handle or by hand picking Physicochemical properties: missing | Findings revealed that there was a correlation between water turbidity and overall snail abundance, with the total number of collected snails (abundance) decreasing dramatically as water turbidity increased |
| 10 | Urude et al., 2021 [21] | Nigeria | Out of the total snails collected in the study, 742 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (138) and Bulinus (604) | Cross-sectional | Missing | pH, temperature, dissolved oxygen and salinity | Snails: collected using long handled net scoop Physicochemical properties: measured using HANNA portable combined waterproof tester (model hi 98129) | High-temperature bodies of water did not favour the existence of freshwater snails in the research area. The distribution of freshwater snails in relation to temperature did not indicate a significant difference (p > 0.05) in this study. The distribution of snails was shown to be significantly related to the DO concentration. There was no significant association (p > 0.05) between pH and snail distribution nor was there a statistically significant relationship between snails and salinity of water bodies |
| 11 | Moser et al., 2014 [22] | Chad | A total of 892 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (108) and Bulinus (784) | Cross-sectional | 2 months | Temperature, pH, electrical, conductivity, turbidity | Snails: collected either with a scoop or with forceps Physicochemical properties: measured using a portable multimeter (Hach, HQ40D) and portable turbidimeter (Hach, 2100P ISo) | Findings revealed that the presence of B. truncatus and B. forskalii was linked to EC. The researchers also discovered that pH had a considerable negative influence on B. pfeifferi snails |
| 12 | Oso and Odaibo, 2021 | Nigeria | Out of the total snails collected in the study, 1741 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (1) and Bulinus (1740) | Cross-sectional | 24 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen | Snails: collected using a scoop (0.2 mm mesh size) net Physicochemical properties: measured using portable electronic multipurpose meter (Extech), CE Portable pH Meter and YSI 550A DO Meter | Findings revealed that DO had a substantial positive correlation with some freshwater snails. It has been observed that there is no correlation between EC and any of the bullinid species. The study showed a substantial negative correlation between temperature and all bullinids, as well as a strong correlation between pH and several bullinids |
| 13 | Amoah et al., 2017 [23] | Ghana | A total of 2612 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (1367) and Bulinus (1245) | Cross-sectional | 8 months | Temperature, pH, electrical, conductivity, total dissolved solids, turbidity, salinity | Snails: collected using standard snail scoops or occasionally by forceps Physicochemical properties: measured in situ using a multiparameter tester (HANNA Combo), refractometer and turbidimeter | Findings revealed that TDS and turbidity had a substantial impact on the abundance of live snail species, whereas only temperature had an impact on the quantity of dead B. pfeifferi. Rainfall, conductivity, salinity and pH, on the other hand, had no effect on the number of snail species |
| 14 | Yigezu et al., 2018 [24] | Ethiopia | A total of 1018 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (905) and Bulinus (113) | Cross-sectional | Missing | Temperature, pH, electrical, conductivity, dissolved oxygen, alkalinity, turbidity, hardness | Snails: collected using a scoop net Physicochemical properties: measured using a multiprobe meter (HQ30d Single-Input Multi Parameter Digital Meter, Hach) and handheld fluorometer/turbidimeter | Findings revealed that B. pfefferi was encountered in habitats with high concentration of electric conductivity. On the other hand, B. globosus occurred in habitats with high pH value |
| 15 | Ofulla et al., 2013 | Kenya | A total of 167 schistosomiasis intermediate host snails were collected in the study. They belonged to the genera Biomphalaria (139) and Bulinus (28) | Cross-sectional | 1 month | Temperature, pH, electrical, conductivity, dissolved oxygen, alkalinity, turbidity, hardness, salinity | Snails: collected using a standard flat wire mesh scoop (2 mm mesh size) Physicochemical parameters: measured with a YSI 556 MPS Handheld MultiPparameter Instrument | Findings revealed that temperature levels in different parts of the lake had a negative connection with Biomphalaria spp. Furthermore, in different parts of the lake, pH had a favourable association with both Biomphalaria spp. and Bulinus spp. In addition, the researchers discovered that DO and turbulence were negatively predictive of Biomphalaria spp. |
| 16 | Ezinna E et al., 2023 | Nigeria | Out of the total snails collected in the study, 533 snail species were schistosomiasis intermediate hosts from the genera Bulinus. | Cross-sectional | 3 months | Temperature, pH, dissolved oxygen | Snails: collected using a long plastic spoon and hand gloves Physicochemical properties: measured in situ using pH meters, turbidity meters and thermometers | Findings revealed that the main parameters that influenced snail breeding in the research area were DO and pH levels |
| 17 | Rowel et al., 2015 [25] | Uganda | A total of 19,355 schistosomiasis intermediate host snails were collected in the study and belonged to the genera Biomphalaria | Cross-sectional | 29 months | Temperature, pH, electrical, conductivity, total dissolved solids | Snails: collected using the snail scoop net Physicochemical properties: measured using a water meter (430 Enterprise) | Along Lake Albert, the snail population exhibited a positive correlation with pH and a negative correlation with EC. Along the borders of Lake Victoria, however, only pH demonstrated a good relationship with snails. Although there was a positive correlation between TDS and EC along the coastlines of the mentioned areas, these correlations were not statistically significant. In addition, there was no correlation between temperature and snail population along the two bodies of water. EC, water depth and pH were discovered to be the physicochemical parameters affecting the Biomphalaria population |
| 18 | Olkeba et al., 2020 [26] | Ethiopia | Out of the total snails collected in the study, 1494 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (580) and Bulinus (914) | Cross-sectional | 2 months | Temperature, pH, electrical, conductivity, dissolved oxygen, alkalinity, turbidity, hardness | Snails: collected using a standard scoop net with a mesh size of 300 µm supported by a metal frame Physicochemical properties: measured using a multiprobe meter (HQ40d Single-Input Multi Parameter Digital Meter and a turbidity meter (Wag-WT3020) | At locations with relatively warmer water temperatures, B. pfeifferi, B. sudanica and B. globosus were numerous. Additionally, B. pfeifferi and B. sudanica were more prevalent at locations with low DO levels. B. pfeifferi was less prevalent and less abundant at locations with a high turbidity. At sites with a high chloride content, B. sudanica and B. globosus were more prevalent than at sites with a high-water alkalinity |
| 19 | Nwoko et al., 2022 [27] | South Africa | Out of the total snails collected in the study, 734 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (246) and Bulinus (488) | Cross-Sectional | 3 months | pH, electrical, conductivity, dissolved oxygen, salinity | Snails: collected using a metal scoop complemented by hand picking Physicochemical properties: measured using a portable multiparameter meter (HANNA Instruments, HI9829) | Snails were found in locations with pH levels ranging from 6.42 to 7.98. pH and B. globosus exhibited a strong positive correlation, while B. tropicus and B. forskalii exhibited a substantial negative correlation with pH. Some snails were shown to have a favourable correlation with DO. Salinity, DO, temperature, precipitation and pH have been identified as physicochemical characteristics that impact snail distribution, abundance and variety |
| 20 | Fuss et al., 2020 [28] | Tanzania | A total of 4888 schistosomiasis intermediate host snails were collected in the study and belonged to the genera Biomphalaria | Cross-sectional | 2 months | Temperature, pH, electrical, conductivity, dissolved oxygen, salinity | Snails: collected using a scoop using a pair of forceps Physicochemical properties: measured using a portable multimeter device (PCE-PHD 1) | Snail abundance was not found to be significantly correlated with any of the measured water quality variables including temperature (p = 0.8), pH (p = 0.6), EC (p = 0.9) or DO (p = 0.8). Salinity was shown to be significantly related to the prevalence of Biomphalaria snail infections (rs = 0.504, p = 0.047, n = 16) |
| 21 | Mereta et al., 2023 [29] | Ethiopia | Out of the total snails collected in the study, 363 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (117) and Bulinus (246) | Cross-sectional | 3 months | Temperature, pH, electrical, conductivity, turbidity | Snails: collected by handpicking live visible snails and using a scoop net with a wire mesh measuring 1.5 mm on an iron frame (40 30 cm) and mounted on a 1.5-m long iron handle Physicochemical properties: measured using a multiprobe meter (HQ30d Single-Input MultiParameter Digital Meter, Hach) | Findings revealed that the abundance of B. pfeifferi was positively connected with oxygen saturation and the abundance of B. globosus was inversely correlated with oxygen saturation. B. globosus was also more numerous in regions with higher EC. The EC of water bodies polluted by human waste and sewage from household wastes was very high |
| 22 | Dida et al., 2014 [30] | Kenya and Tanzania | Out of the total snails collected in the study, 37 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (10) and Bulinus (27) | Cross-sectional | 2 months | Temperature, pH, electrical, conductivity, dissolved oxygen, alkalinity, turbidity, salinity | Snails: collected using a standard flat wire mesh scoop with a mesh size of 2 mm Physicochemical properties: measured using a handheld multiparameter YSI meter (YSI Model 650-01 m Environmental Monitoring Systems, Yellow Springs, OH) | Water samples with B. pfeifferi and B. africanus showed statistically significant variations in mean turbidity, alkalinity and DO but not in pH or conductivity. The alkalinity of water bodies also varied greatly between the two snail species |
| 23 | Oloyede et al., 2016 | Nigeria | Out of the total snails collected in the study, 55 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (37) and Bulinus (18) | Cross-sectional | 6 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen, alkalinity, hardness | Snails: collected using a 0.2-mm scoop net with a long handle Physicochemical properties: measured using a mercury-in-glass thermometer, Consort C933-chemical analyser, Atomic Absorption Spectrometer (Model 210/211 VGP), mixed indicators (methyl red and bromocresol green) and Winkler’s reagent | Findings revealed that there was no association between DO and pH and the populations of any of the freshwater snails. Water depth was positively correlated with B. globosus and negatively correlated with B. pfeifferi. EC was strongly associated with B. globosus |
| 24 | Ikpeze and Obikwelu, 2016 | Nigeria | Out of the total snails collected in the study, 4753 snail species were schistosomiasis intermediate hosts from the genera Bulinus | Cross-sectional | 12 months | Temperature, pH, dissolved oxygen | Snails: collected using a scoop net and hand-picking technique Physicochemical properties: measured using standard laboratory procedures | Findings revealed that temperature had minimal effect on snail density, although temperatures above 30°C were reported to be deadly to snail development. In addition, there was a negative association between DO, pH and the density of snails. |
| 25 | Mereta et al., 2019 [31] | Ethiopia | Out of the total snails collected in the study, 2360 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (2079) and Bulinus (281) | Cross-sectional | 3 months | Temperature, pH, electrical, conductivity, turbidity, hardness | Snails: collected using a scoop net with wire mesh measuring 1.5 mm on an iron frame (40 × 30 cm) and mounted on a 1.5-m-long iron handle Physicochemical properties: measured using a multi probe meter (HQ30d Single-Input MultiParameter Digital Meter, Hach), a fluorometer (Turner Design Aqua Fluor and a 0.45 μm filter paper | Findings revealed that water quality, cleanliness and water contact behaviour of the people all had a significant impact on the abundance, occurrence and infectivity of snail species, pH, EC and DO were major factors of snail occurrence |
| 26 | Abdulkadir et al., 2017 [32] | Nigeria | Out of the total snails collected in the study, 727 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (495) and Bulinus (232) | Cross-sectional | 12 months | Temperature, pH, electrical, conductivity, total dissolved solids, dissolved oxygen | Snails: collected using a scoop net or manually collected with forceps when seen Physicochemical properties: measured using pocket size pH meter, H198103 Checker pH Tester (HANNA instruments, Inc.).and EC/TDS/Temp COMBO meter (Model Com 100,11/05) | Findings revealed that the relative abundance of freshwater snails in the dam was significantly (p < 0.05) influenced by DO, biological oxygen demand (BOD) and pH |
| 27 | Oladejo et al., 2021 [33] | Nigeria | Out of the total snails collected in the study, 171 snail species were schistosomiasis intermediate hosts from the genera Bulinus | Cross-sectional | 8 months | Temperature, pH, turbidity | Snails: collected using a D-shaped dip net (0.2-mm bottom edge and 243 µm mesh) Physicochemical properties: measured using a mercury-in-glass thermometer a Waterproof Multiparameter, PCSTestr 35 and a calibrated Secchi disc | Findings revealed that there was a strong connection between pH and the quantity of freshwater snails. There was no significant relationship between snail density and temperature. There was also a substantial positive association between turbidity and snail abundance |
| 28 | Ablavi et al., 2018 | Benin | Out of the total snails collected in the study, 1345 snail species were schistosomiasis intermediate hosts from the genera Bulinus | Cross-sectional | 12 months | Temperature, pH, total dissolved solids, dissolved oxygen, turbidity, salinity | Snails: collected using metal sieves or strains with elongated (1.5 to 2 m) rounded, 20 cm diameter shallow and round bottom (dipping technique) Physicochemical properties: measured using a multiparameter pH/Oxi340i/SET device and a cond340i/SET conductivity meter | Findings revealed a variable correlation between the dynamic of schistosomiasis intermediate host snails and several physicochemical parameters. B. globosus was negatively influenced by salinity and nitrate rates, while B. forskalii was negatively influenced by pH, DO, TDS and salinity rates. The three species of freshwater snails were strongly influenced by the water temperature |
| 29 | Ebenezer and Ekwuribe, 2022 | Nigeria | Out of the total snails collected in the study, 68 snail species were schistosomiasis intermediate hosts from the genera Biomphalaria (34) and Bulinus (34) | Cross-sectional | 3 months | Temperature, pH, electrical, conductivity, alkalinity, turbidity, salinity | Snails: collected using scoops or hand picking Physicochemical properties: measured using portable electronic multipurpose meter (Extech) (CE Portable pH meter) and YSI 550A DO meter | Findings revealed that Bulinus had a positive relationship with pH, salinity and EC and a negative correlation with temperature, turbidity, BOD and alkalinity. Biomphalaria had a positive correlation with temperature, pH, salinity, turbidity and alkalinity |
| 30 | Obisike et al., 2018 [34] | Nigeria | Out of the total snails collected in the study, 587 snail species were schistosomiasis intermediate hosts from the genera Bulinus | Cross-sectional | 4 months | Dissolved oxygen, pH, temperature, turbidity | Snails: collected using a wire mesh scoop nets of size 2.2 mm and hand picking Physicochemical properties: measured using the Lovibond comparactor pH, centigrade thermometer, Winkler’s method and some taken to the laboratory | The average monthly change in some of the physicochemical parameters observed revealed that the temperature ranged between 26 and 29° C, which was ideal for the development of snails. The number of snails grew as the pH, BOD and DO decreased. This was accompanied by an increase in both phosphate and turbidity levels |
- Note: Summary of studies used to determine the effect of physicochemical properties of water bodies on schistosomiasis intermediate host snails’ distribution in sub-Saharan Africa.
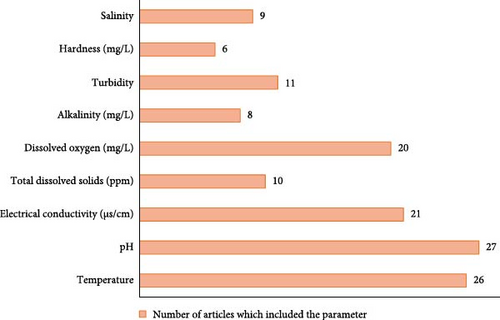
Out of the 30 studies that were analysed, only 27 of them provided relevant information regarding the physicochemical properties of their samples. To compile the results, physicochemical properties such as temperature, pH, EC, total dissolved solids (TDS), DO, alkalinity, turbidity, hardness and salinity were used, as they were the most researched properties. The predominant or extensively studied physicochemical property of water bodies was pH, seen in all 27 investigations, followed by temperature in 26 studies and EC in 21 studies. DO was the fourth most often measured parameter, seen in 20 studies. Turbidity was measured in 11 studies, TDS in 10 studies, salinity in 9 studies, alkalinity in 8 studies and hardness in 6 studies (Figure 3).
3.3. Geographic Distribution of Studies That Measured Physicochemical Properties in SSA
Our current study revealed that only 11 out of 48 sub-Saharan African countries were actively engaged in reporting evidence-based research on the effect of physicochemical properties of water bodies on the distribution of schistosomiasis intermediate host snails, with most of the research conducted in Nigeria and Ethiopia. The review encompasses research from Western Africa (Nigeria, Benin, Côte d’Ivoire and Ghana), Eastern Africa (Ethiopia, Tanzania, Kenya and Uganda), Central Africa (Cameroon and Chad) and Southern Africa (South Africa). The majority of studies featured in this review were conducted in West and East Africa.
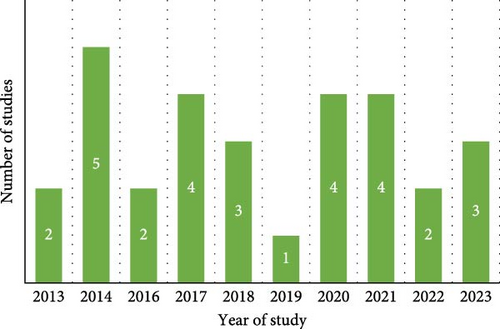
The analysis encompassed articles published from 2013 to 2023, as illustrated in Figure 4. Five studies were undertaken in 2014, while the years 2017, 2020 and 2021 each included four studies. In 2018 and 2023, there were three studies each, whereas 2013, 2016 and 2022 had two studies each. A single study was conducted in 2019. All the research used a cross-sectional study design.
3.4. Physicochemical Influence on Snail Abundance
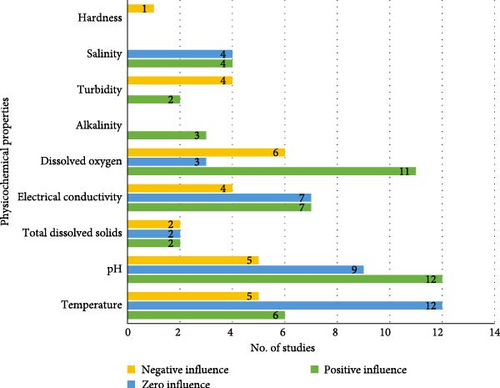
The results of 29 out of 30 studies were thoroughly reviewed, and published findings regarding the effect of physicochemical properties on snail abundance were retrieved. Based on the findings, most studies (n = 12) concluded that water temperature did not have any influence on the abundance of snails [16, 18–21, 23, 25, 28, 33–35]. A few studies (n = 5) indicated that temperature has a negative influence on snail abundance [4, 24, 36–38]. Moreover, most studies (n = 12) indicated that pH had a positive influence on snail abundance [15, 18, 25, 27, 31–33, 36, 37, 39–41], whereas others (n = 9) did not observe any influence [4, 17, 19–21, 23, 24, 28, 42]. In addition, the findings for EC were inconsistent, with some studies (n = 7) indicating a positive influence of EC on snail abundance [15, 22, 24, 29, 31, 39, 41], while others (n = 7) found that EC had no influence [18–20, 23, 28, 36, 42]. Most studies (n = 11) indicated that DO had a positive influence on snail abundance [4, 16, 18, 21, 27, 31, 32, 36, 38–40]; however, other studies (n = 6) found that DO had a negative influence on snails [15, 26, 34, 35, 37, 41]. Most studies (n = 4) indicated that turbidity had a negative influence on snail abundance [20, 23, 24, 26]. Three studies revealed that alkalinity positively influenced snail abundance [15, 17, 26]. Furthermore, the findings on salinity were inconsistent, with n = 4 studies indicating that salinity had a positive influence on snail abundance [15, 21, 27, 28], while another (n = 4) studies indicated that salinity had no influence [19, 20, 23, 24]. In addition, a few studies (n = 2) discovered that TDS had a positive influence on the abundance of snails [15, 18], while other studies (n = 2) found no influence [19, 20]. Only one study reported on the impact of water hardness and found that it had a negative influence on snails [15]. Figure 5 provides a visual breakdown of the reported influence of studies.
The physicochemical data from the included studies were used to determine the overall mean, standard deviation and standard error, as indicated in Table 2. Based on the findings, the average temperature suitable for snail growth and abundance is 27.1°C, a pH level of ~7.5 and an EC level of 252.5 μ/cm. Additionally, we discovered that the average TDS that could conceivably sustain snail abundance is 450.4 ppm, with a DO of 5.3 mg/L, alkalinity of 106.8 mg/L, turbidity of 76.2 NTU, hardness of 72.3 mg/L and salinity of 0.8 ppm.
| Descriptive statistics | Physicochemical properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | pH | Electrical conductivity (μs/cm) | Total dissolved solids (ppm) | Dissolved oxygen (mg/L) | Alkalinity (mg/L) | Turbidity (NTU) | Hardness (mg/L) | Salinity (ppm) | |
| Overall mean | 27.1 | 7.5 | 252.5 | 450.4 | 5.3 | 106.8 | 76.2 | 72.3 | 0.8 |
| Standard deviation | 2.0 | 0.7 | 174.6 | 1043.9 | 1.7 | 121.4 | 58.6 | 41.6 | 1.3 |
| Standard error | 0.4 | 0.1 | 38.1 | 330.1 | 0.4 | 42.9 | 17.7 | 17.0 | 0.4 |
| No. of studies | 26 | 27 | 21 | 10 | 17 | 8 | 11 | 6 | 9 |
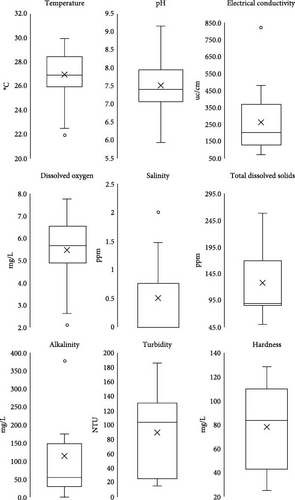
The boxplots illustrate the distribution of the given data set (Figure 6). The statistical measures of central tendency, such as the mean, median, quartiles and range, are used to describe the values represented by the data. Additionally, any outliers are identified and analysed. The analysis revealed a positively skewed distribution for the dataset of temperature, EC, TDS, alkalinity, pH and salinity. Moreover, findings revealed that the hardness and turbidity datasets exhibited a negative skewness, while there was no skew for DO. Furthermore, a data point that deviated significantly from the norm was noted in temperature (22°C), EC (825 μ/cm), alkalinity (378.8 mg/L), salinity (2 ppm) and DO (2.1 mg/L) measurements. The findings indicate that the minimum temperature recorded is 22.5°C, the first quartile (Q1) is 26°C, the median is 26.9°C, the third quartile (Q3) is 28.5°C, and the maximum temperature is 30°C. The pH values range from a minimum of 5.9 to a maximum of 9.1, with Q1 at 7.1, the median at 7.4 and Q3 at 7.9. The minimum value for EC is 74 μ/cm, Q1 is 131.9 μ/cm, the median is 204.1 μ/cm, Q3 is 368.8 μ/cm, and the maximum value is 481.1 μ/cm. The minimum DO level is 2.6 mg/L, Q1 is 4.9 mg/L, the median is 5.7 mg/L, Q3 is 6.6 mg/l, and the maximum DO level is 7.8 mg/L. The salinity values range from a minimum of 0.002 ppm to a maximum of 1.48 ppm, Q1 is 0.004 ppm, the median is 0.012 ppm, and Q3 is 0.77 ppm. The minimum value for TDS is 50.6 ppm, Q1 is 86.7 ppm, the median is 90.1 ppm, Q3 is 169.8 ppm, and the maximum value is 259.4 ppm. The alkalinity values range from a minimum of 2.6 mg/L to a maximum of 176 mg/L, Q1 is 31.6 mg/L, the median is 55.2 mg/L, and Q3 is 148.8 mg/L. The minimum value for turbidity is 16.1 NTU, Q1 is 25.8 NTU, the median is 103.9 NTU, Q3 is 130.6 NTU, and the maximum value is 186.4 NTU. The minimum hardness value is 25.3 mg/L, Q1 is 43.4 mg/L, the median is 83.9 mg/L, Q3 is 110.1 mg/L, and the maximum value is 128.4 mg/L.
4. Discussion
This systematic review provided a summary of the effect of physicochemical properties of water bodies on schistosomiasis intermediate host snails’ distribution in SSA. Under natural circumstances, snails are exposed to multiple environmental factors that have a cumulative effect on snail abundance [43], and this review showed that the nine physicochemical properties of water bodies which included pH, temperature, EC, DO, TDS, salinity, turbidity, alkalinity and hardness had an impact on snail abundance. This review found that most studies that assessed the effects of physicochemical properties on snail abundance were conducted only in 11 out of the 48 countries representing SSA. This finding is alarming and shows the lack of research in this area, and this may be due to the fact that the treatment and control of schistosomiasis in endemic regions of Africa are based on preventive chemotherapy (PC) through mass drug administration (MDA) with praziquantel (PZQ) [44]. In light of this, the majority of research is concentrated on the prevalence of PC. Although the utilization of MDA in conjunction with PZQ appears to provide advantages, individuals may rapidly reinfect themselves if they return to water bodies that contain infectious cercariae after treatment. Therefore, in order to transition from widespread control to elimination, a multifaceted intervention approach is required, which includes the following: snail control, assessment of water physicochemical properties, effective risk mapping and epidemiological surveillance and treatment [44]. Moreover, the review indicates that most studies were conducted in Nigeria (n = 13), followed by Ethiopia (n = 5). Only one study was undertaken in Ghana, and no studies were conducted in the Democratic Republic of Congo (DRC), which is concerning given that both Ghana and the DRC rank third and fourth in schistosomiasis prevalence in SSA, respectively [6]. In addition, the review indicates that the sampling period for most studies that investigate the effect of physicochemical properties on the distribution of intermediate host snails is either 3 months or 12 months. It was also discovered that the most frequently employed instrument for snail collection was the long-handed scoops or dip nets, while visible snails were collected by hand or with the use of forceps. Measurements of physicochemical properties were predominantly conducted using the HANNA portable multimeter and the Hach Lange multimeter.
Based on the review, the most frequently investigated or quantified physicochemical properties in SSA are pH, temperature, DO and EC. These physicochemical properties are frequently investigated and quantified due to their significance as fundamental indicators of environmental health and water quality. For example, pH is a metric that quantifies the acidity or alkalinity of water, which can have an impact on the health of aquatic organisms and the solubility and toxicity of chemicals [36]. Temperature impacts the solubility of gases in water, the metabolic rates of organisms and the rates of chemical reactions [34, 45]. Meanwhile, DO is crucial for aquatic life, with low levels signifying pollution and substandard water quality [36], whereas EC indicates the water’s capacity to conduct electricity, correlating with ion concentration. Elevated conductivity may signify increased concentrations of dissolved salts or contaminants [46]. Collectively, these metrics offer an extensive overview of the water’s quality, facilitating the identification of pollution sources, evaluation of ecosystem health and informing environmental management strategies.
Most studies (n = 12) reported that temperature did not have any influence on snail abundance since the snails were collected in various temperature ranges. However, other reviewed studies (n = 6) contradicted this conclusion and suggested that temperature has a positive impact on snail abundance, meaning that whenever there is an increase in water temperature above 27°C, snail populations thrive. According to Yigezu et al. [24], increased temperature significantly raises the snail metabolic rate and, as a result, increases the snail population size by shortening the duration of the development periods. This could be because a higher temperature enhances the availability of food [26]. Rubaba et al. [47] further highlighted that Bulinus snails can endure greater temperatures owing to their ability to aestivate and adapt, which explains why S. haematobium is more prevalent in warmer regions than S. mansoni. Breeding is typically inhibited at temperatures below 20°C. The interaction between snail microhabitats, temperature and infection may be complex since snails may also go deeper into a water body where the temperature is high [48]. The influence of pH on snail abundance is still debatable, as most of the studies (n = 12) in this systematic review reported that pH has a positive impact on snail abundance. However, other reviewed studies (n = 9) suggested that pH does not influence snail populations. According to Marie et al. [43], a change in pH below or above 7.0 and 9.0, respectively, reduces snail production from 68.94% to roughly 1.35% and 13.28%, respectively. In contrast, Levitz et al. [49] found that a lower pH (more acidic) was related to a greater snail population size. This was supported by Joof et al. [50], who also discovered that the abundance of Bulinus snails increased with a decline in water pH.
In the context of EC, some reviewed studies (n = 7) found that it does not have any influence on snail abundance, whereas other studies (n = 7) disputed this, as they found EC to have a positive impact on the snails. EC has been regarded as an indicator or factor that may limit the dispersal of snail species, and tolerance to this characteristic varies between species and phases of development. This is observable in egg masses and hatchlings, which are more vulnerable to high conductivity than adults [36]. In addition, a rise in EC levels leads to a decrease in DO, which has a negative impact on snail abundance [23, 51]. This is consistent with a few reviewed studies (n = 4) that reported EC having a negative impact on snails. Most reviewed studies (n = 11) found that DO has a positive impact on snail abundance. According to Mereta et al. [31], the amount of DO in the water is an indicator of the level of organic pollution, and it can have a significant impact on the existence of aquatic organisms. Other reviewed studies (n = 6) found that low DO levels may also impact the snails negatively. Furthermore, Olkeba et al. [26] discovered that B. pfeifferi and B. sudanica snails favour low DO habitats. The snails’ ability to colonise places rich in organic matter may explain the unfavourable association between snail abundance and low DO levels [11].
Some reviewed studies (n = 2) reported that TDS has no influence on snail abundance. In contrast, it was discovered that a positive association between TDS and the abundance of intermediate host snails exists [11], which is consistent with other reviewed studies (n = 2) that found that TDS has a positive impact on snails. The wide deviation in the box plots for TDS could be attributed to the fact that the dataset was limited in comparison to other parameters. High levels of alkalinity are believed to be connected with organic pollution, and most snails thrive in water bodies contaminated by human waste and sewage [30]. Alkalinity was found to have a positive impact on snail abundance, meaning that snails thrive in more alkaline water [15, 17, 26]. This may be related to the fact that organic matter boosts the growth and abundance of algae, which is one of the most nutritious foods for snails [30]. The wide deviation in the box plots for alkalinity could be attributed to the fact that the dataset was limited in comparison to other parameters. All natural water bodies are known to possess a certain level of turbidity, which may be attributed to the high planktonic volume, flooding or sediments from soil erosion [52]. Increased turbidity may have a negative impact on the oviposition, development and hatching of snail eggs, resulting in a decline in snail abundance [53]. This is consistent with most reviewed (n = 4) studies, as they also found that turbid water has a negative impact on snail abundance. The wide deviation in the box plots for turbidity could be attributed to the fact that the dataset was limited in comparison to other parameters.
The salinity of aquatic habitats affects the survival and abundance of intermediate host snails [52], since they are less resistant to salinity [23]. However, reviewed studies (n = 4) contradict this, as they suggest that salinity has a positive impact on snail abundance, according to most studies. The positive impact may be explained by the fact that conductivity and salinity are usually correlated, and polluted waters have higher conductivity than nonpolluted waters. The wide deviation in the box plots for salinity could be attributed to the fact that the dataset was limited in comparison to other parameters. Water hardness has a negative impact on snail abundance, as reported in this systematic review [15]. This contradicts the findings by El Deeb et al. [45], who discovered that reduced water hardness led to a decrease in the number of snails and a thinning of their shells. The wide deviation in the box plots for hardness could be attributed to the fact that the dataset was limited in comparison to other parameters. As a result, we may conclude that intermediate host snails rely on certain physicochemical properties for survival; however, how they are affected by these parameters may vary depending on other factors such as snail species, habitat, other environmental conditions and anthropogenic activities.
5. Conclusion
This review shows that while the significance of an appropriate intermediate host is paramount, limited research has been undertaken to explore the interaction between snail species and various physicochemical factors that affect their development and abundance in the aquatic ecosystems of SSA. This is due to the fact that the number of studies that were germane to the research question and included in this review belonged to only 11 countries out of the 48 countries in SSA. Consequently, future research should also concentrate on the identification of these environmental factors to facilitate the implementation of control measures. We also found that pH and DO have a positive influence on snail abundance, while temperature does not have any influence on snail abundance. However, evidence gathered from both the reviewed studies and other literature searched indicates that snail abundance is not simply dependent on a single physicochemical property. These properties appear to have an effect only on snails when combined, and this demonstrates that various parameters influence one another. For example, temperature is directly proportional to EC, whereas an increase in conductivity can reduce DO levels. As a result, in the future implementation of control measures, physicochemical properties such as temperature and DO in water can be used to manipulate other physicochemical properties, making waterbodies unsuitable for snail growth and thus reducing disease transmission. Temperature, pH, turbidity and DO were among the values/properties with contradictory findings on their effect on snail abundance. In addition, no conclusive evidence could be derived from hardness, EC, TDS or salinity; therefore, additional meta-analyses may be required.
6. Limitations and Strengths
The studies included in this systematic review did not examine the same number or types of physicochemical properties; hence, they do not create enough room for further data interrogation or comparative evaluations. In addition, only 27 of the 30 reviewed studies documented their measured physicochemical values; therefore, only those 27 could be used in computations (mean, standard deviation and error). Also, only studies that employed a cross-sectional design approach were included. Moreover, the studies covered in this systematic review are not representative of all the 48 ‘sub-Saharan’ nations as classified by the World Bank Group [54].
The findings of this systematic review could help in determining which physicochemical parameters have the most impact on intermediate host snails. This information can help relevant authorities implement measures in schistosomiasis-endemic areas to break the transmission circle. In addition, physicochemical parameters can be used to forecast snail abundance or whether certain bodies of water are suitable for snail survival. These are crucial elements to consider when looking for potential transmission sites, because an increase in the population of snail species may be supported if the physicochemical parameters and climatic factors of the water bodies are favourable for their diversity, distribution and abundance.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Prince Samkelo Campbell oversaw the original draft’s authoring as well as modifications, methodology, data analysis, validation and data curation. Maryline Vere did the review and editing. Wilma ten Ham-Baloyi, Melusi Thwala and Janine Barbara Adams did the validation, review, editing and supervision, while Paula Ezinne Melariri oversaw the conceptualization, validation, review, editing and supervision.
Funding
We would like to thank the Water Research Commission of South Africa for funding the project entitled ‘Water quality-based predictive tool for disaster management of waterborne infections during extreme weather events’ (Project Number 2022/2023-00834).
Acknowledgments
The National Research Foundation of South Africa, with the support of the DSI/NRF Research Chair in Shallow Water Ecosystems, supported Janine Barbara Adams and is thanked for funding (UID 84375).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data supporting the conclusions of this study are available upon reasonable request from the corresponding author (Prince Samkelo Campbell).




