Serpin B9-Insensitive Granzyme B Mutant Delivered by Engineered Capsid AAV Vectors Demonstrates Selective Killing of EGFR-Positive Cancer Cells
Abstract
Cellular immunotherapy strategies that harness the cytotoxic properties of T and NK cells by releasing granzyme B have emerged as a crucial approach in cancer therapy for both hematological and solid tumors. However, the effective release and antitumor activities of granzyme B are significantly influenced by protease inhibitors including serpin B9. Gene delivery mechanisms that can counteract these limitations of cell-based immunotherapies are highly desired. In this regard, genetic modification of adeno-associated virus (AAV) capsids as vehicles for targeted delivery of therapeutic genes is an emerging area of research in the field of gene therapy of both monogenic and polygenic diseases. The insertion of antigen-specific antibody fragments and peptides into capsid sequences has been shown to redirect capsid tropism from native antigens to specific targets. Herein, we report on genetically engineered AAV2 capsid modified by inserting two antibody fragments: single-chain variable and single-domain antibodies and GE11 peptide, all specific for targeting epidermal growth factor receptor, at the N terminus of VP2. We observed an inverse correlation between the size of the inserted anti-EGFR moiety, capsid structure conservation, and vector yield. After demonstrating the proof of concept by confirming antigen-dependent transduction of cancer cells using AAV-GFP vectors, we further demonstrated that targeted AAV vectors encoding a protease-insensitive granzyme B mutant DNA cargo selectively eliminated cancer cells expressing serpin B9 in comparison to vectors encoding wild-type granzyme B in vitro. Our findings suggest that the challenges posed by intracellular expression of serpin B9 to antitumor T(NK) cell–dependent immunotherapies can be curtailed by utilizing the R201K granzyme mutant in immune-based therapy development.
1. Introduction
T cell-based immunotherapy has become a pivotal cancer therapeutic strategy against both hematological and solid tumors. Its mechanism of action includes cytotoxic activities by activated T cells through checkpoint blockade, adoptive cellular transfer, cytokine immunomodulation, bispecific T cell engagers, and therapeutic vaccines [1–3]. At the cellular level, the antigen-directed cytotoxicity that is mediated by activated effector T and natural killer (NK) cells is elicited by granzyme B (GrB) release and deposition into the targeted cancer cells. Thus, the reliance on GrB secretion to initiate cytotoxicity has become an important factor for the efficacy of T cell-dependent immune therapies. In this regard, GrB-based recombinant fusion protein and nanoparticle-based therapies have been extensively explored for cancer therapy [4–7]. However, the efficacy of GrB is controlled intracellularly by an endogenous expression of serpin B9 [8, 9], which serves as a protective mechanism for T cells from their own GrB secreted upon activation and the major dictating factor influencing their lifespan following lysosomal stress [10]. The upregulation of serpin B9 by tumor cells represents a significant limitation to GrB-mediated cell death through multiple immune escape cues and a critical regulator of immunotherapy responses [11, 12]. This phenomenon has recently been exemplified in a strategy involving anti-serpin B9 immunotherapy [13].
Gene therapy of human cancers is an emerging strategy of delivering antitumor nucleic acids to treat different malignancies [14]. This approach can be divided into two main categories: in vivo and ex vivo gene therapy. In ex vivo gene therapy, immune effector cells (including activated T cells, macrophages, NK cells, and tumor-infiltrating lymphocytes) are collected, engineered, and reinfused into the patient, which have resulted in remarkable outcomes in the treatment of various diseases, including hematological cancers and autoimmune diseases [15, 16]. However, this form of cell-based therapy is limited by autologous sourcing and lengthy manufacturing processes. On the other hand, in vivo gene therapy involves the direct delivery of anticancer nucleic acids by genetically or chemically modified viral and nonviral vectors to kill tumor cells [17–19]. This category includes the direct (either through locoregional or systemic administration) delivery of therapeutic payloads by engineered biological and synthetic nanocarriers that recognize cancer-specific markers and the delivery of the therapeutic transgene to target cells. A leading example of a viral vector for gene delivery is the adeno-associated virus (AAV) [20]. Structurally, the icosahedral-shaped AAV capsid consists of three viral proteins (VP1-3) of molecular weights (MWs) of 87, 72, and 64 kDa in the ratio of 1:1:10, respectively, assembled into a 60-mer vector. Its ssDNA genome (4.7 kb) is flanked at both ends by 145 base pairs of structural elements referred to as inverted terminal repeats (ITRs) whose primary function is to “sandwich” the gene of interest (GOI). Currently, AAV is the leading vector for gene delivery in preclinical models and in clinical trials targeting certain cancers [21] and cardiomyopathies [22]. In the context of cancer treatment, AAV-based vectors have gained prominence due to their nonpathogenic properties, capacity to carry different therapeutic payloads, and limited risk of genotoxicity.
The potential of AAV vector–mediated delivery of genetic materials that code for proteins that can directly induce cell death has been exemplified by previous studies. Lu et al. recently demonstrated AAV-mediated delivery of the cytotoxic gasdermin payload leading to the induction of pyroptosis in preclinical glioblastoma models [23]. Similarly, previous approaches have involved the delivery of a transgene encoding bacterial diphtheria toxin which nonselectively eliminated selected cancer cell lines [24]. However, in addition to the issue of immunogenicity, the accumulation of vectorized particles in off-target organs resulting from the broad tissue tropism mediated by AAV capsid proteins poses a significant challenge. To overcome these limitations in the application of AAV vectors for cancer therapy, extensive exploration has been carried out based on rational design or directed evolution of capsid viral proteins to precisely direct the AAV vector to the intended targets for cancer immunotherapy [25, 26]. The approaches include the incorporation of antigen-specific ligands into the capsid structure to redirect viral capsid tropism toward defined cell targets, resulting in decreased off-target accumulation and enhanced tissue specificity and entry [27, 28]. However, these previous studies did not explore the insertion of antigen-specific ligands of varrying sizes by comparing parameters critical to vector biomanufacturing such as transfection efficiency, packaging fitness, capsid structure conservation, and production titers.
In this study, we bioengineered recombinant AAV vectors consisting of modified capsids integrating three anti-EGFR moieties of different molecular sizes. We then conducted a comprehensive assessment of their biological properties, including antigen-dependent transduction of target cancer cells. The anti-EGFR moieties were inserted at site T138 (N-terminus of VP2). A missense mutation was introduced to abrogate the primary antigen heparin sulfate proteoglycan (HSPG) binding recognition domain as previously described [29] without observed changes to the capsid structure (Figure S1). Following the establishment of proof of concept using GFP encoding AAV vectors, the deployed GrB-vectorized particles demonstrated EGFR-dependent cytotoxicity at unprecedented, significantly low genome copy numbers. The mechanism of action of the induced cell death by AAV-mediated delivery of GrB includes the release and expression of GrB gene cargo resulting in selective cytotoxicity. More importantly, the study shows that capsid-modified vectors possessing a serpin B9-resistant human GrB mutant (GrBmut) referred to as GrB R201K efficiently eliminated serpin B9-overexpressing cancer cells when compared to AAV-GrB wild-type vectors. Thus, our study successfully demonstrated a proof of concept for the delivery of protease inhibitor–resistant GrB mutant cargo which can be employed to replace the wild-type GrB in T- and NK cell-based immunotherapy strategies.
2. Material and Methods
2.1. Cell Lines
The following tumor cell lines were used in this study: SKOV3 (HTB-77), A431 (CRL-1555), MDA-MB-468 (HTB-132), and noncancerous HEK 293T cells. SKOV3 is an ovarian adenocarcinoma cell line. HEK 293T (CRL-11288) is a human embryonic kidney cell line containing the SV40 large T antigen and characterized by minimal EGFR expression [30]. Both the A431 (melanoma cell line) and MDA-MB-468 (triple-negative breast cancer cell line, TNBC) are confirmed EGFR-positive cell lines [31]. All tumor cells were cultured with Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher) and supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1% (v/v) 100 U/mL penicillin–streptomycin (PS) cocktail with or without 25% GlutaMAX (Gibco). HEK 293T cells were cultured in Roswell Park Memorial Institute (RPMI-1640) media and supplemented the same as above. Cells were grown in 95% relative humidity, 37°C, and 5% CO2. Cells were tested to rule out mycoplasma contaminations. Live cells were quantified by staining dead cells using Trypan blue solution 0.4% (Thermo Fisher) and counted on a TC20 automated cell counter (Bio-Rad). All tissue culture procedures were performed under sterile conditions.
2.2. Plasmid Mutagenesis
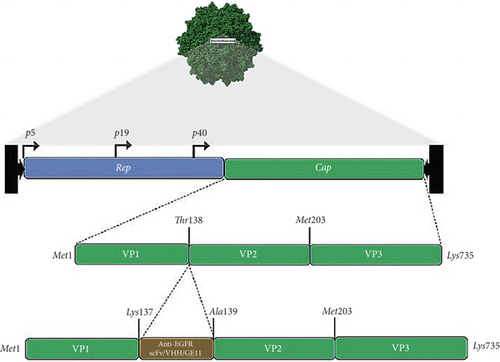
The pAAV-cap/rep and pAdeno-helper plasmids were obtained from the Weinberg group (formerly of AGTRU, University of Witwatersrand, Johannesburg). The pAAV-GFP (#32395) was purchased from Addgene, United States. The cap/rep plasmid variant with mutated HSPG domain is named pAAV-CapKO, and variants containing anti-EGFR fragments and peptide were named accordingly elsewhere in this study. The parent transgene plasmids and their variants containing GrBmut were assembled as described below. Gene fragments of the 1711(scFv)-in VH-linker-VL orientation, G10(VHH), and GE11 were amplified from respective sources using the primers listed in Table S2 (supporting information). The sequences of the single chain and single-domain antibody fragments referred to as 1711(scFv) and G10(VHH) were extracted from US patents US6235883B1 and US20170190787A1, respectively. The GE11 is a 12-amino acid (Tyr, His, Trp, Tyr, Gly, Tyr, Thr, Pro, Gln, Asn, Val, and Ile) peptide. After three rounds of splicing by overlap extension (SOE)–PCR employing standard conditions, the three gene fragments were ligated (using T4 ligase, NEB) to the pAAV-Cap/Rep plasmid by insertion at site T138. Following successful PCR amplification, the spliced gene fragments (2557, 2216, and 2196 bp, respectively) and pAAV-CapKO (a cap/rep gene containing a mutation at the HSPG binding domain) backbone were digested with EcoNI and XcmI restriction enzymes by incubation at 37°C for 16 h. The final plasmid was transformed in Escherichia coli (NEB5-alpha) cells overnight. The following day, a single transformant colony was cultured in LB media (Sigma-Aldrich) supplemented with 100 μg/mL of ampicillin sulfate (Sigma-Aldrich). The extracted and purified plasmids, pAAV-GrB WT, 1711(scFv)Cap, G10(VHH)Cap, and GE11-Cap, were confirmed by Sanger sequencing (Ingaba Biotech, South Africa).
2.3. Packaging of Recombinant AAV in HEK 293T Cells
Recombinant AAV2 vectors were produced in HEK 293T semiadherent cells by the PEI-mediated triple transfection method. Cells were seeded in supplemented media until 70%–80% confluency was reached and maintained at 37°C, 5% CO2, and 95% relative humidity. Cells were transfected with recombinant AAV plasmids encoding capsid/replication proteins (pAAV-1711(scFv)Cap, G10(VHH)Cap, and GE11-Cap; transgene (pAAV-GFP and pAAV-GrB (R201K)); and pAdeno-helper plasmid in the ratio of 1:1:2, respectively). One hundred milligrams of PEI (linear, 2 g, Polysciences, United States) with a MW of 25,000 was dissolved in 100 mL of sterile ddH2O, stirred while adding HCl to adjust to pH 7.0, to a concentration of 1 mg/mL. The mixture was filtered using a 0.22-μm sterile filter, and 1-mL aliquots were placed into 1.5-mL vials which were stored at −80°C. Aliquots were thawed for immediate use and then stored at −20°C. Vectorization of targeted AAV particles was performed by transfecting HEK 293T cells with anti-EGFR-Cap, pAdeno-helper, and AAV-GrB (the transgene which was generated by cloning GrB/GrBmut gene sequence into the pAAV-GFP plasmid [32] between AgeI and NotI restriction sites leading to the generation of anti-EGFR-AAV vectors). The transgene is preceded by a Kozak sequence for translation initiation under the CMV promoter and terminated by a TAA stop codon (Figure S4).
2.4. Harvesting and Bioprocessing of AAV Preps
On Day 7 post transfection, 5-mL 1x phosphate-buffered saline (1x PBS, 137 mM NaCl, 8.8 mM Na2HPO4, 2.7 mM KCl, and 1.75 mM KH2PO4) stored at 4°C was added to each plate and scrapped gently and then transferred into 50-mL conical tubes. Extra 2 mL was added to each plate to collect remaining cells. Cells were then pelleted by centrifugation at 4000 × g for 15 min at 4°C. Supernatant was discarded and cells were resuspended in 5 mL of cell lysis buffer and then stored at −80°C. The cell culture supernatant (CCSN) was filtered by using sterile 0.45-μm filters. A 40% PEG solution containing 40 g of polyethylene glycol (Sigma-Aldrich, MW: 25 K) in 100 mL, 60 mL of 0.5 M NaCl (14.653 g) solution was added to the mixture. The solution was autoclaved and shaken at 150 rpm at 37°C for 16 h to mix thoroughly and stored at the same temperature to prevent precipitation of the PEG out of the solution. A 5x PEG solution was added to CCSN in the ratio of 1:4, respectively. The mixture was stored at 4°C for 16 h to allow AAV particles to settle at the bottom. The next day, AAVs were pelleted by centrifugation at 5000 rpm for 30 min. PEG particles were removed by performing three cycles of buffer exchange in 100 kDa MW Amicon centrifugation columns in 1x PBS.
2.5. AAV Extraction and Purification
Recombinant AAVs were purified by using Takara Bio AAVpro purification kits (cat # 6675, 6678 according to the manufacturer’s protocols) and by using iodixanol gradient. In summary, cell pellets containing viruses were subjected to five cycles of freeze-thaw (5x at 37°, 5x at 196°C in liquid nitrogen) and then sonicated for 5 min at 40 amplitude. Next, virus suspension was gently transferred into a quick-seal round-top polypropylene centrifugation tube (Beckman Coulter, Lifesciences) using a Pasteur pipette. Each of the iodixanol solutions was added in the following order: 7 mL of 15%, 4 mL of 25%, 5 mL of 40%, and 4 mL of 60% iodixanol solutions to a total volume of 20 mL. Bubbles were eliminated by constantly flicking the tubes. Afterwards, the Pasteur pipettes were carefully removed, and the tubes were filled up with a lysis buffer and sealed by melting with a Bunsen burner. Afterwards, the mixture was layered, creating a gradient by ultracentrifugation in 70.1 Ti rotor at 50,000 rpm for 120 min at 4°C. Virus particles were collected from the 40% phase of the iodixanol gradient using a 20 G sterile needle (0.8 × 40 mm) by puncturing slightly through the 60% iodixanol phase in the upper 40% phase. Viral particles were concentrated in 1x PBS in 100 kDa cut-off Amicon centrifugation columns (Sigma-Aldrich, South Africa) by centrifugation at 4000 rpm × 10 min in three cycles. An aliquot of 100 μL of viruses was taken and stored at −80°C.
2.6. Quantification of AAV Vector Titers by Quantitative Polymerase Chain Reaction (qPCR)
Extracted and purified AAV particles were estimated by qPCR. The molecular size and mass concentration of the transgene plasmids (pAAV-GFP, pAAV-GrB) were determined. Molarity of GOI plasmids was calculated from DNA copy numbers and the intended dilution by using an online calculator (Thermo Fisher). From this, serial dilutions of 102–1010 were prepared as standards. Viral DNAs were extracted and purified from 50 μL of viral preparations by using AAVpro purification kits (Takara Bioscience, Japan) according to the manufacturer’s protocol. DNA impurities were denatured by incubation of the viral particles with DNase I (Sigma-Aldrich) at 37°C for 60 min. The volume of viral preps was topped up to 200 μL with 1x PBS (pH 7.4). A 5 μL of purified DNA was run with the standards on the standard PCR thermocycling conditions.
2.7. Branched Chain Amino Acid (BCAA) Assay
The BCAA assay is a biochemical procedure for the determination of protein concentrations in whole cell lysates and other biological samples. The assay was used to quantify the intracellular expression of serpin B9 protein in whole tumor cell lysates. Briefly, HEK 293T, A431, MDA-MB-468, and SKOV3 cells were grown in T75 flasks containing supplemented DMEM until 70%–80% confluency was reached. Cells were detached using trypsin and then washed in 1x PBS pH 7.4 by centrifugation at 2500 rpm for 7 min. The cell pellet was lysed using lysis buffer 1:1000 (990 μL radioimmunoprecipitation assay buffer + 10 μL protease inhibitor) (Thermo Scientific, United States) and then incubated on ice for 30 min. Clarified cell lysates were recovered by centrifugation at 4500 × g for 30 min. Next, serial dilutions of BSA of working concentration ranging from 200 to 2000 μg/mL of BSA were used as standards. Afterwards, 10 μL of clarified cell lysate and 20 μL lysis buffer were mixed in a sterile flat-bottom 96-well plate. A 200-μL BCAA reagent (Reagent A:Reagent B) in the ratio of 1:50 was added to each sample in the dark. The plate was shielded and incubated at 37°C for 30 min before taking spectrophotometric readings at 560 nm. The standard curve was plotted from which the concentration of cell lysates was determined as an average of three samples.
2.8. Western Blotting
Western blot was performed for the detection of serpin B9 (42 kDa) and α-tubulin (~50 kDa) proteins separated by SDS-PAGE. Briefly, cell lysates separated by SDS-PAGE were transferred from the polyacrylamide gel onto a Whatman nitrocellulose membrane (Sigma-Aldrich) using the Xcell II Blot Module (Bio-Rad) for electro-tank blotting at 120 V for 90 min in transfer buffer. This allows the movement of negatively charged proteins from the gel toward the positively charged anode of the blotting chamber. Afterwards, unspecific binding of the detection antibody to the membrane was prevented by blocking it with 5% nonfat dry milk for 60 min at RT. The membrane was washed three times with 1x PBST and incubated with anti-serpin B9 polyclonal primary antibody (Thermo Fisher, United States) or α-tubulin monoclonal antibody (Thermo Fisher, United States) at 4°C for 16 h and 20 rpm. Thereafter, unbound antibodies were removed by washing the membrane in TBS-Tween buffer (three times) and incubated with a horseradish peroxidase–conjugated anti-rabbit or mouse-IgG secondary antibody (1:5000; Sigma, United States) at RT. A second set of washes was performed in TBS-Tween buffer (three times) prior to staining with the chemiluminescent substrate. For signal detection, the nitrocellulose membrane was covered evenly with the enhanced chemiluminescence mixture for 1 min and washed with ddH2O for 5 min. Protein band signals were captured by a Gel Doc XR imaging system (Bio-Rad).
2.9. Tumor Cell Viability and Cytotoxicity Assays
2.9.1. XTT-Based Cell Viability Assay
Live cells were seeded in a 96-well plate at a density of 5 × 103 cell/well in DMEM or RPMI supplemented with 10% (v/v) heat-inactivated FBS and 1% (v/v) 100 U/mL PS and allowed for 24 h at 37°C and 5% CO2 in a humidified atmosphere. Untreated cell populations were considered negative controls (100% cell viability), while zeocin-treated (100 mg/mL) cells were designated as positive controls (0% cell viability). The Cell Proliferation Kit II (XTT) (Roche, Switzerland) was used (according to the manufacturer’s protocol) to assess cell death. In this assay, cleavage of the tetrazolium salt XTT occurs in the presence of metabolically active cells and resulting formation of orange-colored formazan crystals, which absorb light at 450 nm. At 68 h posttreatment, the XTT reagents were mixed in the ratio of 50:1 and added to the cells. Beyond 72 h, absorbance readings (at 450 nm as the measurement filter and 650 nm as the reference filter) were taken using a spectrophotometer (iMark Microplate Absorbance Reader, Bio-Rad, United States) and results were exported as Excel files for analysis. The 50% inhibitory concentration (IC50) was calculated by normalizing data with untreated controls (100% cell viability) and zeocin (100 mg/mL)–treated or positive control (0% cell viability).
2.9.2. Annexin V Apoptosis Assay
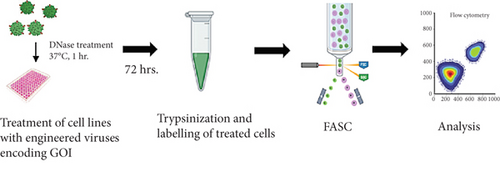
The calcium ion–dependent binding of annexin V to phosphatidylserine on cell surfaces, in addition to the cell membrane integrity–dependent propidium iodide staining, has been a reliable method for differentiating apoptotic cells (or at initial stage of apoptosis) or necrotic cell death. This assay is based on the ability of the anticoagulant protein annexin V to specifically bind to phosphatidylserine phospholipid that is exposed on the surface of cells undergoing apoptosis. Upon binding, the FACSCalibur equipment (BD Biosciences) can detect the Alexa 488 fluorophore that is conjugated to annexin V. By combining with 7-AAD live/dead cell marker, a single cell quantitative determination of apoptotic cell death was obtained by flow cytometry. Briefly, tumor cells were treated with GrB bearing 1711(scFv)/CapKO rAAV vectors at 2000 vg/cell or zeocin (100 mg/mL) as positive control or no treatment. Untreated cells served as negative controls. 1 × 105 live cells each of HEK 293T, A431, and MDA-MB-468 cells were seeded in a flat bottom 48-well plate in 500 μL of supplemented media. After 72 h posttreatment, cells were lifted by the addition of Accutase solution and incubated at 37°C for 5 min. Cells were washed with 1x PBS pH 7.4 by centrifugation at 1500 × g for 7 min and decanted into labeled fluorescence-associated cell sorting (FACS) tubes. Cells were washed in 200 μL of annexin V buffer pH 7.4 (10 mM HEPES, 150 mM NaCl, 2.5 mM CaCl2, 1x PBS, Cold Spring Harbor Laboratories). Live–dead cell populations were used for gating. Next, cells were resuspended in annexin V buffer containing 7-AAD live dead marker (1000:1) for 30 min. Cells were stained with annexin V-Alexa 488 conjugate in PBS (1:40) for 60 min on ice. Finally, cells were washed in annexin V buffer and proceeded for FACS analysis. The percentage of GFP-positive cells was determined by FACS using FlowJo (v8) software.
3. Results
3.1. Production and Packaging of Capsid-Modified AAV Vectors
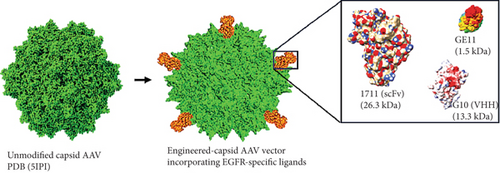
Three anti-EGFR moieties were incorporated at the N-terminus of VP2 through site-directed mutagenesis at T138 of the AAV2 cap gene, which led to the ligation of donor gene fragments to VP2 via PCR-amplified gene fragments bearing complementary sequences as overhangs. The site of integration has been previously demonstrated to tolerate the installation of noncanonical ligands [33]. Figure 1a illustrates the cloning strategy used to integrate the donor gene sequences of the anti-EGFR single chain variable fragment (1711(scFv)), variable heavy domain of heavy chain (G10 (VHH)), and GE11 peptide into the unmodified plasmid (pAAV-CapKO), resulting in the generation of circularized pAAV-anti-EGFR-Cap representing pAAV-1711(scFv)Cap, pAAV-G10(VHH)Cap, and pAAV-GE11-Cap, respectively (Figure S2). Note that the unmodified plasmid represents the wild-type AAV cap/rep gene with an abrogated primary antigen-binding domain. The pAAV-anti-EGFR-Cap products, adeno-helper, and pAAV-GFP (Addgene, United States) plasmids were transfected into HEK 293T cells using a triple transfection protocol, as previously described [34]. Successful transfection of AAV plasmids showed satisfactory transfection efficiency, as observed by strong GFP signals across all vector types with increasing intensity 24 h post transfection (hpt) up to Day 5 (Figure S3). The resulting targeted viruses externally displayed the incorporated ligands with an expressed stoichiometric ratio of 5 as a ligated product of ligand–VP2 fusion protein (Figure 1b) correlating to the anti-EGFR ligands extending from the fivefold symmetry axis [35].
3.2. Semiquantitative Analysis Correlates the Size-Dependent Cell Transduction Capacity of Anti-EGFR Moiety-Targeted AAV Vectors
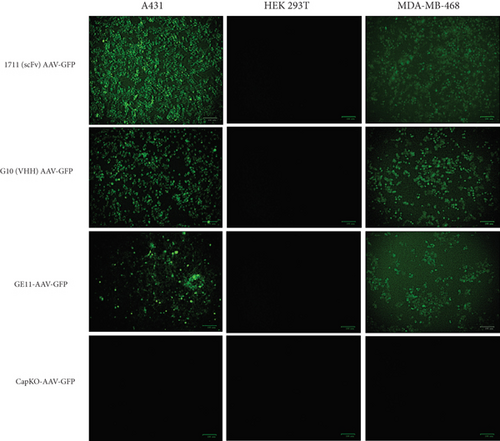
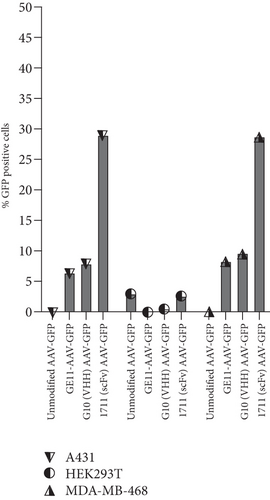
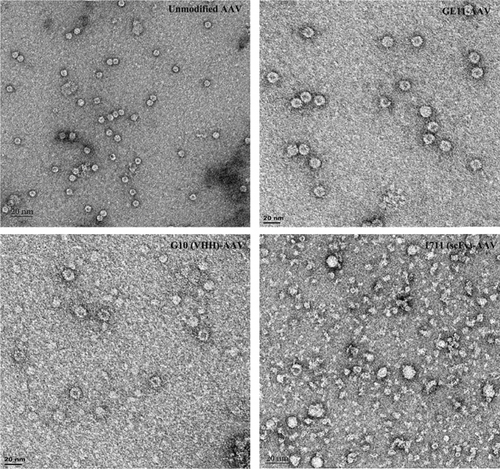
To investigate and compare the transduction potential of capsid-modified vectors, three cell lines (A431, HEK 293T, and MDA-MB-468) cultured in supplemented media were treated with targeted (1711(scFv)AAV-GFP, G10(VHH)AAV-GFP, and GE11-AAV-GFP) and nontargeted (CapKO-GFP) viruses at a multiplicity of infection (MOI) of 100 gc/cell. MDA-MB-468 and A431 cells treated with all three targeted viral vectors showed GFP expression signifying EGFR-dependent uptake of capsid-modified vectors compared to unmodified vectors (Figure 2a). However, EGFR-positive cells (A431 and MDA-MB-468) treated with 1711(scFv)AAV-GFP showed the strongest GFP signals based on microscopic image analysis. Semiquantitative analysis was conducted and plotted as a bar chart to determine the relative transduction demonstrated by GFP signals. The results confirm the highest antigen-dependent transduction by the scFv capsid-modified vectors (39%) compared to viruses displaying VHH antibody fragments and GE11 peptide (Figure 2b). Conceivably, this finding points to the larger surface area exposed by the 1711(scFv)AAV-GFP vectors in interactions with the EGFR antigen leading to higher uptake and transduction by the A431 and MDA-MB-468 cells. In addition, the introduced missense mutation at the HSPG domain abrogated transduction of the HEK 293T cells by the unmodified vectors confirming these residues as critical for HSPG-driven cell uptake and transduction by wild-type AAV2. Furthermore, acquired transmission electron microscopic images characterized the icosahedral shape of particles specifically in unmodified AAV vectors. As anticipated, various contaminants were observed in modified-capsid AAVs including visible protein debris, empty capsid, and intermediate viruses compared to unmodified capsid AAVs (Figure 2c), a major limitation in engineered capsid AAV products [36]. Extensively modified capsid AAVs appear to harbor significant percentages of impurities which may be attributed to residual and poorly packaged particles resulting from challenges faced by the host cell expression machinery during capsid assembly processes as well as purification bottlenecks.
3.3. Targeted GrB-Vectorized AAVs Demonstrate Antigen-Dependent Cell Death In Vitro
Because targeted vectors decorated with the 1711(scFv) antibody fragment demonstrated the highest EGFR-dependent transduction of the targeted cells, it was selected for the packaging of GrB-vectorized AAV vectors for targeted delivery of the transgene to confirm whether cytotoxicity was EGFR-dependent compared to AAV-GFP vectors in a small-scale vector transduction assay. This approach also confirms the induction of apoptosis following the release and translation of the GrB gene delivered by the EGFR-targeting AAV particles in addition to the time taken for the initialization of cell death mechanisms [32].
To investigate and compare the cytotoxic potentials of the targeted 1711(scFv)AAV-GrB to 1711(scFv)AAV-GFP vectors, A431, MDA-MB-468, and HEK 293T cells were treated at an MOI of 200 gc/cell. Targeted vectors containing GrBmut gene cargo induced antigen-dependent cell death as determined by the annexin V assay 72 hpt. Following FACS analysis, complete cell death was observed in A431 (29.5%) and MDA-MB-468 (10.2%) while 68% and 71% of cells were at an early stage of apoptosis, respectively (Figure 3), denoting that cell death induced by GrB-vectorized particles occurred in a relatively shorter period of time (Table 1). Our observation further validates efficiency in GrB-mediated apoptosis of target cells [37, 38]. In contrast, no significant cell death was observed in EGFR-positive cells that were treated with 1711(scFv)AAV-GFP, denoting that the observed cell death was solely due to the cytotoxic activities of the released GrB molecules. Similarly, the transduction of HEK 293T cells with the GrB-encoding vectors did not induce cell death, thereby further confirming the abrogation of HSPG-driven cellular uptake and a switch to EGFR-dependent transduction.
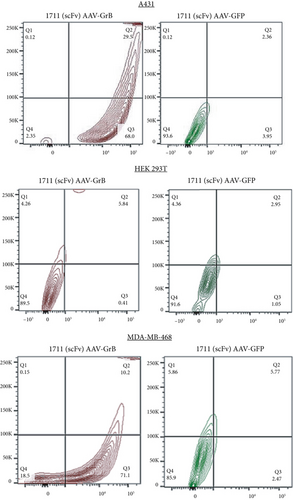
| Percentage (%) of cells that undergo cell death at 72 h posttreatment | ||
|---|---|---|
| Cell line | 1711(scFv)AAV-GrB | 1711(scFv)AAV-GFP |
| A431 | 29.5 | 2.4 |
| HEK 293T | 5.8 | 3.0 |
| MDA-MB-468 | 10.2 | 5.8 |
3.4. GrBmut (R201K) Eliminates Serpin B9-Overexpressing Tumor Cells by Targeted rAAV-Mediated Delivery
Serpin B9 (42 kDa) is recognized for its intracellular inhibition of GrB-induced apoptosis and has been utilized by tumor cells as a mechanism for evading cytotoxic T cell-mediated cell death. Herein, the effectiveness of the GrBmut variant gene cargo administered via targeted AAV vectors to selectively target and eliminate serpin B9-overexpressing cancer cells compared to the wild-type GrB was explored. The GrBmut was generated from the wild-type GrB gene as previously described by Lossaso et al. [39]. Firstly, we investigated the ideal vector genome copy number per cell required to induce selective cell death in antigen-positive cells while minimizing vector-induced and bystander toxicities. Three starting MOIs (200, 100, and 10 gc/cell) of 1711(scFv)AAV-GrB vectors were used to treat cells seeded at the density of 1 × 104 per 48-well plate in a single experimental run. Our observations indicated that the 100 gc/cell treatment selectively induced antigen-dependent cell death in EGFR-positive cells without harming antigen-negative cells. Next, the intracellular overexpression of Serpin B9 by MDA-MB-468, A431, SKOV3, and HEK 293T cells was investigated by using the BCAA assay to normalize total protein in cell lysates for western blotting. Intracellular overexpression of serpin B9 by MDA-MB-468, A431, and HEK 293T cells was observed at the expected MW ~42 kDa mobility and not by SKOV3 cells (Figure 4a). The relatively high overexpression of serpin B9 in both cancer cell lines correlates with a previous study [8]. Additionally, the lack of serpin B9 expression by SKOV3 ovarian cancer cell lines correlates with the findings by Kimman et al. [9]. With these findings, further evaluation was conducted by comparing the induction of cytotoxicity by targeted (1711(scFv)AAV, G10(VHH)AAV, GE11-AAV) vectors encoding either GrBmut or GrB wild type at the three starting MOIs.
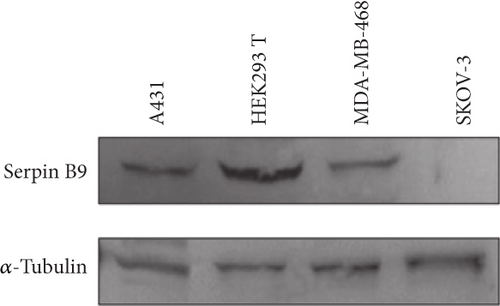
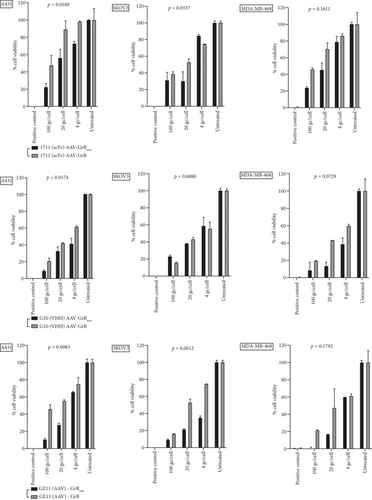
The selected cancer cell lines (MDA-MB-468, SKOV3, and A431) except HEK 293T are known for aberrant overexpression of EGFR [40–42]. The three targeted vectors induced EGF receptor–dependent apoptosis but not in HEK 293T cells with baseline EGFR expression (Figure 4b and Figure S5). Targeted AAV vectors encoding GrB wild type and GrBmut eliminated SKOV3 cells depending on increasing genome copy numbers per cell. Specifically, the targeted vectors reduced cell viability across all three cell lines as determined by XTT-based apoptosis assays. Comparatively, induced cytotoxicity by the targeted vectors encoding GrB wild-type vectors was lower in serpin B9-overexpressed (A431 and MDA-MB-468) cells (Figure 4b). These findings indicate antigen-dependent killing of serpin B9-positive cells by the targeted AAV-GrBmut vectors. For example, 1711(scFv)AAVs, G10(VHH)AAVs, and GE11-AAVs encoding GrBmut cargo significantly eliminated A431 cells by 2.5, 2.2, and 4.8-fold decrease, respectively, compared to vectors encoding GrB wild type at 100 gc/cell. Similar findings were observed across all cell lines specifically in serpin B9-overexpressing cell lines treated with the engineered viruses at MOIs 20 and 4 gc/well. In contrast, while all the targeted vectors induced cell death in SKOV3 cell lines regardless of the MOI, the modified vectors failed to induce cell death in HEK 293T cells as well as unmodified capsid AAVs encoding either GrB or its mutant (CapKO-GrB/CapKO-GrBmut in the selected cell lines) (Figure S5) signifying that cell death induction by AAV-mediated gene delivery is first dependent on efficient receptor uptake and cell entry.
4. Discussion
Given the critical role of effector/activated T cells in the clinical efficacy of immunotherapies, the overexpression of serpin B9 by tumors presents a significant challenge to T cell-based therapies that depend solely on GrB-mediated cell death. This immune evasion mechanism is prevalent in multiple solid cancers, necessitating the development of innovative strategies to outsmart its negative impact on immune-based anticancer therapies. To address these challenges, a practical and alternative approach to ex vivo gene delivery mechanisms that does not rely on autologously sourced immune cells is highly desired. Herein, we demonstrated a shift from clathrin and caveolin-mediated cell entry by wild type AAVs [43] to receptor-dependent transduction by the engineered vectors. The mechanism of antitumor activity by the GrBmut gene expression following vector transduction involves sequential steps, including the vector evasion of endosomal cleavage, uncoating, and subsequent deposition of the GrB gene into the cytosol. Subsequently, the percentage of the expressed transgene was translated into functional GrB protein, which then triggers one or more of its multiple apoptosis signaling pathways [44] resulting in EGFR-dependent cytotoxicity as confirmed by two independent cell death and viability assays. By applying the annexin V assay, we uncovered time-dependent and multiple GrB-induced cell death mechanisms in the selected cancer cell lines. Importantly, the GrBmut protein effectively avoided interaction with serpin B9, ultimately inducing cell death in serpin B9-positive cancer cells at a relatively low genome copy number of up to 100 gc/cell. This finding corresponds to the observed cytotoxic activities of the fused GrB-antibody recombinant protein [45] thereby confirming the potential of the R201K as the preferred GrB mutant to overcome serpin B9-overexpressing cancer cells. In the present study, the AAV vector delivery of GrBmut gene cargo as a novel modality for surmounting the inhibitory effects of serpin B9 overexpression demonstrates its potential applicability to the development of T cell-based immunotherapies. Cell death mechanisms induced in the EGFR-positive cells occurred approximately 72 h posttreatment, which further confirms the cytotoxic capacity of GrB in a relatively short duration. The observed cell death (approximately 6%) induced by the targeted GFP vectors at a concentration of 100–200 gc/cell in the MDA-MB-468 cell lines may be attributed to its aggressive phenotype and shorter doubling time. In addition, its rapid growth rate resulted in bystander toxicity and cell death due to contact inhibition. However, there was an approximately twofold increase in cell death induction in this cell line when comparing the two vector types. Furthermore, the findings correlate differential cell surface EGFR overexpression density on A431 and MDA-MB-468 cells compared to the relatively low expression by HEK 293T cells, thereby signifying the crucial role in receptor-mediated cytotoxicity. The observed transduction of HEK 293T cells at approximately 3% by the 1711(scFv)AAV vectors points to the presence of EGF receptors, albeit in low expression density. Similar transduction characteristics were demonstrated by CapKO-AAVs, denoting that the HSPG receptor binding was not disrupted entirely and that the HSPG domain extends beyond the knockout residues to cover the highly conserved regions, including the critical roles of R484, R487, and N587 residues implicated in AAV2-HSPG antigen interactions [46].
Delivering nucleic acids which translate to cytolytic protein instead of corrective gene (in the case of monogenic diseases) by employing AAV offers a paradigm shift in the gene therapy landscape specifically for the treatment of recalcitrant solid cancers. In this study, we observed that AAV-mediated delivery of the transgene selectively induced apoptosis in antigen-positive cells. Consequently, by employing computer-guided simulations to evaluate the tolerability of AAV capsid modifications for the insertion of three anti-EGFR moieties with MWs ranging from 1.5 to 26.3 kDa, we observed significant variations in capsid structure conservation among the three anti-EGFR moiety modifications attributed to the structural rearrangements during capsid assembly. The EGF receptor was selected as the target of interest due to its overexpression in various tumor types including melanoma, TNBC, and ovarian cancer, among others, thereby establishing it as an ideal therapeutic target [47, 48]. In relation to the above, follow-up comparative experiments show that the functionality of the resulting vectors mapped the correlation between the size of the incorporated EGFR ligand and the antigen recognition, uptake, and transduction of the targeted cells. On this basis, capsid integrity and overall titers of the resulting vectors were inversely proportional to the size of the incorporated anti-EGFR ligand (Figure S7). This finding is conceived to have had effects on capsid VP stoichiometry [49] even though it was estimated that the insertion at the N-terminus of VP2 yields a stoichiometry of 5 per vector. This finding further suggests that extensive modification of AAV capsid can lead to destabilizing the structural integrity, overall titers, and high proportion of empty capsid virions which can affect the particle-to-infectivity ratio. Although the AAV2 capsid can be genetically manipulated, the sites that can accommodate the insertion of larger ligands (> 20 kDa) and with retained biological functions are yet to be identified [50]. In our study, we reasoned that incorporating the anti-EGFR moieties at the N-terminus would enable fusion with the VP2 subprotein, which initiates translation from a weak and unconventional start codon (T138) which led to the formation of particles with EGFR-dependent infectivity. Ongoing drug development campaigns include the combination of both genetic and chemical AAV capsid modification to overcome the current challenges.
The vectorization strategy consisting of the Kozak sequence preceding the GrB gene and a STOP codon for translation termination seems to have abrogated cytotoxicity of producing host cells within the first 96 hpt by restraining the gene cargo specifically in cases of truncated ITRs and empty capsids. This resulted in a reduction of GrB-induced cell death of production cell lines when compared to previous studies [23]. Under the CMV promoter, this approach resulted in the confinement of GrB expression in producing host cell machinery without or reduced leakage into the cytosol. The consequences of induced cell death in the host cells led to a reduction in titers of fully encapsulated vectors. In this context, the recovered vectors obtained from repeated production experiments were subjected to DNase treatment prior to conducting cytotoxicity assays [42]. In this regard, the differences in cell death percentages between the two assays raise concerns about the timing and the techniques used to assess the efficacy of cell and gene products. This underscores the need for further studies in animal models as well as for evaluating biodistribution, immune response activation, and off-target toxicities.
5. Conclusion
Therapy-resistant solid cancers due to serpin B9 overexpression remain a major but overlooked limitation to activated or engineered T and NK cell-based therapies. Since in vivo gene therapy can lead to direct and selective induction of cell death of desired targeted tumors, our preliminary in vitro approach described here demonstrated a nucleic acid–based strategy for the treatment of serpin B9-positive cancers. In this study, we demonstrate that targeted delivery of nucleic acid encoding for the GrBmut can withstand tumor overexpression of serpin B9, providing an escape from its limitations when applied in protein-based therapy development [51] and also avoiding dependence on osmolytic agents to achieve its intracellular trafficking and endosomal escape [52]. This innovative method of AAV vector–mediated delivery of GrB cargo involved the genetic modification to allow the packaged AAV capsid to target cell surface EGF receptors. However, this study did not assess the impact of this modification on immunogenicity in relation to high natural immunity within the general population.
An important future consideration regarding the modification of AAV capsids with large MW ligands (> 30 kDa) requires striking a balance between maintaining capsid assembly, stability, and retrievable vector titers in large quantities. Obtaining full capsid vectors with large ligand domain insertions presents challenges as the likelihood of successfully realizing intact capsid vectors that are packaged is low compared to empty capsid vectors. Additionally, expanding the capsid gene sequences to accommodate exogenous large gene sequences can result in compromising the icosahedral shape of the vectors due to increased burden on the packaging machinery of producing cells. These crucial factors are to be taken into consideration when engineering the next generation of AAV vectors for gene delivery. Nevertheless, in this study, the highest percentage of EGF receptor–dependent cell transduction was by the vectors incorporating the panitumumab-derived scFv antibody fragment, despite the high number of empty and partially filled capsids as well as high instability in capsid assembly observed in these vectors when stored at beyond 3 months (Figure 2c, Panel 4). As such, this study validates the successful integration of an anti-EGFR moiety at the N-terminus of VP2, resulting in the engineered targeted and stable vectors which demonstrated EGFR-dependent killing of cancer cells. Taken together, our study demonstrates an exemplary approach to pave the way into in vivo gene therapy of serpin B9-positive cancers that would otherwise be resistant to the repertoire of T and NK cell-based immunotherapies.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work is based on research supported by the South African Research Chairs Initiative of the Department of Science and Technology (DST) and the National Research Foundation (NRF) of South Africa, Grant Number 47904, Google PhD Fellowship funding, and the Harry Crossley Foundation for the award of postdoctoral fellowship funding for D.M.D.
Acknowledgments
Our sincere acknowledgement goes to Dr. Betty Maepa, Prof. Patrick Arbuthnot, and all members of the Antiviral Gene Therapy Research Unit, University of Witwatersrand, South Africa, for their kind support in the form of training. We also acknowledge the valuable inputs of Alex Olusiji Akinrinmade, PhD.
Open Research
Data Availability Statement
All data generated from this study are included in the supporting information.