From Sunshine to Wellness: Understanding Vitamin D Impact on Menstrual Health
Abstract
Background: Vitamin D, a fat-soluble nutrient essential for normal bone development and maintenance, plays a crucial role in the body’s absorption of calcium, magnesium, and phosphate. Recent research has highlighted its significant influence on menstrual health, an area of human well-being that has often been overlooked.
Objective: This review aims to explore the multifaceted role of vitamin D in menstrual health, examining its effects on various aspects of the menstrual cycle, mood regulation, and reproductive health, as well as its involvement in gene pathways and the modulation of stress.
Methods: A comprehensive review was conducted using databases such as Ovid Medline, OVID EMBASE, OVID Cochrane Library, PubMed, Scopus, and Web of Science to identify peer-reviewed articles published up to 2024. Keywords included “Vitamin D,” “Women’s menstrual phases,” “Menstrual cycle,” “Hormonal regulation,” and “Reproductive health.” Studies were screened and selected based on eligibility criteria, including a focus on the relationship between vitamin D and menstrual phases in women, and were subjected to a quality assessment for methodological rigour. The results show that vitamin D was found to have a significant impact on menstrual health, including the regulation of menstrual cycle phases, alleviation of premenstrual syndrome (PMS) symptoms, reduction of abdominal pain during menstruation, and support for bone health. Additionally, vitamin D exerts anti-inflammatory effects, promotes cardiovascular health, and has a modulatory influence on stress and mood.
Conclusion: This review highlights the pivotal role of vitamin D in women’s reproductive health, highlighting its comprehensive involvement in the menstrual cycle and its potential to enhance overall well-being. The findings suggest that ensuring adequate vitamin D levels, whether through sunlight exposure, diet, or supplementation, may offer significant benefits for menstrual health and related aspects of women’s health.
1. Introduction
Vitamin D colloquially referred to as the “sunshine vitamin” stands as an indispensable micronutrient essential for a myriad of physiological functions critical to human health and well-being [1]. Its significance spans a broad spectrum of bodily processes, encompassing immune system modulation, calcium absorption, and the maintenance of skeletal health [2]. Recent research has unveiled the remarkable impact of vitamin D on an often-overlooked facet of human health—menstrual health [3]. Vitamin D synthesis is intriguingly intertwined with exposure to ultraviolet radiation (UVR) from sunlight. This synthesis occurs with specificity when an organism encounters UVR possessing wavelengths shorter than 315 nanometers (nm) [4]. Vitamin D manifests in two principal forms: Vitamin D2, scientifically known as ergocalciferol, is generated through the UV) irradiation of ergosterol, a plant sterol [5]. Conversely, Vitamin D3, also recognized as cholecalciferol, is synthesized within the epidermal layers when 7-dehydrocholesterol interacts with UV rays [6]. The synthesis of vitamin D unfolds through a meticulous two-step progression. Initially, vitamin D undergoes hydroxylation within the liver, culminating in the formation of 25-hydroxy vitamin D3, denoted as 25[OH]D3. Subsequently, this metabolite undergoes further transformation to attain its active form, 1,25-dihydroxy vitamin D3, represented as 1,25[OH]2D3. It is imperative to underscore that while sunlight remains the primary source of vitamin D, dietary intake constitutes a crucial avenue for meeting the daily recommended intake of this vital nutrient. Roughly 80% of the requisite vitamin D is biosynthesized when 7-dehydrocholesterol encounters UV-B radiation from sunlight. The remaining 20% is procured through dietary sources [7]. Notably, animal-derived products serve as the primary reservoir of vitamin D, with diverse food items contributing to its dietary availability. These include various fish species, mushrooms, reindeer lichen, fish liver oil, cattle liver, eggs, dark chocolate, yogurt, and fortified cheese [8].
Beyond its established roles in calcium metabolism and bone health, vitamin D has garnered significant attention for its potential impact on menstrual health. It influences the menstrual cycle, enhances mood, alleviates premenstrual syndrome (PMS) symptoms, and promotes bone health. Additionally, it helps reduce abdominal pain during menstruation, exerts anti-inflammatory effects, and supports cardiovascular health (Table 1) [11–13]. This comprehensive review delves into the intricate interplay between vitamin D and menstrual health, encompassing its biosynthesis, dietary origins, safety considerations, and multifaceted involvement in gene pathways and behavioral regulation. Furthermore, we elucidate the effects of vitamin D on stress and its modulatory effects on the menstrual cycle, underlining its pivotal role in women’s reproductive health in Figure 1.
| Region | Vitamin D deficiency prevalence (%) |
|---|---|
| North India | 28 |
| South India | 14.3 |
| United States | 41.3 |
| South America | 35 |
| North Africa | 10 |
| European countries | 78 |
| Northern Europe | 70 |
2. Methods
2.1. Literature Search/Data Source/Search Criteria
A narrative review of our literature search methodology, we conducted a review search using Ovid Medline, OVID EMBASE, OVID Cochrane Library, PubMed, Scopus, and Web of Science databases, focusing on studies published up to 2024. We employed a combination of keywords, including “Vitamin D,” “Women’s menstrual phases,” “Menstrual cycle,” “Hormonal regulation,” and “Reproductive health,” using Boolean operators (AND, OR) to refine our search strategy. Our eligibility criteria were stringent, including only peer-reviewed articles that specifically explored the relationship between vitamin D and menstrual phases in women, with studies available in English. Reviews, meta-analyses, and editorials were excluded to ensure the inclusion of primary research articles. The search process involved title and abstract screening, followed by a full-text review to assess eligibility. Key data points, such as study design, population characteristics, and main findings, were extracted.
2.2. Plan and Protocol
This review was planned by Dr. Rajeshkumar Shanmugam, and the study was protocolized by Dhanyaa Muthukumaran. The data extraction procedure was implemented by the authors. They employed the study’s objective, type, details, vitamin D, and reproductive health.
2.3. Review and Categorization
To ensure a thorough analysis of the literature, we conducted a narrative review and categorization of the identified studies. After the initial data extraction, studies were categorized based on key themes such as the impact of vitamin D on different menstrual phases, its role in hormonal regulation, and its overall effect on reproductive health. Each study was evaluated for its methodological quality, and studies with robust designs were prioritized in the synthesis of evidence. The categorization process also involved grouping studies according to the specific outcomes measured, such as changes in hormone levels, menstrual regularity, and reproductive health indicators. This approach allowed for a structured and narrative review, ensuring that the findings presented in our paper are well-organized and reflective of the current state of research on the relationship between vitamin D and menstrual phases in women.
3. Vitamin D Deficiency Status
Scientific investigations have demonstrated the widespread prevalence of vitamin D deficiency in India and globally (Table 1). Vitamin D deficiency arises from many factors, each intricately linked to the body’s ability to produce or absorb this essential nutrient. The foremost contributor is insufficient exposure to sunlight, particularly prevalent in individuals with limited outdoor activities, the elderly, and those residing in institutions or higher latitudes [14]. The synthesis of cutaneous vitamin D is hindered in individuals with darker skin pigmentation, intensifying the risk of deficiency. Inadequate dietary intake, especially in populations with lower vitamin D consumption, can significantly contribute to the deficiency, frequently observed in the elderly [15].
Beyond lifestyle and dietary factors, malabsorption conditions such as inflammatory bowel disease, celiac disease, cystic fibrosis, and certain gastrointestinal disorders impede the absorption of vitamin D, further exacerbating deficiency risks [16]. Age plays a crucial role, as older adults often experience diminished sun exposure, reduced dietary intake, and a decreased efficiency in converting sunlight into vitamin D. Conditions affecting the kidneys or liver, such as kidney or liver failure, disrupt the proper conversion of vitamin D into its active form, contributing to deficiency [17]. Moreover, certain medications and chronic diseases, including obesity, can accelerate vitamin D catabolism, compounding the risk of deficiency [18]. Thus, a comprehensive understanding of these multifaceted factors is essential for addressing and preventing vitamin D deficiency, necessitating tailored interventions based on individual circumstances and health profiles.
3.1. Synthesis of Vitamin D
When the human body is exposed to UVR from the sun, it undergoes a process to synthesize vitamin D. This synthesis occurs precisely when the incoming UV radiation has wavelengths shorter than 315 nanometers (nm) [4]. On the other hand, Vitamin D3, or cholecalciferol, is produced within the epidermis when 7-dehydrocholesterol is exposed to UVR [6]. Research indicates a complex interplay between skin color and vitamin D deficiency. Melanin, the pigment responsible for skin coloration, is a significant factor influencing vitamin D production. Melanin absorbs UVR, which is necessary for initiating vitamin D synthesis [19]. Consequently, individuals with darker skin, characterized by higher melanin levels, require increased sun exposure to generate equivalent vitamin D levels compared to those with lighter skin. This heightened reliance on sun exposure for adequate vitamin D synthesis contributes to a greater risk of deficiency in people with darker skin. However, skin pigmentation is just one facet of the intricate equation governing vitamin D levels [20]. Other variables such as available UVB radiation, exposure time, exposure pattern, skin area exposed, and age also impact vitamin D synthesis. Considering these factors is essential when determining the suitable amount of sun exposure or vitamin D supplementation needed to maintain optimal vitamin D levels [21]. Consulting a healthcare provider becomes crucial to navigating this complexity and ensuring personalized recommendations. For individuals with darker skin aiming to enhance their vitamin D levels, a multifaceted approach is recommended. Increasing sun exposure, especially during peak hours when the sun is at its peak, can be beneficial. However, caution is advised to avoid overexposure and mitigate the risk of skin cancer. Dietary adjustments, such as incorporating vitamin D-rich foods like fatty fish, egg yolks, and fortified items such as milk and cereal, can contribute to an improved vitamin D status [22].
Supplementation emerges as another viable option, with vitamin D supplements serving as a practical means to address deficiency. Nevertheless, the dosage should be determined in consultation with a healthcare provider, considering individual factors and requirements [23]. In summary, while skin pigmentation significantly influences vitamin D production, a comprehensive approach considering various factors and tailored guidance from healthcare professionals is imperative to effectively manage and prevent vitamin D deficiency, particularly in individuals with darker skin. The synthesis of vitamin D in the body involves a two-step process. Initially, vitamin D is hydroxylated in the liver, forming 25-hydroxy vitamin D3, denoted as 25[OH]D3 [24]. Subsequently, 25[OH]D3 is further converted into its active form, 1,25-dihydroxy vitamin D3, represented as 1,25[OH]2D3. The impact of latitude on vitamin D production is crucial in understanding the dynamics of maintaining optimal vitamin D levels. Latitude exerts a direct influence on the availability of UV radiation, a key factor in the synthesis of vitamin D. As one moves towards higher latitudes, there is a notable decrease in the amount of UV radiation, particularly during the winter months. This reduction in UV radiation poses a challenge for vitamin D synthesis in humans, necessitating a larger dose of UV relative to erythemal UV to produce an equivalent amount of vitamin D. Consequently, individuals residing at higher latitudes may encounter difficulties sustaining adequate vitamin D levels solely through sun exposure. Conversely, within the latitudinal range of 40° North to 40° South, there exists a relatively high and certainly sufficient presence of UVB radiation [25]. This signifies that sunlight exposure can serve as a primary and effective source of vitamin D production in tropical regions falling within this latitude belt. The consistent availability of UVB radiation in these areas supports the synthesis of vitamin D in the skin, reducing the reliance on alternative methods. The implications of latitude on vitamin D levels underscore the significance of geographical location in determining the effectiveness of sun exposure in obtaining this essential nutrient. Individuals residing at higher latitudes may find relying solely on sunlight to maintain adequate vitamin D levels challenging. In such cases, incorporating dietary sources rich in vitamin D and considering supplementation become crucial strategies to ensure a sufficient supply of this vital nutrient. The latitude-dependent variations in UV radiation thus highlight the need for tailored approaches to address vitamin D requirements, considering geographical considerations for individuals residing at different latitudes.
3.2. Food Sources
Fortified foods may contain either vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol), both of which are forms of vitamin D. These vitamins are predominantly found in animal-derived products. Here are the approximate vitamin D content levels in various food sources (Table 2) [8, 31].
| Food Item | Vitamin D content (µg per 100 g) | Reference |
|---|---|---|
| Fish | 5–25 | [26] |
| Mushrooms | 21.1–58.7 | [27, 28] |
| Reindeer lichen | 87 | [8] |
| Fish liver oil | 250 | [8] |
| Beef liver | 1.3–2.9 | [8] |
| Eggs | 1.3–2.9 | [8] |
| Dark chocolate | 4 | [29] |
| Yoghurt | 25 | [30] |
- Note: This information is valuable for individuals seeking to incorporate vitamin D-rich foods into their diets and meet their nutritional needs.
3.3. Vitamin D Safety Levels
Utilizing the optimal vitamin D supplement dosage is of paramount importance. Toxicity concerns typically arise when daily doses exceed 10,000 IU (International Unit) [32]. Conversely, the administration of dosages exceeding 50,000 IU per day over several weeks or months is frequently associated with severe adverse effects, notably confirmed hypercalcemia [3]. It is noteworthy that the majority of documented cases of vitamin D toxicity have involved daily intakes exceeding 40,000 IU [32].
The impact of vitamin D supplementation, either as a standalone treatment (with doses ranging from 1000 IU per day to 60,000 IU per week) or in conjunction with co-supplements, was examined in a recent comprehensive analysis published in 2018. The study compared the effects of these interventions to the use of placebos in a cohort of women diagnosed with polycystic ovary syndrome (PCOS) [33]. The study included 11 studies, including a sample of 601 women diagnosed with PCOS. The results indicated that the administration of vitamin D supplements substantially reduced fasting glucose levels and enhanced the HOMA-IR score within this particular group.
Evidence derived from randomized controlled trials indicates that continuous low doses of vitamin D at 4000 IU per day or the supplementation of vitamin D as a cotreatment for individuals with PCOS may lead to enhancements in insulin sensitivity. More precisely, there have been noticeable enhancements in fasting glucose levels (when vitamin D is added alongside other micronutrients) and HOMA-IR scores (when vitamin D is given in consistently low daily dosages or as a supplementary treatment) [2]. Furthermore, administering higher doses of vitamin D, exceeding 4000 IU per day, for at least 12 weeks has been associated with favorable outcomes. This dosage regimen has beneficial effects on glucose levels, insulin sensitivity, hyperlipidemia, and hormonal balance [34]. It is imperative to take into account various population parameters such as body mass index (BMI), ethnicity, as well as baseline levels of vitamin D and hormones when making decisions regarding the supplementation of vitamin D. These factors play a pivotal role in determining the appropriate and effective dosage for individuals with PCOS [35, 36].
4. Vitamin D in a Gene Pathway
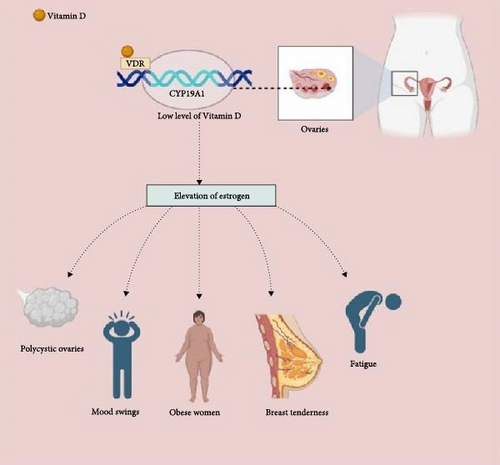
The biological effects of vitamin D3 are activated when it binds to the vitamin D receptor (VDR) in humans. This interaction changes the expression of over 3000 genes in different tissues, including reproductive organs such as the ovaries, uterus, and vagina [37]. Research has shown that low levels of 25-hydroxy vitamin D in the blood are linked to symptoms of insulin resistance, hirsutism, and infertility [38, 39]. Ovulatory disturbances, if left unaddressed quietly, can ultimately result in sterility and the development of PCOS [40]. In addition, changes in insulin, luteinizing hormone, sex hormone-binding globulin, and testosterone levels in the blood have been associated with genetic variants in the VDR [41]. Vitamin D and calcium blood concentrations may improve reproductive function in PCOS patients [2]. According to human studies, Vitamin D may play a role in controlling the menstrual cycle, treating PCOS, and implantation of embryos. This overview highlights the several functions of vitamin D in promoting reproductive health in women.
Calcitriol (1,25[OH]2D3) possesses the capability to directly influence the production of reproductive hormones and the characteristics of the estrus cycle. This metabolite is prominent in reproductive tissues, particularly in ovarian granulosa cells, where the VDR is also found [42]. Beyond its well-known role in calcium and phosphorus metabolism, vitamin D exerts regulatory influence over a spectrum of metabolic functions [43]. Significantly, vitamin D is involved in many cell type’s development and proliferation. Period abnormalities in women may be caused by vitamin D deficiency, which interrupts the menstrual cycle. In addition, via controlling the aromatase gene and influencing extracellular calcium homeostasis, vitamin D is pivotal in regulating estrogen synthesis [44]. Furthermore, it may be shown that vitamin D has a noticeable influence on the function of insulin by regulating the expression of VDR receptors in pancreatic cells. Calcitriol, the biologically active variant of vitamin D, interacts with these receptors, increasing insulin secretion [10, 45] (Figure 2).
4.1. Low Vitamin D Levels Affect the Menstrual Cycle
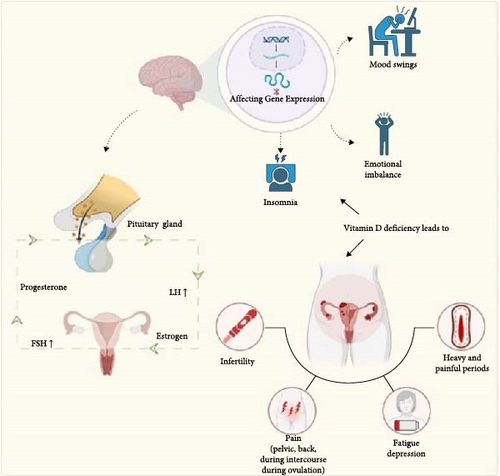
1,25[OH]2D3 also plays a crucial role in calcium metabolism. In females afflicted with PCOS, the combination of vitamin D insufficiency and disturbances in the body’s calcium regulation mechanisms contributes to the inhibition of ovarian follicular development [46]. There is compelling evidence indicating that vitamin D supplementation may offer potential benefits for individuals with PCOS in managing their menstrual cycles [47].
Furthermore, it is noteworthy that menstrual irregularities, even in women not diagnosed with PCOS, can be influenced by the actions of vitamin D3 on the production of anti-Müllerian hormone (AMH), which serves as a marker for ovarian reserve [48]. Research findings suggest a correlation between low vitamin D levels and diminished AMH levels. Reduced AMH levels are, in turn, associated with a decline in ovarian reserve and an early-stage increase in follicle-stimulating hormone (FSH) during the menstrual cycle [49]. These interconnected factors can collectively result in a substantial reduction in fertility (Figure 3).
4.2. Stress Affecting Menstrual Health
In menstrual health, vitamin D has emerged as a pivotal player, exerting a significant influence on various aspects of women’s well-being. An extensive study has shed light on its profound impact on women attempting to conceive, especially those facing prolonged challenges in this endeavor [46]. The research indicates that women who have been engaged in the arduous journey of trying to conceive over an extended period tend to experience more pronounced adverse effects on their mental health when they encounter interruptions in their treatment regimen [50]. This highlights the delicate interplay between reproductive health and emotional well-being.
Notably, the number of confidants a woman has in her social circle does not appear to directly correlate with the extent of distress caused by treatment suspensions. Instead, what emerges as a crucial factor is the emotional support she receives, particularly from her partner and other individuals in her network [51]. Those who received more robust emotional support reported a mitigated negative impact on their mental health. This aligns seamlessly with prior research findings that emphasize the link between high-quality social support and reduced distress in the context of infertility [52].
It is worth highlighting that the study uncovered a relatively modest mean number of reported confidants within the surveyed group, with a mere 42% of respondents characterizing their confidants as even “somewhat” helpful in their coping efforts during fertility treatment suspensions. Interestingly, women found their partners to be somewhat more useful, with 58% rating them as at least “somewhat” useful. Additionally, a mere 22% of women reported seeking social support from friends and loved ones “often.” [46]. These statistics collectively point towards a scarcity of accessible, quality social support for the majority of women grappling with the complexities of infertility [53].
In summary, these findings highlight the pivotal role of high-quality social support in alleviating the burdens faced by women navigating the challenging terrain of infertility. However, they also shed light on the unfortunate reality that such support remains elusive for many of these women. This aligns with previous research, which consistently highlights the prevalence of unsupportive social interactions experienced by women contending with infertility and underscores their role in exacerbating distress in this vulnerable population.
4.3. Vitamin D in Behavioral Regulation
In the realm of brain health and function, vitamin D emerges as a multifaceted player, exerting its influence through diverse mechanisms. Critical to this involvement is the VDRs residing within the cellular nucleus, orchestrating the expression of target genes upon binding to the active form of vitamin D. Remarkably, VDRs find their expression in key cerebral regions integral to behavioral regulation, encompassing the cortex, cerebellum, and limbic system [54]. In illuminating animal models, genetically modified mice with impaired VDRs manifest striking anomalies in brain morphology, and reduced levels of neural growth factors exhibit behavioral deviations that include heightened anxiety, diminished grooming behaviors, and impaired social interactions [51]. In another context, a compelling observation emerges, a significant majority of women, specifically 82%, fall short of meeting the recommended dietary allowances (RDAs) for vitamin D [55]. Intriguingly, a discernible correlation comes to the fore, as the consumption of ≥400 IU of vitamin D per day aligns with superior mental health-related quality of life (QOL) scores in comparison to those who intake less than 400 IU daily [56]. This underscores the proposition that adhering to established dietary guidelines for vitamin D intake holds promise to enhance overall well-being, particularly in older women [51]. Furthermore, the intricate interplay between vitamin D and mental health extends to the modulation of brain-derived neurotrophic factor (BDNF), a pivotal protein encoded by the BDNF gene. Vitamin D emerges as a critical player in the intricate landscape of brain health, exerting influence through various mechanisms that impact neuronal growth, survival, and cognitive function. One of the pivotal aspects of vitamin D’s role in brain health is its involvement in the production of BDNF, a protein crucial for the growth and maintenance of neurons [57]. Studies have unveiled the presence of VDRs in the brain, emphasizing the direct regulatory role of vitamin D in the expression of genes associated with neuronal growth and differentiation, including those contributing to BDNF production [58]. The administration of vitamin D3 has been linked to elevated BDNF levels, correlating with improved memory and reduced concentrations of Aβ (amyloid beta) in the hippocampus. This suggests a potential avenue for utilizing vitamin D as a modulator of cognitive function and memory [59]. Furthermore, vitamin D exhibits anti-inflammatory properties, which may contribute to BDNF production. Chronic inflammation has been associated with decreased BDNF levels, and the ability of vitamin D to mitigate inflammation may play a role in enhancing BDNF production [60]. However, the relationship between vitamin D and BDNF is intricate and context-dependent, as evidenced by contradictory findings in studies on depressed rats, where vitamin D did not consistently affect BDNF protein levels in the hippocampus [61]. Correlation studies in individuals with type 2 diabetes mellitus further complicate the understanding of the vitamin D–BDNF relationship [62]. While some studies indicate a positive effect of vitamin D on BDNF levels, others report contradictory or non-significant effects [61], highlighting the need for comprehensive exploration in diverse contexts.
In summary, the association between vitamin D and BDNF production is multifaceted, encompassing the regulation of gene expression, anti-inflammatory properties, and context-dependent effects. Although numerous studies demonstrate a positive correlation between vitamin D and BDNF levels, the intricacies of these interactions and the impact of various conditions on this relationship necessitate further investigation to comprehensively understand the role of vitamin D in brain health.
Empirical evidence accentuates the significance of BDNF, as deficiencies in this protein have been linked to age-related declines in spatial learning, neurodegenerative processes, cognitive impairment, and the emergence of depression [63]. Consequently, the profound implications of vitamin D resonate not only within the domain of menstrual health but also as a potential avenue for bolstering neurological and mental well-being. It underscores the critical importance of meeting dietary recommendations for vitamin D intake, especially for older women, as a means to promote holistic health [52].
Vitamin D’s potential to positively impact human mental health is postulated to be mediated through its regulatory influence on systemic inflammation [64]. An insightful review article, drawing from the insights of five extensive cross-sectional studies, has unearthed compelling evidence of significant inverse associations between 25(OH)D levels and markers of inflammation [65, 66]. These associations have been particularly pronounced in individuals with low 25(OH)D concentrations and adults characterized by elevated inflammation levels. A growing body of research has illuminated a strong nexus between vitamin D deficiency and a spectrum of mood disorders. Notable among these are major depressive disorder, seasonal affective disorder (SAD), and PMS. PMS, for instance, encompasses a cluster of distressing symptoms, with one prominent facet being a notably depressed mood during the final week of the luteal phase, occurring close to the onset of menses [67, 68]. The ample evidence suggests that optimizing 25(OH)D levels may contribute to alleviating the burdens of various mood-related conditions, including but not limited to depression, SAD, PMS, postpartum depression, perinatal depression, and depressive disorders, particularly in women of reproductive age. This underscores the potential therapeutic significance of 25(OH)D in managing mental health and highlights the relevance of vitamin D supplementation in promoting emotional well-being (Figure 2).
4.4. Vitamin D-Related Dysmenorrhea
Primary dysmenorrhea, characterized by menstrual pain in the absence of pelvic pathology, adversely impacts individuals’ daily lives, leading to work and school absenteeism, diminished QOL, and increased health and socioeconomic burdens. Its prevalence among menstruating women ranges from 20% to 90%, affecting up to 25% of this population [69]. Several studies have explored the potential of high-dose vitamin D administration in alleviating menstrual pain. Weekly intake of elevated vitamin D doses has demonstrated varying degrees of effectiveness. For instance, a single 300,000 IU dose was found to reduce dysmenorrhea severity in the initial month, while another regimen involving 300,000 IU vitamin D taken 5 days before each menstrual cycle for three consecutive cycles exhibited positive outcomes only in the second and third months post-intervention [70–73]. The biologically active form of vitamin D exerts its impact by diminishing prostaglandin production within the endometrium and influencing prostaglandin receptors, thereby limiting their biological activity [74]. Additionally, vitamin D exhibits anti-inflammatory effects through diverse pathways. Despite these mechanisms, the question remains whether vitamin D can effectively mitigate menstrual bleeding [3]. Some studies have indicated a reduction in the number of individuals experiencing heavy menstrual flow with vitamin D supplementation, although statistical significance was not achieved. Notably, this study’s findings indicate that high doses of vitamin D effectively alleviate menstrual pain but do not influence menstrual bleeding. It is speculated that vitamin D might exhibit more significant efficacy in individuals experiencing severe menstrual bleeding.
4.5. Vitamin D-Related Menorrhagia
Menorrhagia, characterized by a blood loss exceeding 80 mL per menstrual cycle, is a prevalent issue leading to considerable morbidity and diminished QOL among reproductive-aged women [9, 75, 76]. While its origins can be traced to pelvic or systemic pathologies, the etiology remains enigmatic in around half of the affected women. Emerging research underscores a significant interplay between Vitamin D and menstrual cycle disorders. Vitamin D plays a pivotal role in ovarian function and the regulation of the menstrual cycle [3]. Current knowledge firmly establishes that Vitamin D activates the VDR, thereby influencing the expression of approximately 3000 genes across diverse tissue types, including those in female reproductive tissues [10]. The amelioration of menorrhagia through vitamin D supplementation supports the notion that this condition may be mediated by dysregulation in the expression and signaling of endometrial mediators [77]. Notably, vitamin D supplementation has shown efficacy in addressing gynaecological issues such as irregular menstrual cycles and menorrhagia, particularly in women exhibiting low vitamin D levels. Consequently, its administration is recommended for individuals with vitamin D deficiency to harness its regulatory effects on menstrual disorders. It is crucial to recognize that vitamin D deficiency often goes unnoticed by a significant portion of the female population, highlighting the need for enhanced awareness and understanding of this medical condition within communities.
4.6. Other Medications Utilized in Menstrual Health
Menstrual dysfunction is an important symptom of PCOS and is a therapeutic strategy to restore regular menstrual cycles, focusing on ovulation induction and improving insulin sensitivity. Clomiphene citrate (CC) is commonly used as a first-line pharmacological agent to induce ovulation and regulate menstruation. However, in cases where women are resistant to CC, letrozole, an aromatase inhibitor, is more effective in increasing ovulation and menstrual regularity, particularly in women with a higher BMI. Additionally, insulin sensitizers like metformin are frequently employed to enhance menstrual cycle regularity, especially when combined with ovulation-inducing agents. Metformin’s ability to improve insulin sensitivity and reduce hyperinsulinemia has a positive impact on menstrual function, with studies reporting significant improvements in ovulatory cycles and overall reproductive outcomes in women with PCOS [78, 79]. Treatments like oral contraceptives are commonly used to regulate menstrual cycles, but they carry potential side effects, including weight gain and cardiovascular risks [80].
5. Limitations
Despite the promising findings regarding the influence of vitamin D on menstrual health, several limitations must be considered. First, the exact mechanisms by which vitamin D regulates various aspects of the menstrual cycle remain inadequately understood, and more healthy, longitudinal studies are necessary to establish causality and determine optimal dosages for different populations. Furthermore, while dietary intake and sunlight exposure are essential for maintaining adequate vitamin D levels, factors such as geographic location, seasonal variations, skin pigmentation, and lifestyle choices can significantly affect individual vitamin D status, potentially complicating the generalizability of the research findings. Additionally, the potential interactions between vitamin D supplementation and other nutrients or medications have not been thoroughly investigated, which may influence the overall effectiveness of vitamin D in managing menstrual health issues. Finally, there is a need for more comprehensive research to explore the long-term safety and efficacy of high-dose vitamin D supplementation, particularly in women with varying baseline levels of the nutrient. Addressing these limitations is crucial for developing evidence-based guidelines for the use of vitamin D in improving menstrual health.
6. Conclusion
In conclusion, the review highlights the pivotal role of vitamin D in maintaining menstrual health and its broader implications for women’s reproductive well-being. The findings highlight the intricate relationship between vitamin D levels and menstrual cycle regulation, hormonal balance, and reproductive function. Notably, vitamin D deficiency is prevalent and can exacerbate menstrual disorders such as dysmenorrhea and menorrhagia. The review emphasizes the importance of addressing vitamin D deficiency through tailored interventions, including adequate sun exposure, dietary modifications, and supplementation, especially for women with darker skin or those living in higher latitudes. However, further research is necessary to fully elucidate the mechanisms by which vitamin D influences menstrual health and to determine optimal supplementation strategies. The review advocates for increased awareness and proactive management of vitamin D levels to enhance women’s reproductive health and overall QOL.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Search strategy, supervision, and manuscript correction were handled by R.S. Writing, data extraction, and visualization were done by D.M. Data curation, supervision, and concept of the study developed by C.K. Format analysis and manuscript correction responsible for J.A.b.J. All authors approved the final version of the manuscript.
Funding
The authors received no funding support for this article.
Acknowledgments
We would like to thank Saveetha Institute of Medical and Technical Sciences, Chennai for the support and encouragement. We used Biorender for the graphical abstract and Quillbot for editing the manuscript.
Open Research
Data Availability Statement
Data are available on request from the authors.




