First-Principles Calculations to Investigate Structural, Electronic, Mechanical, Optical, and Magnetic Properties of RhNbSb Half-Heusler Compound
Abstract
In this work, the structural, mechanical, electronic, optical and magnetic properties of the RhNbSb half-Heusler compound were examined and analyzed using density functional theory (DFT). According to the results, type I atomic arrangement is structurally the most stable for the compound. In all three of its atomic arrangement types, the compound is mechanically stable and ductile according to the analysis of its mechanical properties. Generalized gradient approximation (GGA) + U approach was applied in addition to GGA approach, where U is Hubbard parameter, to increase the accuracy of results in electronic band structure, density of states (DOS), and magnetic moments. Therefore, electronic band structure and DOS calculations demonstrate that the compound exhibits metallic properties in both its type I and type II atomic arrangements with GGA predictions. However, under GGA + U calculations, the compound becomes half metal when in type I but it still reflects metallic nature when in type II. The compound’s half-metallic nature in type I under the GGA+U method suggests that it may be a good fit for spintronics applications in this type I of its atomic arrangement. The calculated total magnetic moment of the compound under GGA + U approach exactly fits with Slater–Pauling rule of half-metallic nature in its type I atomic arrangement, a result that supports half-metallic nature of the compound in type I atomic arrangement for electronic properties under GGA + U prediction. Furthermore, the compound might be taken into consideration for optoelectronic applications according to the results of computed optical characteristics.
1. Introduction
Since their discovery in 1903 [1], Heusler compounds’ fascinating features have attracted a lot of attention for applications in spintronic [2], optoelectronic [3, 4], and thermoelectric systems [5–7]. Half-Heusler compounds are members of the Heusler compound family with the chemical formula XYZ, where X is transition metal, Y is transition or rare earth element, and Z is a nonmagnetic element from main groups III, IV, or V [8, 9].
Half-Heusler compounds have tunable electronic band structures that can be semiconducting, insulators, metallic, or half-metallic [10], because of this, they have high potential to be considered for applications, such as optoelectronics and spintronics, where control over electron spin and charge is crucial, because their band gap can be engineered [11, 12].
Numerous theoretical predictions suggest that a variety of materials, such as ferromagnetic metallic oxides [13], diluted magnetic semiconductors [14], and Heusler compounds [15], can exhibit half-metallic properties. It has been observed that these compounds contain half-metallic elements [16].
Another characteristic of half-metallic materials is that they typically have an integer total magnetic moment. Naturally, half-metallic materials have metallic nature for the majority spin channel (spin-up) at the Fermi level (EF), but semiconducting/insulating nature for the minority spin channel (spin down) [17]. Theoretically, materials that are half-metallic can have 100% spin polarization at the Fermi level, which makes them excellent candidates for use in spintronic devices. Magnetic random access memory (MRAM), for instance [18], in 1983, de Groot and his colleagues discovered the half-metallic property in the NiMnSb half-Heusler compound while examining the material’s electronic band structure [19]. Tailoring magnetic properties of half-Heusler compounds by either chemical substitution or by other external factors is also another approach to make them suitable for spintronics applications [20]. Furthermore, since half-Heusler compounds have high thermal stability, they can have applications in spintronic devices in extreme environments [21].
Half-Heusler compounds’ band gap tunability qualities also make them appropriate for a number of optoelectronic uses [22, 23], such as photovoltaics [24, 25], light-emitting diodes (LEDs) [26], and photodetectors [27, 28]. Optically, half-Heusler compounds have high absorption coefficient and low electron energy loss in low electromagnetic energy spectrums (visible and infrared), qualifying them appropriate for some optoelectronic applications, such as infrared detectors and solar cells [29, 30].
Numerous studies on the properties of half-Heusler compounds, such as those based on Rh families, were theoretically carried out under zero pressure [31, 32]. For instance, the structural, elastic, electronic, and vibrational properties of the LiTiAl half-Heusler compound were investigated in [33] using the first principles approach. For spintronics applications, CoMnAl, CrTiAs, and RhCrSn half-Heusler compounds were studied using the DFT approach in [34]. N. Mehmood et al. carried out an ab initio studies on the structural, elastic, mechanical, electronic, magnetic, and optical properties of RhCrZ (Z = Si, Ge) half-Heusler compounds, both of these compounds exhibited half-metallic behavior with 100% spin polarization at the Fermi level [35]. It was recently observed by I. Muhammad et al. [36] that the RhMnSb half-Heusler compound shifted from metallic to half-metallic behavior when pressure exceeded 21 Gpa. The study examined the structural, mechanical, thermal, magnetic, and electronic properties of the compound under pressure using the DFT technique.
Even with the significant advancements in our knowledge of half-Heusler materials, there are still untested candidates with possibly better qualities. To the best of our knowledge, no theoretical or experimental research has been done on RhNbSb compound. The unique elemental combination in RhNbSb, which indicates a promising interaction of structural, electronic, mechanical, optical, and magnetic properties, makes this absence of research unexpected. The capacity of Rh and Nb to contribute to half-metallicity, which is a highly desirable feature for spintronic applications is well-known, but Sb frequently plays a crucial role in stabilizing the structure.
The goal of this work is, therefore, to close this gap by thoroughly examining RhNbSb. Through first-principles computations based on DFT, we examined this compound’s structural, mechanical, electronic, magnetic, and optical properties. According to our results, RhNbSb is a half-metallic material that has a lot of potential for use in spintronics. This work expands our knowledge of this significant class of materials and establishes the foundation for further experimental and theoretical research by presenting RhNbSb as a new member of the half-Heusler family.
In order to put its performance in context and emphasize its special qualities, we also contrast its attributes with those of other thoroughly researched half-Heusler compounds. For example, our results were compared to those of closely related half-Heusler compound, like RhNbAs [37], in order to offer an alternate validation. Consistent patterns in structural, electronic, mechanical, optical, and magnetic properties are shown by this comparison, which supports the validity of our findings. Despite the fact that the lack of previous studies highlights the uniqueness and importance of our work, this work is the first theoretical investigation of RhNbSb and lays the groundwork for future experimental and theoretical research on this material.
2. Computational Details
Software tools, such as XCrySDen and VESTA, were used for crystal modeling, design, and visualization.
3. Results and Discussion
3.1. Structural Properties
This work considers the RhNbSb half-Heusler compound with space group F43m (No. 216) and face-centered cubic structure. According to certain experimental research, the atomic arrangement of half-Heusler structures affects their properties. [38]. Studying the RhNbSb half-Heusler compound in its three most prevalent atomic configurations is therefore crucial [39]. Therefore, we examined this compound’s structural properties by accounting for these three most prevalent varieties, which are type I, type II, and type III. Figure 1 depicts the atomic configurations of these three kinds of the arrangements.
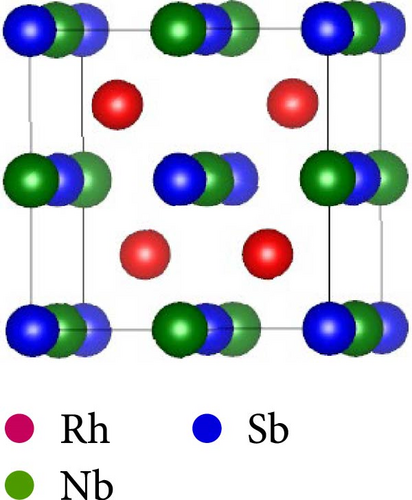
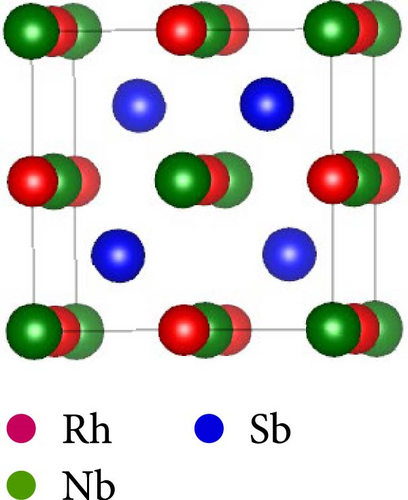
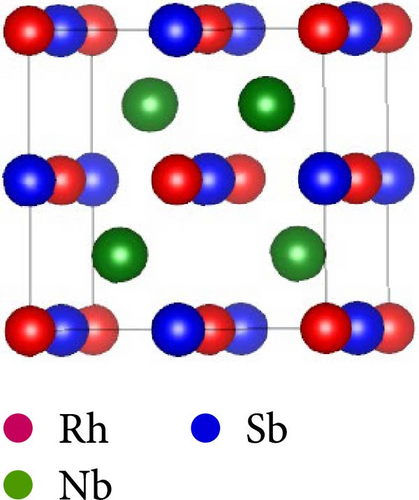
The positions of atoms in these types of atomic arrangement are illustrated in Table 1.
| Types | Rh | Nb | Sb |
|---|---|---|---|
| Type I | (0.25, 0.25, 0.25) | (0.50, 0.50, 0.50) | (0.00, 0.00, 0.00) |
| Type II | (0.50,0.50, 0.50) | (0.00,0.00, 0.00) | (0.25,0.25, 0.25) |
| Type III | (0.00,0.00, 0.00) | (0.25,0.25, 0.25) | (0.50,0.50, 0.50) |
| Types | (a0) (a.u) | V0 (a.u)3 | E0 (Ry) | B (Gpa) | B′ |
|---|---|---|---|---|---|
| Type I | 11.7524 | 405.81 | −1152.19053 | 153.0 | 4.36 |
| Type II | 11.8995 | 421.24 | −1152.07581 | 133.0 | 5.42 |
| Type III | 11.9803 | 429.87 | −1152.10300 | 126.3 | 5.45 |
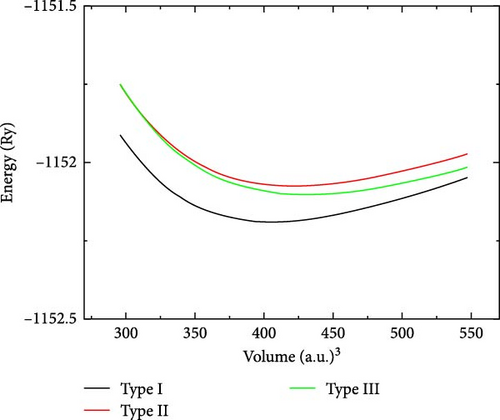
Figure 2 displays the volume against energy of the RhNbSb compound for each of the three possible atomic arrangements. Given the lowest value of minimum energy in type I atomic arrangement when compared to other types of atomic arrangement, it can be concluded from Table 2 and Figure 2 that the compound is most stable in this arrangement.
| Compound | Types | Total energy of the compound (Ry) | Individual elements | Energy per atom (Ry) | Formation energy (Ry) |
|---|---|---|---|---|---|
| RhNbSb | Type I | −1152.18068622 | Rh | −470.17407732 | −0.11803237 |
| Type II | −1152.06567328 | Nb | −334.24603482 | −0.00301943 | |
| Type III | −1152.09614099 | Sb | −347.64254171 | −0.03348714 |
3.2. Phonon Calculation
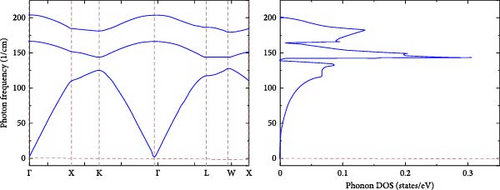
Figure 3 shows the calculated phonon band structure of RhNbSb, which offers important information about the dynamical stability and lattice vibrational properties of the compound. A system with three atoms per primitive unit cell is shown by the phonon dispersion curves (left figure), which show three acoustic and three optical branches. Interestingly, the acoustic branches start at zero frequency at the Γ-point, which is a basic prerequisite for any crystalline material’s dynamical stability. The sharp dip in the lowest acoustic branch near the Γ-point, where the frequency naturally falls to zero, is a prominent property of the phonon dispersion. Given that translational symmetry requires acoustic phonons to have zero frequency at Γ, this behavior is expected. Crucially, the RhNbSb compound’s dynamic stability is confirmed by the lack of imaginary (negative) frequencies. Furthermore, there is a noticeable phonon band gap between the optical and acoustic branches, measuring around 100 cm−1. Such a gap is frequently caused by a significant variation in bond stiffness or a noticeable mass contrast between constituent atoms, both of which affect the behavior of thermal transport. The material’s vibrational properties are further clarified by the phonon DOS), which is displayed in the right figure. At low frequencies (<100 cm−1), a considerable portion of the total phonon states are concentrated, suggesting that the acoustic modes primarily contribute to lattice dynamics. The optical branches can be seen in two different frequency ranges, roughly 150 cm−1 and 200 cm−1. A degree of phonon localization is suggested by their comparatively flat dispersion, which is probably caused by the lighter Nb atom participation in high-frequency vibrations.
3.3. Mechanical Properties
Any material’s mechanical properties must be investigated in order to determine how much the material will react to external stress [41]. Elastic constants, Cij, and other associated mechanical parameters provide more details regarding the mechanical properties of a particular material [42]. Even if many elastic constants are required for other, more complex geometry, only three independent elastic constants, namely C11, C12, and C44 are sufficient for cubic materials like half-Heusler compounds [43]. These three elastic constants can be used to easily derive other mechanical parameters [44]. Table 4 lists the computed values of these three elastic constants as well as other mechanical parameters for each of the three atomic arrangement types of the compound RhNbSb. The Born–Huang mechanical stability criteria is satisfied by the three elastic constants for the three types of atomic arrangements of the RhNbSb compound, which are C11 + 2C12 > 0, C11 − C12 > 0, and C44 > 0 [45]. Furthermore, for all sorts of atomic arrangements, these elastic constants follow the cubic stability condition (C12 < B < C11) [46]. As a result, the compound exhibits mechanical stability across all three atomic arrangement types and therefore can be used in building thermoelectric devices, where materials must endure mechanical and thermal stress. In other words, for thermoelectric devices in the real world, mechanical stability guarantees toughness and reliability under mechanical and thermal stress. Mechanical stability of the compound also ensures its suitability under high pressure applications, such as in aircraft or energy technologies.
| Mechanical parameters | Type I | Type II | Type III |
|---|---|---|---|
| C11 | 205.7 | 191.32 | 177.57 |
| C12 | 128.26 | 105.21 | 101.01 |
| C44 | 83 | 56.66 | 67.9 |
| B | 154.07 | 133.91 | 126.53 |
| GV | 65.29 | 51.22 | 56.05 |
| GR | 56.95 | 50.3 | 51.85 |
| G | 61.12 | 50.76 | 53.95 |
| B/G | 2.52 | 2.64 | 2.35 |
| Y | 161.95 | 135.2 | 141.71 |
| ν | 0.32 | 0.33 | 0.31 |
| H | 7.52 | 6.4 | 7.61 |
| A | 2.14 | 1.32 | 1.77 |
| CP | 45.26 | 48.55 | 33.11 |
Pugh’s ratio, B/G, with an equilibrium value of 1.75, is used to distinguish between ductile and brittle materials. Pugh’s ratio for the compound RhNbSb in our current study is bigger than this equilibrium value in all three of its atomic arrangement types; as a result, the compound is ductile in nature in all three of its atomic arrangement types.
Since the Young modulus quantifies the stiffness or rigidity of a material, RhNbSb is at its stiffest when its atomic arrangement is in type I since in this type, Y has the highest value in relation to the other types. The Poission’s ratio, which typically runs from 0.0 to 0.5, is used to identify the type of bonding present in a material. The material is covalently bound if v = 0.1, has an ionic bond if ν = 0.25, and a metallic bond if ν = 0.33 [50]. As a result, in all three forms of its atomic arrangement, RhNbSb exhibits metallic nature. As we will see later under electronic section, this nature is also demonstrated. It is important to note that the mechanical property calculations were carried out using the GGA approximation. Consequently, the ν result needs to be compared to Figures 4 and 5.

Defects become more likely as A’s value rises above 1, since 1 is its equilibrium value. Therefore, in the type I atomic arrangement, RhNbSb has a significant likelihood of defects. All three of the atomic arrangement types under investigation have positive values for CP, suggesting that RhNbSb is ductile by nature in each of the three atomic arrangement types.
Table 5 displays the computed fusion temperature values for the chemical in each of its three atomic arrangement types, with type I having the highest value. This compound is stable at high temperatures because it generally has a high melting temperature in all three of its atomic arrangement types.
| Mechanical parameters | Type I | Type II | Type III |
|---|---|---|---|
| ρ | 8782 | 8460 | 8291 |
| vm | 2956.50 | 2747.29 | 2854.33 |
| vl | 5179.72 | 4881.46 | 4892.86 |
| vt | 2638.42 | 2449.49 | 2551.05 |
| θD | 324.46 | 297.87 | 307.09 |
| Tm ± 300 K | 1768.89 | 1683.89 | 1602.62 |
The Debye temperature, θD, provides information about a material’s thermal stability. It is a basic parameter that gives information on a material’s lattice vibrational properties. It is strongly associated with important mechanical and thermal qualities, like specific heat capacity, thermal conductivity, and structural stability, and represents the lattice’s greatest vibrational frequency. When evaluating a material’s viability for high-temperature applications, the Debye temperature is especially crucial for RhNbSb and similar materials.
The computed values of the compound RhNbSb’s density (ρ kg/m3), transverse, longitudinal, and average velocities (m/s), Debye (θD) and melting temperatures (K) for the three atomic arrangements are displayed in Table 5.
The mechanical stability of this compound is consistent with the Debye temperature that is being described here (Table 5). The material’s capacity to tolerate heat stress is demonstrated by the high value of θD, which reflects the strong interatomic bonding indicated by the mechanical stability of the compound from elastic constants (Table 4 and Born–Huang criteria). The suitability of RhNbSb for high-temperature applications, such as thermoelectric and heat-resistant materials, is highlighted by these findings.
3.4. Electronic Properties
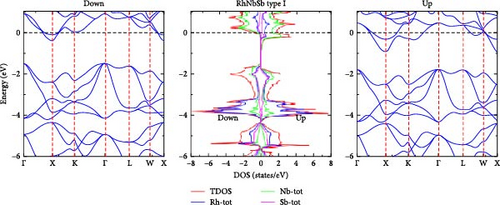
We have computed the electronic band structure, total DOS and PDOS for the RhNbSb half-Heusler compound by using both the GGA and GGA+U methods. These properties enable us to identify whether the compound is semiconductor, half-metallic or metallic. In the irreducible Brillouin zone at the equilibrium lattice parameters, the electronic band structure of the RhNbSb compound with both majority (spin-up) and minority (spin-down) spin channels are depicted in Figures 4 and 6 for type I and in Figures 5 and 7 for type II along the main symmetry directions. The GGA approach was used to compute the spin-polarized electronic band structure of the compound for the type I atomic arrangement (Figure 4). The results indicate that the bottom of the conduction band crosses the Fermi level (EF) in both the majority and minority spin channels, indicating a metallic nature in both spin channels. That is, the GGA method indicates that the compound has a metallic property. However, under the GGA+U method, the compound’s type I atomic arrangement (Figure 6) exhibits metallic behavior for the majority spins (spin-up) due to band lines crossing EF, but for the minority spin channel, EF was not crossed by the electronic band structure, indicating that the compound is semiconductor. As a result, the compound exhibits half-metallic behavior under GGA+U treatment in type I atomic arrangement. Electronic band structures under the GGA and GGA+U approaches for both the majority and minority spin channels in the case of type II atomic arrangement clearly cross the Fermi level, indicating that the compound has metallic nature at its equilibrium lattice constant when it is in type II atomic arrangement.
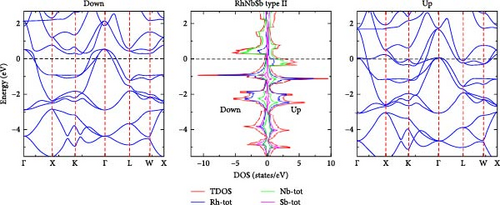
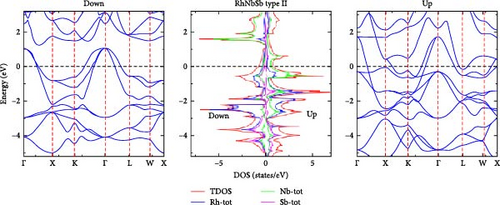
In order to gain a deeper understanding of the electronic properties of the RhNbSb half-Heusler compound, the GGA and GGA+U approaches were used to calculate the compound’s DOS and PDOS, which are shown in Figures 4 and 6 and Figures 5 and 7 for type I and type II atomic arrangements, respectively. For both majority and minority spin channels in both atomic configurations, the trend of these DOS (both PDOS and DOS) closely matches the electronic band structure. For example, under GGA+U approximation in type I atomic arrangement (Figure 6) both DOS (DOS and PDOS) did not cross the Fermi level in the case of minority channel (spin-down) indicating semiconductor property of the material but the Fermi level is well crossed by both DOS and PDOS in the majority spin channel (spin-up) that shows metallic character of the compound resulting in half-metallic behavior of the compound which well agrees with both the electronic band structures in this type atomic arrangement (Figure 6). With type II atomic arrangements, where the compound exhibits metallic character when treated by both GGA and GGA+U, the agreement between electronic band structures and DOS as well as PDOS also remains true.
Furthermore, information about the contribution of electronic properties of component atoms in the compound on their chemical bonding can be provided both by DOS and PDOS. For both type I and type II atomic arrangement below Fermi level large contribution comes from Rh PDOS whereas above Fermi level large contribution to DOS comes from Nb PDOS. Below and far from Fermi level Sb PDOS largely contributes to DOS. The overall contribution to DOS comes from Rh PDOS in both types and under both approaches.
Generally speaking, structural properties of a material have a significant impact on its electronic properties. In the RhNbSb half-Heusler combination under current research, for instance, we have found that varying atomic configurations within a particular material structure (cubic RhNbSb) can impact the electronic property of the material. Under the GGA+U technique, this material readily reflected half-metallic electronic properties in the type I atomic arrangement; nevertheless, under the GGA and GGA+U approaches, its band structure continued to reflect metallic properties in the type II atomic arrangement.
In type I atomic configuration, RhNbSb is recognized as a half-metallic material, displaying a spin-polarized band gap in one spin channel (spin down) and metallic behavior in the other (spin-up). In the semiconducting spin channel, the computed band structure reveals an indirect band gap, with the conduction band minimum at the X point and the valence band maximum at the Γ point. RhNbSb’s half-metallic nature, which is defined by 100% spin polarization at the Fermi level, emphasizes its potential for use in spintronics. Figure 6 shows the detailed electronic band structure and DOS.
3.5. Optical Properties
To find RhNbSb half-Heusler compound applications in optoelectronics, it is essential to analyze its optical properties. Using a GGA technique, we examined the optical properties of this material in a type I atomic arrangement over the photon energy range of 0–40 eV. The findings are shown in Figure 8. The complex dielectric function, ε is the fundamental optical parameter for optical response of materials and is defined as [55],
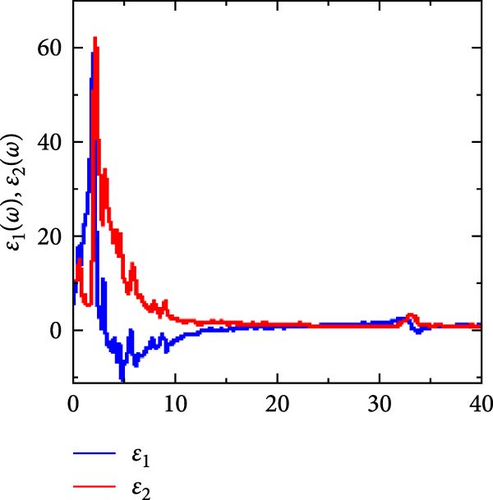
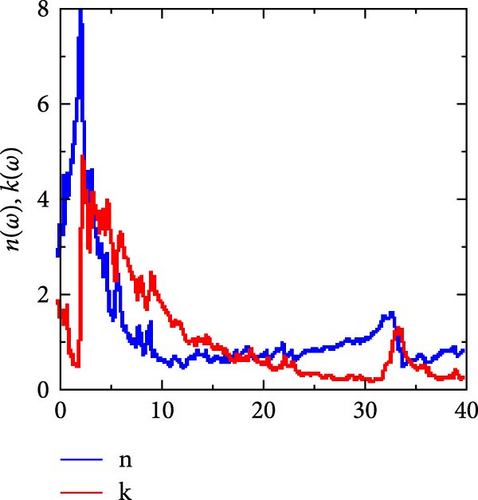
The computed results of n (ω) and k (ω) are shown in Figure 8b. The extinction coefficient quantifies the loss of absorbed electromagnetic energy when a wave propagates in a given medium, whereas the refractive index is a crucial optical parameter for determining the quantity of light that refracted (bent) in a specific material. The obtained static refractive index, n (0) is 2.74, which is in line with the values of popular optoelectronic materials like Ge. This refractive index decreases as photon energy rises after reaching its maximum value at 2.14 eV. In the low energy ranges (0–1.78 eV), the extinction coefficient is very low, indicating very little photon energy absorption, which is advantageous for the material’s transparent qualities in these energy ranges. RhNbSb can be regarded as light absorbent as photon energy increases because it significantly increases after these ranges, peaks at 2.40 eV, and subsequently falls.
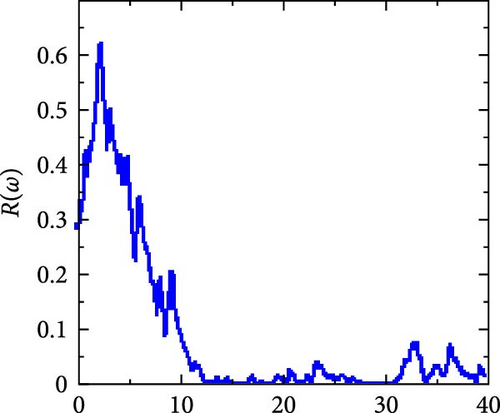
It is displayed in Figure 8c with a static value of 0.28 (the value at which photon energy is zero, or R (0) = 0.28). From this constant value, it rises abruptly to its maximum peak at 2.27 eV, after which it falls as photon energy rises to 23.87 eV.
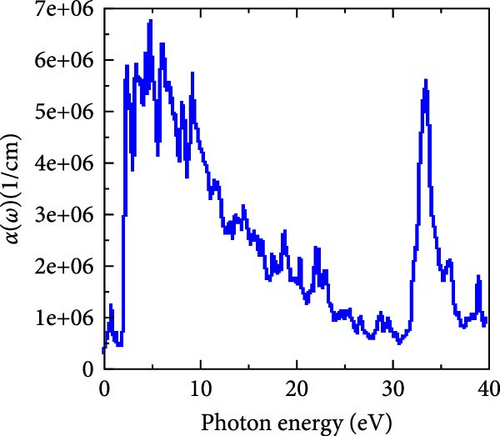
The computed results of α(ω) are displayed in Figure 8d, where the visible and some ultraviolet regions have the highest absorption values, with the visible region having the maximum absorption value. It declines until photon energy reaches 31 eV, at which point it sharply rises to 33.23 eV before eventually declining. Generally speaking, RhNbSb can be regarded as a promising option for optoelectronic applications, for example, for construction of photovoltaic devices that absorbs solar energy efficiently due to its high absorption capacity in the visible energy domain. Absorption coefficient starts increasing from zero photon energy (Figure 8d) indicating that RhNbSb has metallic character as observed by electronic band structure (Figure 4). It should be noted that the calculations for Figure 4 (electronic properties) and optical properties were carried out by GGA approaches.
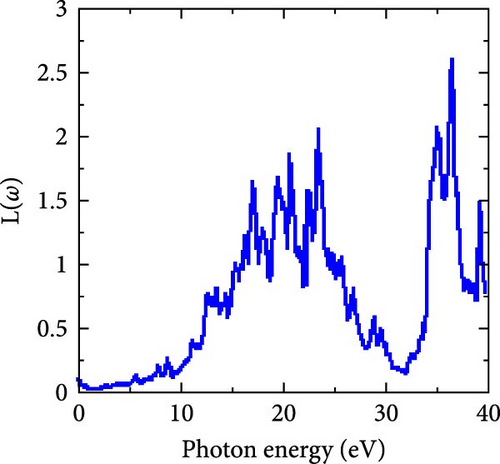
Figure 8e shows the electron energy loss vs photon energy. Its low values at lower photon energies suggest that the RhNbSb compound loses little electron energy at first, increasing to have several peaks around 20 eV. Following numerous oscillations, it reaches its highest peak at 36.39 eV before declining.
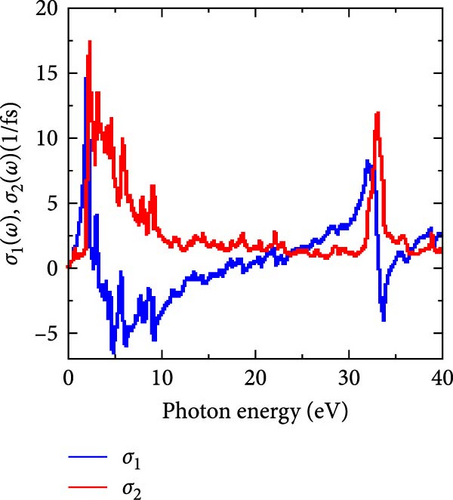
According to the figure, the threshold point for both components of optical conductivity is at 0 eV, indicating that the compound has no band gap (metallic character), which is consistent with the electronic band structure shown in Figure 4 under the sections on electronic properties.
3.6. Magnetic Properties
The magnetic moment of a substance can be used to determine its magnetic properties [61]. Table 6 presents the computed total and partial magnetic moments for the RhNbSb half-Heusler compound in type I of its atomic arrangement. Table 6 shows that Nb atom significantly contributes to the total magnetic moment in both the GGA and GGA+U methods. Under the treatment of GGA+U approach, both Rh and Sb atoms do not favor for magnetism of RhNbSb compound. The estimated total magnetic moment correctly matches with the Slater–Pauling rule of half-metallicity [62], Mt = Zt − 18, where Mt denoting the total magnetic moment and Zt the total number of valence electrons in a given half-Heusler compound, in type I under the GGA+U approximation. According to the Slater–Pauling rule, the total valence electron count of the RhNbSb half-Heusler compound is 19, with Rh = 9, Nb = 5, and Sb = 5. This means that Mt = 1, which is precisely equivalent to the total magnetic moment of the RhNbSb compound in type I under the GGA+U method. This suggests that when RhNbSb is treated with GGA+U, it exhibits half-metallic properties, which is consistent with the band structure calculation found in the section on electronic properties (Figure 6).
| Type | Method | μRh(μB) | μNb(μB) | μSb(μB) | μtotal (μB) |
|---|---|---|---|---|---|
| Type I | GGA | 0.0444 | 0.4944 | 0.0032 | 0.75 |
| GGA+U | −0.1684 | 0.9369 | −0.0655 | 1.00 | |
| Slater–Pauling rule | — | — | — | 1.00 |
By adjusting for the delocalization error in the d-electrons, the U values that were determined by computations guarantee that the magnetic moment of RhNbSb is precisely predicted. Table 6 shows this predicted result by GGA+U comparison with Slater–Pauling rule which exactly matches with this rule. The impact of these values is also reflected in the electronic structure computations, which give a realistic account of the compound’s electronic properties by calculating the band gap and DOS. Using these U values, we were able to get results that were in line with half-Heusler compound trends.
The half-metallic features of type I atomic arrangements for the RhNbSb compound can considerably assist spintronic devices because of their unique electronic and magnetic properties which lead to high percentage of spin polarization at Fermi level. For one spin channel (e.g., spin-up), half-metallic materials, like the RhNbSb half-Heusler compound, behave metallically, while for the other spin channel (e.g., spin-down) they show semiconducting or insulating properties. This leads to 100% spin polarization at the Fermi level, which is a crucial prerequisite for effective spintronic devices. By reducing spin relaxation and scattering, the high spin polarization makes it possible to manipulate spin currents effectively and strongly without suffering a large loss of spin information.
Type I atomic arrangements of RhNbSb compound are perfect for devices, such as spin valves, magnetic tunnel junctions (MTJs), and spin-transfer torque magnetic random-access memory (STT-MRAM) because of this feature, which guarantees maximal spin injection efficiency into nonmagnetic materials. Compared to traditional charge-based logic, devices based on type I RhNbSb half-metal can achieve reduced energy consumption and higher switching speeds in spin-based logic circuits.
Dilute magnetic semiconductors and transition metal oxides are competitors for half-Heusler half-metallic compounds in the construction of spintronic devices; however, these materials frequently have low Curie temperatures or restricted scalability. Half-Heusler half-metallic compounds (e.g., RhNbSb) have high Curie temperature which allows them to work properly even above room temperature. Comparing to their competitor materials, half-Heusler half-metallic compounds have excellent structural, mechanical, and chemical stabilities enabling them incorporation into current semiconductor.
4. Conclusions
The RhNbSb half-Heusler compound’s structural, mechanical, electronic, optical and magnetic properties were calculated using the quantum espresso simulation software, which is based on DFT. The compound is most stable structurally when its atoms are arranged in type I. The compound is mechanically stable in all three forms of its atomic arrangement and ductile in nature. To get good results on the electronic and magnetic properties, the GGA+U technique was applied in addition to the conventional GGA approach. Under the GGA technique, the compound’s type I and type II atomic arrangements were observed to have metallic character. In type I atomic configuration, the compound in GGA+U computations show a half-metallic behavior; however, in type II atomic arrangement, it retains its metallic nature. Under the GGA+U technique, the compound’s total magnetic moment precisely satisfies the Slater–Pauling rule of half-metallic nature in its type I atomic arrangement. This outcome is consistent with the half-metallic attribute derived from the electronic band structure in the type I atomic arrangement under the GGA+U forecast. When the compound is in type I atomic arrangement, it can therefore be viewed as a possible front-runner in the realm of spintronic applications. The compound has a high absorption coefficient and minimal electron energy loss in the visible and certain ultraviolet light areas, according to the results from calculations of optical characteristics, indicating that it can be employed in optoelectronic applications. It should be noted that standard DFT calculations,such as LDA and GGA, approaches have their own limitations in predicting electronic (underestimating band gap), mechanical, optical, and magnetic properties of any compounds including half-Heusler compounds. To minimize these limitations, we used DFT+U approach for electronic and magnetic properties calculations. To further reduce the constraints of standard DFT, we could refine the projected mechanical and magnetic properties of the RhNbSb half-Heusler compound in our next work by incorporating temperature-dependent effects and spin–orbit coupling respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Adem Beriso Bejo, calculations, data analysis, conceptualization, methodology and manuscript writing; Megersa Wodajo Shura, supervising, manuscript review and editing; Kumneger Tadele, supervising, manuscript review and editing; Fekadu Tolessa Maremi, supervising, manuscript review and editing; Mesfin Asfaw Afrassa, manuscript review and editing; Kunsa Haho Habura, manuscript review and editing.
Funding
The authors received no specific funding for this work.
Acknowledgments
Madda Walabu University (MWU) and Adama Science and Technology University (ASTU) are greatly acknowledged for their support during this work.
Open Research
Data Availability Statement
The data that support the findings of this work are available within the article.




