Morphology, Phylogeny, and Evolution of the Rarely Known Genus Admetella McIntosh, 1885 (Annelida, Polynoidae) with Recognition of Four New Species from Western Pacific Seamounts
Abstract
The polynoid genus Admetella constitutes a deep-sea assemblage of polychaetes, notable for their large bodies adorned with antennal scales positioned dorsally to the bases of lateral antennae. Furthermore, the genus exhibits swimming proficiencies facilitated by elongated parapodia and flattened chaetae. Despite the frequent encounters with Admetella members during various deep-sea explorations, a substantial gap in our comprehension of their diversity, phylogeny, and evolutionary trajectories still exists. Our thorough morphological and phylogenetic investigations of specimens obtained from three seamounts located in the tropical western Pacific have unveiled six species belonging to the genus Admetella, four of these being newly identified as Admetella multiseta sp. nov., A. levensteini sp. nov., A. nanhaiensis sp. nov., and A. undulata sp. nov. The other two species of Admetella remain unidentifiable at the species level due to the loss of crucial details. Our phylogenetic analysis, grounded on 13 mitochondrial protein-coding genes and the inclusion of 12S, 16S, 18S, 28S rRNA, and ITS1–ITS2 genes, substantiates the monophyly of Admetella. Admetella is positioned at an intermediate node within the phylogenetic tree, situated between representative shallow-water and deep-sea subfamilies. The independent evolution of antennal scales within Admetella among polynoids constitutes a synapomorphy for this genus. Ancestral state reconstruction (ASR) analyses suggest that deep-sea polynoids evolved from shallow-water ancestors that possessed lateral antennae, which were subsequently lost in members inhabiting extreme marine environments, such as deep-sea hydrothermal vents and anchialine caves. The analysis further implicates that swimming ability independently evolved at least four times within the Polynoidae family.
1. Introduction
The family Polynoidae, comprising a diverse assemblage of scale worms, demonstrates extensive variability across diverse marine benthic habitats, notably in the deep sea [1]. Among the members of this family, Admetella McIntosh, 1885 [2], one of the two genera encompassed within the subfamily Admetellinae Uschakov, 1977 [3], is distinguished by the distinctive feature of antennal scales or sheaths, which are adhered dorsally to the bases of lateral antennae [4]. Furthermore, Admetella stands out for its conspicuously large and flattened body, reaching dimensions of up to 120 mm, as well as its remarkable swimming proficiency, facilitated by elongated parapodia and fan-shaped chaetal bundles.
Numerous images and videos of Admetella species have been consistently captured by the remotely operated vehicle (ROV) during deep-sea explorations (https://www.ncei.noaa.gov/waf/okeanos-animal-guide/gal_Annelida.html), yet little is known about the taxonomy and diversity of this genus. Up until now, merely three species have been formally documented from the deep sea by early taxonomists [5]. Among these, A. hastigerens Chamberlin, 1919 [6] was recorded in the deep sea of Central America, Admetella longipedate (McIntosh, 1885) [2] was initially described from the deep sea off the Prince Edward Islands of Canada, and A. brevis Levenstein, 1978 [7] from the Hjort Trench of the Antarctic Ocean, with the latter two species distributed in high latitudes.
Within the family Polynoidae, the distinctive feature of antennal scales positioned superior to the lateral antennae is exclusively observed in the subfamily Admetellinae. The feature was previously considered homologous to the scales found at the base of the tentacle in the polychaete family Sigalionidae [2]. However, no molecular phylogenetic studies have been conducted to date on any species belonging to the subfamily Admetellinae. Furthermore, Admetella stands out prominently among polynoids due to its substantial body size, coupled with exceptionally long parapodia and chaetae. Notably, the neurochaetae of Admetella are flexible, transparent, and flattened, featuring spinous regions that taper to two dagger-like tips. These morphological characteristics distinguish Admetella from other polynoid genera. Given these unique morphological characteristics, the patterns of morphological evolution and phylogenetic relationships between Admetella and other polynoid groups represent significant areas of ongoing research interest. Further studies, particularly molecular phylogenetic analyses, are necessary to elucidate these relationships and provide insights into the evolutionary history of the subfamily Admetellinae within the broader Polynoidae family.
Despite over three decades of intensive deep-sea explorations in the western Pacific, no species belonging to the genus Admetella had previously been documented in this region. Our examination of specimens recently collected from seamounts in the tropical western Pacific, including the South China Sea, has revealed six previously undescribed species of Admetella. Four novel species are described and named herein as Admetella multiseta, A. levensteini, A. nanhaiensis, and A. undulata. The remaining two specimens, designated as A. sp.1, and A. sp.2, could not be definitively identified at the species level owing to the absence of critical taxonomic details. The systematic status of Admetella was evaluated employing a molecular phylogenetic approach that encompassed 13 mitochondrial protein-coding genes, in addition to the 12S, 16S, 18S, 28S rRNA, and ITS1–ITS2 genes. Furthermore, we explored the relationships among the polynoid subfamilies and traced the evolution of lateral antennae, antennal scales, habitats, and swimming ability, thereby elucidating the ancestral and derived character states of the major polynoid clades.
2. Materials and Methods
2.1. Specimen Collection and Morphological Examination
Between May 2019 and August 2022, specimens were systematically gathered from three distinct deep-sea seamounts (116°35′–140°11′E, 10°03′–15°06′N, depth ranging from 938 to 2,000 m) located in the tropical western Pacific. The acquisition procedure entailed five dives using the ROV FaXian (Discovery), which was deployed from the R/V KeXue (Science). Following acquisition, the specimens underwent preservation in an 80% (v/v) ethanol solution and were subsequently archived at the Marine Biological Museum of the Chinese Academy of Sciences (MBMCAS), situated within the Institute of Oceanology, Chinese Academy of Sciences (IOCAS).
The morphologies of the head, elytra, pygidium, parapodia, branchiae, and chaetae were meticulously observed utilizing a Zeiss Discovery V20 stereomicroscope and a Zeiss Imager Z2 compound microscope. Digital photographs were subsequently captured using an Axiocam 512 color camera securely mounted on these microscopes. To enhance clarity and contrast, stacks of images from distinct focal planes were meticulously combined employing Helicon Focus 7 software. Additionally, morphological details were stained with methyl green to further accentuate the visibility of intricate features.
2.2. DNA Extraction, Library Preparation, and Genome Skimming Sequencing
Specimens of Admetella multiseta sp. nov., A. levensteini sp. nov., A. nanhaiensis sp. nov., A. undulata sp. nov., A. sp.1, and A. sp.2 were utilized for genome skimming sequencing (Table 1). Genomic DNA was extracted using the TIANamp Marine Animal DNA Kit (Tiangen Bio. Co., Beijing, China) in accordance with the manufacturer’s instructions. A total of 200 μL of the genomic DNA solution (OD260/280 = 1.8–2.0, ≥3 μg) was submitted to Berry Genomics Co., Ltd. (Beijing, China) for library preparation and whole-genome sequencing (WGS). Paired-end libraries were constructed with an approximate insert size of 300 bp and sequenced (2 × 150 bp) on the Illumina NovaSeq 6000 platform. Approximately 10 Giga bases (Gb) of raw sequencing data were generated for each library.
| Family | Subfamily | Species | Mitogenome | 18S-28S-ITS | Reference |
|---|---|---|---|---|---|
| Polynoidae | Admetellinae | Admetella levensteini | PQ221480 | PQ211133 | This study |
| Polynoidae | Admetellinae | Admetella multiseta | PQ221478 | PQ211131 | This study |
| Polynoidae | Admetellinae | Admetella nanhaiensis | PQ221483 | PQ211136 | This study |
| Polynoidae | Admetellinae | Admetella undulata | PQ221482 | PQ211135 | This study |
| Polynoidae | Admetellinae | Admetella sp.1 | PQ221481 | PQ211134 | This study |
| Polynoidae | Admetellinae | Admetella sp.2 | PQ221479 | PQ211132 | This study |
| Polynoidae | Lepidonotinae | Lepidonotus clava | SRR16188824 | SRR16188824 | Filée et al. [45] |
| Polynoidae | Lepidonotinae | Lepidonotus sp. | KY753831 | KY753851 | Zhang et al. [8] |
| Polynoidae | Lepidonotinae | Halosydna sp. | KY753830 | KY753845 | Zhang et al. [8] |
| Polynoidae | Lepidonotinae? | A. gelatinosa | OX465594 | PRJEB61555 | Genbank |
| Polynoidae | Lepidonotopodinae | Branchipolynoe onnuriensis | NC_064376 | SRR18456218 | Kim et al. [44] |
| Polynoidae | Lepidonotopodinae | Branchinotogluma sp. | SRR16188833 | SRR16188833 | Filée et al. [45] |
| Polynoidae | Lepidonotopodinae | Thermopolynoe branchiata | SRR16188829 | SRR16188829 | Filée et al. [45] |
| Polynoidae | Lepidonotopodinae | Branchinotogluma segonzaci | SRR16188828 | SRR16188828 | Filée et al. [45] |
| Polynoidae | Lepidonotopodinae | Lepidonotopodium fimbriatum | SRR16188823 | SRR16188823 | Filée et al. [45] |
| Polynoidae | Lepidonotopodinae | Lepidonotopodium williamsae | SRR16188822 | SRR16188822 | Filée et al. [45] |
| Polynoidae | Lepidonotopodinae | Levensteiniella iris | KY753827 | KY753848 | Zhang et al. [8] |
| Polynoidae | Lepidonotopodinae | Lepidonotopodium sp. | KY753828 | KY753842 | Zhang et al. [8] |
| Polynoidae | Lepidonotopodinae | Branchinotogluma japonicus | KY753824 | KY753841 | Zhang et al. [8] |
| Polynoidae | Lepidonotopodinae | Branchipolynoe pettiboneae | KY753825 | KY753840 | Zhang et al. [8] |
| Polynoidae | Macellicephalinae | P. iliffei | MW794261 | MW794264 | Gonzalez et al. [9] |
| Polynoidae | Macellicephalinae | G. jameensis | MW794260 | MW794263 | Gonzalez et al. [9] |
| Polynoidae | Polynoinae | Pettibonesia furcosetosa | SRR16188832 | SRR16188832 | Filée et al. [45] |
| Polynoidae | Polynoinae | Harmothoe extenuata | SRR16188827 | SRR16188827 | Filée et al. [45] |
| Polynoidae | Polynoinae | Harmothoe fuligineum | SRR16188826 | SRR16188826 | Filée et al. [45] |
| Polynoidae | Polynoinae | Harmothoe sp. | SRR16188825 | SRR16188825 | Filée et al. [45] |
| Polynoidae | Polynoinae | Eunoe nodosa | NC_060302 | SRR14996616 | Kim et al. [44] |
| Polynoidae | Polynoinae | Acholoe squamosa | OX439053 | PRJEB60118 | Genbank |
| Polynoidae | Polynoinae | Harmothoe impar | OX381722 | PRJEB57909 | Genbank |
| Polynoidae | Polynoinae | Melaenis sp. | KY753829 | KY753849 | Zhang et al. [8] |
| Polynoidae | Polynoidae incertae sedis | Drieschia cf. elegans | MW794259 | MW794262 | Gonzalez et al. [9] |
| Polynoidae | Lepidonotopodinae | Branchipolynoe longqiensis | MW753826 | MW753847 | Zhang et al. [8] |
| Aphroditidae | — | Laetmonice producta | KY753833 | KY753853 | Zhang et al. [8] |
| Iphionidae | — | Iphione sp. | KY753835 | KY753852 | Zhang et al. [8] |
| Sigalionidae | — | Sthenelais limicola | OW804103 | PRJEB52697 | Genbank |
| Sigalionidae | — | Pisione sp. | KY753836 | KY753844 | Zhang et al. [8] |
| Sigalionidae | — | Euthalenessa festiva | KY753837 | KY753839 | Zhang et al. [8] |
| Acoetidae | — | Panthalis oerstedi | KY753832 | KY753846 | Zhang et al. [8] |
| Eulepethidae | — | Eulepethus nanhaiensis | KY753834 | KY753850 | Zhang et al. [8] |
| Syllidae | — | Trypanobia cryptica | KR534503 | SRR2006109 | Aguado et al. [43] |
| Nereididae | — | Neanthes sp. | SRR11669576 | SRR11669576 | Genbank |
2.3. Genome Skimming Sequencing Data Assembly
In addition to the six newly generated genome skimming sequencing datasets, we retrieved raw sequencing data for 14 species that had been utilized in previous polychaete phylogenomic investigations (Table 1) from the NCBI Sequence Read Archive (SRA) database.
Prior to assembly, raw sequencing reads were filtered to eliminate adaptors and low-quality reads utilizing Trimmomatic v 0.39 [10]. Subsequently, sequences pertaining to the mitochondrial genome and ribosome were assembled employing multiple k-mer strategies through MegaHit v 1.2.9 [11]. Contigs corresponding to mitochondrial and nuclear ribosomal sequences were identified utilizing BLAST + v 2.12.0 [12] and verified by mapping them to the respective reference sequences with Unipro UGENE v. 42.0 [13]. Furthermore, sequences of individual mitochondrial protein-coding genes, alongside the 12S, 16S, 18S, 28S, and ITS1–ITS2 genes, were individually extracted, resulting in 18 distinct datasets. Lastly, nucleotide sequences of each gene were aligned using MAFFT v 7.475 [14] with default parameters, followed by manual refinement within Unipro UGENE.
2.4. Species Delimitation and Phylogenetic Analyses
Two sequence datasets were created for genetic distance and phylogenetic analyses. The first dataset consisted of six COI sequences derived from specimens of Admetella, specifically designed for DNA barcoding analysis. The second dataset encompassed 41 concatenated sequences, incorporating 13 mitochondrial protein-coding genes along with 12S, 16S, 18S, 28S rRNA, and ITS1–ITS2 genes, aimed at phylogenetic analyses. The ingroup encompassed 32 species belonging to the family Polynoidae, while nine species from closely related families were selected to serve as the outgroup (Table 1).
The novel sequences, complemented by those downloaded from GenBank (Table 1), underwent alignment utilizing the MAFFT version 7 webserver [15], employing the “–auto” strategy with default parameters. The reading frame of each protein-coding gene was adjusted by excising one or two bases from the 5′ terminus to guarantee the absence of intragenic stop codons within the alignments. Subsequently, the alignment of each gene was manually standardized to a uniform length. For the first dataset, interspecific pairwise distances were computed using MEGA X, adopting the Maximum Composite Likelihood model [16]. Highly divergent and inadequately aligned segments within the 12S, 16S, 18S, 28S rRNA, and ITS1–ITS2 alignments were excised using GBlocks 0.91b [17] (GBlocks parameters: minimum length of a block = 5; allowed gap positions = with half). The trimmed alignments from each gene were concatenated into a dataset consisting of 18 genes (12S/16S/18S/28S/ITS/13PCGs) using Sequence Matrix 1.8 [18].
Phylogenies were inferred from both maximum likelihood (ML) and Bayesian inference (BI) methods, based on the concatenated dataset (the second dataset). The dataset was partitioned by gene, and the best-fit nucleotide substitution models and optimal partition schemes were selected utilizing ModelFinder [19], implemented within IQ-TREE 2.2.0.3 [20]. The ML analysis was conducted using IQ-TREE 2, and the node supports were evaluated by performing ultrafast bootstrap approximation (UFBoot) with 10,000 replicates [21]. The BI tree was reconstructed using MrBayes 3.2.6 [22]. Two independent runs, each consisting of four Markov Chains, were performed for 10,000,000 iterations, with sampling conducted every 1,000 iterations. Following the removal of the first 25% (2,500) trees as burn-in, the remaining trees were employed to construct the 50% majority rule consensus tree and estimate the posterior probabilities (PPs). The effective sample size (ESS) values for all sampled parameters were diagnosed using Tracer 1.7.1 [23] to ensure convergence. The resulting phylogenetic trees were subsequently visualized using FigTree 1.4.3 [24] and annotated with Adobe Photoshop CS4®.
2.5. Ancestral State Reconstruction (ASR)
Four pertinent characters concerning lateral antennae, antennal scales, habitats, and swimming ability of the studied polynoid species were summarized for phylogenetic analysis (Table S1). Ancestral states were calculated by stochastic character mapping, employing the empirical Bayes method within the R package phytools [25]. PP of these ancestral states were generated from 100 stochastic character maps for each trait using make.simmap function within phytools. These reconstructions were undertaken based on the topology previously derived from BI analyses of the fully partitioned sequencing dataset. Ultimately, the stochastic character maps for each trait were visually represented onto the inferred phylogeny, with PP clearly depicted at each node through pie charts.
The morphological characters and states used were as follows:
Antennal scales: State 0, antennal scales absent; State 1, antennal scales present.
Lateral antennae: State 0, lateral antennae absent; State 1, lateral antennae present.
Habitat: State 0, shallow water; State 1, deep-sea seamounts; State 2, hydrothermal vents; State 3, anchialine cave.
Swimming ability: State 0, swimming; State 1, nonswimming. The choice of this character and states followed Gonzalez et al. [9], who defined the term “swimming” for scale worms that either sink quickly upon cessation of active swimming or exclusively inhabit water columns, and “nonswimming” for those always staying in the bottom or swimming only for brief time when disturbed.
2.6. Nomenclatural Acts
The new names contained in this article are available under the International Code of Zoological Nomenclature. This work and the nomenclatural acts it contains have been registered in ZooBank. Zoobank Life Science Identifier (LSID) for this publication is urn:lsid:zoobank.org:pub:8023FDA4-A583-40F5-BE6E-971E180E782B. The LSID registration and any associated information can be viewed in a web browser by adding the LSID to the prefix http://zoobank.org/.
3. Results
3.1. Genetic Distance and Phylogenetic Analyses
The complete COI gene sequences of the six species of Admetella described herein were retrieved from their corresponding mitochondrial genomes, encompassing 1,536 nucleotide sites. Genetic distances among the species of Admetella varied between 0.0580 and 0.1508 (Table S2). Notably, three pairs of species displayed relatively low interspecific genetic distances: 0.0580 between A. levensteini and A. nanhaiensis, 0.1248 between A. multiseta and A. undulata, and 0.1251 between A. sp.1 and A. sp.2. These genetic affinities align with their morphological similarities (as depicted in Table 2) and their corresponding sister group relationships within the phylogenetic tree (Figure 1).
| Species | References | Lt (mm) | Wm (mm) | Seg | Elyt | Ansc | Dorsal cirri | neP shape | neP start | S1 acicula | S3 notopodia | S4 notopodia | Notochaetae start | Distribution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. levensteini sp. nov. | This study | 47 | 22 | 52 | 21 | Long, digitiform | Extend beyond the chaetae | Tapered to blunt tips | S5 | Absent | Reduced to acicular lobes | Reduced to acicular lobes | S5 | Tropical western Pacific |
| A. multiseta sp. nov. | This study | 120 | 42 | 72 | 28 | Short, subtriangular | Extend beyond the chaetae | Tapered to blunt tips | S6 | Absent | Reduced to acicular lobes | Reduced to acicular lobes | S5 | Tropical western Pacific |
| A. nanhaiensis sp. nov. | This study | 27.2 | 16 | 49 | 20 | Long, digitiform | Extend beyond the chaetae | Ovate or knob-like | S3 | Absent | Reduced to acicular lobes | Fused with neuropodia | S5 | South China Sea |
| A. undulata sp. nov. | This study | 95 | 27 | 67 | 26 | Short, subtriangular | Extend beyond the chaetae | Cirriform | S6 | Absent | Absent | Reduced to acicular lobes | S5 | South China Sea |
| A. sp.1 | This study | 40.4 | 23 | 45 | 19 | — | Extend beyond the chaetae | Ovate or knob-like | S9 | Absent | Reduced to acicular lobes | Reduced to acicular lobes | S7? | Tropical western Pacific |
| A. sp.2 | This study | 74.1 | 33 | 56 | 22 | Long, digitiform | Extend beyond the chaetae | Tapered to blunt tips | S11 | Absent | Reduced to acicular lobes | Reduced to acicular lobes | S5 | Tropical western Pacific |
| A. brevis | Levenstein [7] | 45 | 20 | 50 | 20 | Short, subtriangular | — | — | S5 | Absent | — | — | — | Hjort Trench of Antarctic Ocean |
| A. hastigerens | Chamberlin [6] | 60–80 | 24–27 | 57–70 | 23–25 | Short, subtriangular | Extend beyond the chaetae | Tapered to blunt tips | S6 | Absent | — | — | Absent | Central America |
| A. longipedata | McIntosh [2] | 65 | 30 | 50–60 | 24 | Short, subtriangular | Not extend beyond the chaetae | Tapered to blunt tips | — | Present | Reduced to acicular lobes | Reduced to acicular lobes | Absent | Prince Edward Islands of Canada |
- Abbreviations. Lt, total length of body; Wm, maximum width of body; Seg, number of segments; Elyt, number of pairs of elytra; anSc, antennal scale; neP, nephridial papilla; neP start, first segment with nephridial papilla; S1 referring to segment 1; notochaetae start, first segment with notochaetae.
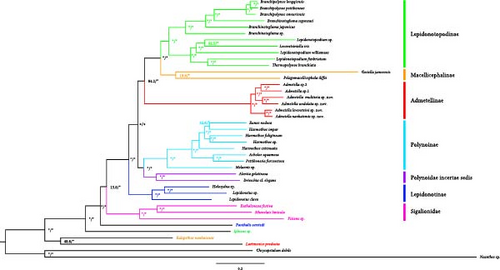
Both the ML and BI analyses yielded congruent phylogenetic trees, with BI PP and ML bootstrap values indicated on each node (Figure 1). Both the ML and BI phylogenetic trees consistently identified Admetella as a monophyletic group. The phylogenetic trees provided support for the sister-group relationships between A. levensteini and A. nanhaiensis, A. multiseta and A. undulata, and A. sp.1 and A. sp.2, respectively. Our phylogenetic analyses further unveiled six generally robustly supported clades within Polynoidae, encompassing five subfamilies and Polynoidae incertae sedis. The family Sigalionidae also exhibited as a well-supported clade.
Lepidonotopodinae (BP/PP = 99.9/100): As the most representative polynoid subfamily inhabiting deep-sea chemosynthetic environments, Lepidonotopodinae formed a well-supported clade and was sister to the Macellicephalinae clade.
Macellicephalinae (BP/PP = 56/100): Gesiella jameensis and Pelagomacellicephala iliffei formed a clade, despite exhibiting a low UFBoot support value. This clade, along with the Lepidonotopodinae clade, formed a strongly supported clade (BP/PP = 100/100).
Admetellinae (BP/PP = 100/100): The six species of Admetella described in this study formed a well-supported clade and were found to be the sister group to the clade consisting of the two aforementioned clades (BP/PP = 87/100).
Polynoinae (BP/PP = 100/100): As the most diverse subfamily of Polynoidae, Polynoinae formed a well-supported clade. This clade along with the clade of Polynoidae incertae sedis formed a large clade (BP/PP = 92/100).
Polynoidae incertae sedis (BP/PP = 99/100): Alentia gelatinosa and Drieschia cf. elegans formed a strongly supported clade and were identified as the sister clade to the Polynoinae clade (BP/PP = 92/100). However, the systematic status of these two species remains uncertain. Consequently, they are currently considered as species belonging to Polynoidae incertae sedis and require further investigation.
Lepidonotonae (BP/PP = 100/100): The species of Lepidonotonae formed a basal clade in the phylogenetic trees of Polynoidae. This clade was identified as the sister to the group comprising all the aforementioned clades (BP/PP = 100/100). Notably, the genus Halosydna was found to be nested within Lepidonotus, which was identified as a paraphyletic group.
Sigalionidae (BP/PP = 100/100): A well-supported clade consisting of three species from the family Sigalionidae was identified. This clade was determined to be the sister group to the Polynoidae clade (BP/PP = 58/98).
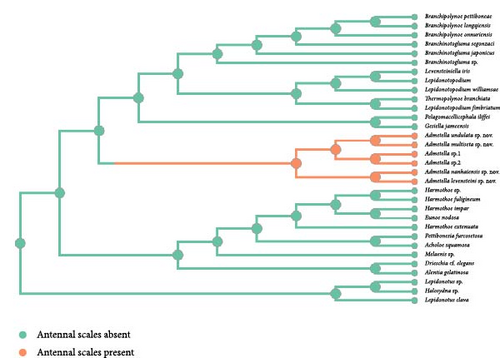
3.2. ASR
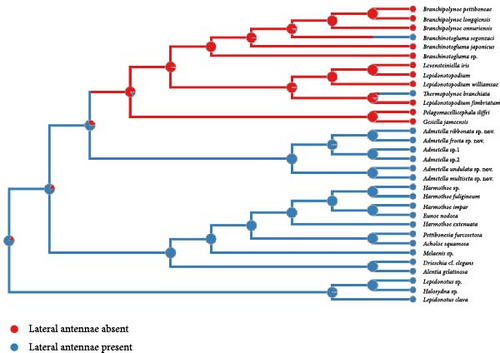
The exclusive presence of antennal scales within the polynoid subfamily Admetellinae was inferred as a synapomorphy for this group (Figure 2(a)). Correspondingly, the absence of antennal scales was deemed to represent an ancestral state of polynoids. In the phylogenetic tree, most polynoids, especially those in basal clades, exhibit lateral antennae situated on the anterior region of the prostomium. Consequently, the presence of lateral antennae is likely to represent the ancestral state of polynoids, whereas the loss of lateral antennae is deemed a derived characteristic observed exclusively in clades of Macellicephalinae and Lepidonotopodinae (Figure 2(b)). Within the subfamily Lepidonotopodinae, only two species, Branchinotogluma segonzaci and Thermopolynoe branchiate, possess lateral antennae, suggesting secondary acquisitions of this trait.
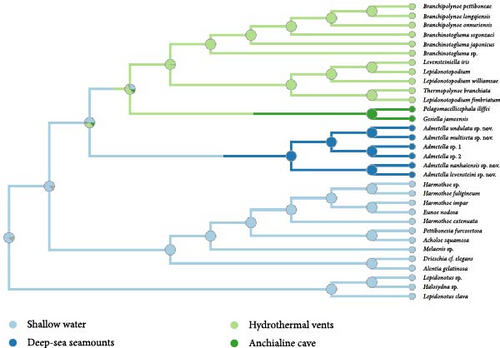
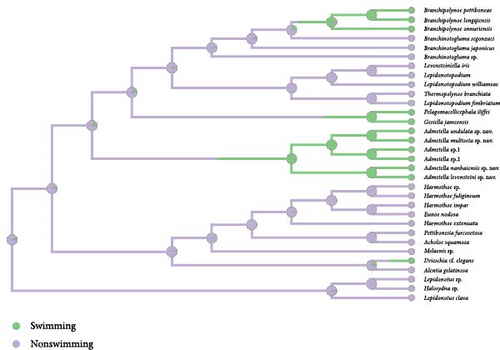
The analysis indicates that the most recent common ancestor (MRCA) of polynoids likely inhabited shallow water (Figure 2(c)). While most species of Polynoinae and Lepidonotinae are predominantly found in shallow waters, the subfamilies Admetellinae, Macellicephalinae, and Lepidonotopodinae are representatives of deep-sea environments. However, due to limited taxa sampling, only two species of Macellicephalinae living in anchialine caves were included in the analyses, which may not fully represent the main habitat of this subfamily. Considering the potential sampling biases, it is suggested that deep-sea polynoids likely evolved from their shallow-water ancestors.
Polynoids exhibiting active swimming capabilities have been documented in at least four distinct taxonomic groups: Drieschia, characterized as holopelagic inhabitants of the pelagic zone; Pelagomacellicephala and Gesiella, uniquely confined to the water columns within inland anchialine caves; Branchipolynoe, demonstrating benthopelagicism behavior in the vicinity of hydrothermal vents; and Admetella, displaying benthopelagicism behavior in the depths of deep-sea seamounts (Figure 2(d)). The trait of nonswimming has been inferred as an ancestral characteristic, whereas the capacity for swimming has been demonstrated to have independently evolved at least four times within the family Polynoidae.
3.3. Systematics
Class Polychaeta Grube, 1850.
Order Phyllodocida Dales, 1962.
Family Polynoidae Kinberg, 1856.
Subfamily Admetellinae Uschakov, 1977.
3.3.1. Genus Admetella McIntosh, 1885
Type species: Admetella longipedata (McIntosh, 1885) [2].
Diagnosis (modified after Pettibone [4]): body large, oval, flattened, with more than 49 segments (49–82) and more than 19 pairs of elytra (19–31). Elytra translucent, usually smooth, arranged on segments 2, 4, 5, 7, alternately to 23, and then on every third segment to the end of body. Prostomium with two long palps and three antennae; median antenna with large ceratophore; lateral antennae slender, inserted on anterolateral extensions of prostomium, with paired antennal scales attached behind ceratophores of lateral antennae. Antennal scales either short, subtriangular, or long, digitiform. Tentacular segment (first segment) achaetous, with two pairs tentacular cirri inserted laterally into prostomium. Tentacular sheathes absent. Buccal segment (second segment) achaetous, with paired, long, ventral buccal cirri. Parapodia very long, biramous with small notopodia; both notopodia and neuropodia with elongated digitiform acicular processes. Notochaetae form small bundles or fan-shaped bundles. Neurochaetae numerous, forming fan-shaped bundles. Both noto- and neurochaetae long, flattened, fragile, and transparent, with faint spinous row; neurochaetae distally smooth with tapered tips. Nephridial papillae on ventral bases of parapodia starting from segments 3–11, small, tapered, or ovate.
Remarks. The generic diagnosis underwent a modest expansion, adhering to Pettibone’s [4] framework, to encompass the species detailed in this study. Specifically, the minimum numbers of elytra and segments have been revised downwards to 19 (from the previous 20) and 49 (from the previous 52), respectively. Furthermore, the shape and start of nephridial papillae constitute crucial taxonomic features for differentiating Admetella species, which are now incorporated into the diagnostic criteria. This refinement enhances the precision of species identification within the genus. Additionally, the presence of notopodia does not invariably correlate with a reduction of chaetal bundles. For instance, in the newly described species A. multiseta and A. undulata, the notopodia are well-developed, featuring fan-shaped bundles of notochaetae. Notably, both notochaetae and neurochaetae are easily broken or detached due to their extreme delicacy and fragility, particularly in specimens subjected to prolonged preservation. Consequently, meticulous examination is warranted to ascertain whether the anterior parapodia possess chaetae or are achaetous.
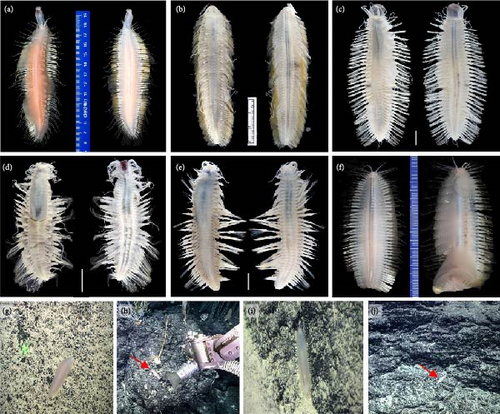
3.3.2. Admetella levensteini sp. nov. (Figures 3, 4; and Table 2).
Zoobank LSID: http://zoobank.org:act:E7A7E60D-6C7D-4FA2-BC9E-D308BDAD15F7

Material examined: Holotype. MBM286807, Dive 226, 140°04′28″E, 10°37′50″N, 1,506 m, 14 June 2016.
Etymology. The species is named to honor the late Dr. R. J. Levenstein, in recognition of his contribution to the polynoid taxonomy.
Description. Holotype, well-preserved with 52 segments, length 47.0 mm, width 22.2 mm (including chaetae) and 13.6 mm (excluding chaetae).
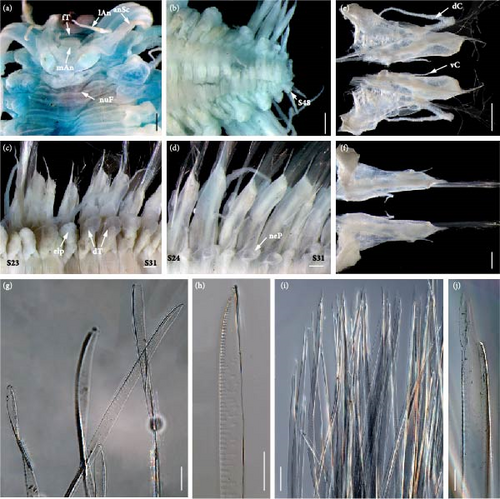
Body (Figures 3(c) and 3(h)) oval shaped, tapering anteriorly and posteriorly, slightly arched dorsally, and flattened ventrally. Parapodia (Figure 4(e)) elongated, two-thirds as long as width of body. Elytra (mostly missing) attached to distinct inflated elytrophores (Figure 4(b)), 21 pairs, arranged on segments 2, 4, 5, and 7, then alternate segments to 23, and then on every third segment to segment 50; elytra delicate, easily curled, translucent, surface smooth without tubercles or papillae. Dorsal tubercles (Figure 4(b)) on cirrigerous segments slightly inflated.
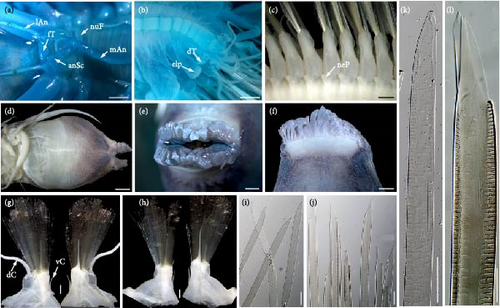
Prostomium (Figure 4(a)) bilobed, much wider than long. A pair of ocular areas prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 4(a)) with large and cylindrical ceratophore, inserted in middle of prostomium, styles much thicker and longer than lateral antennae. Ceratophores of lateral antennae (Figure 4(a)) formed by continuations of anterolateral borders of prostomium. Median antenna and lateral antennae cylindrical, tapering distally to slender tips, with slight subterminal enlargements. Paired, flattened, and elongated antennal scales attached behind ceratophores of lateral antennae; antennal scales (Figure 4(a)) slightly broad basally, tapering to blunt tips. Palps (Figure 4(a)) smooth, stout, and tapered with fine tips. Facial tubercle (Figure 4(a)) bulbous between bases of palps. Pharynx broken during dissection, with around 36 distal papillae, and two pairs of light-brown interlocking jaws.
First or tentacular segment (Figure 4(a)) achaetous, with two pairs of tentacular cirri inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small tapering processes; styles long, and smooth, with slight subterminal enlargements. Second segment with rudimentary parapodia, achaetous, with dorsal nuchal folds between elytrophores. Third and fourth segments with biramous parapodia not elongated, notopodia reduced to digitiform acicular processes, notochaetae absent; neuropodia similar to following ones with bundles of neurochaetae. Starting from segment 5, parapodia (Figure 4(e)) gradually elongated, flattened transversely, biramous. Notopodia (Figures 4(c) and 4(d)) cylindrical, much shorter than neuropodia, with long digitiform acicular processes and bundles of notochaetae. Notochaetae (Figure 4(f)) equal to neurochaetae in width, delicate, flexible, transparent, flattened with faint spinous rows, tapered to blunt hooded tips. Neuropodia (Figures 4(c) and 4(d)) elongated, postchaetal lobes subtriangular, prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figure 4(g)) numerous, forming fan-shaped bundles; delicate, flexible, transparent, flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Dorsal cirri (Figure 3(c)) translucent, long, extending beyond tips of chaetae, with slight subterminal enlargements. Ventral cirri on segments 2–5 elongated, extending beyond neuropodia (excluding acicular processes); on segment 2 similar to ventral tentacular cirri, much thicker and longer than subsequent ventral cirri. Starting from segment 6, ventral cirri (Figures 4(c), 4(d), and 4(e)) not extending neuropodia, subulate, and inserted in middle of elongated neuropodia.
Pygidium (Figure 4(b)) with a pair of anal cirri. Nephridial papillae (Figure 4(e)) on ventral bases of parapodia, starting from segment 5, small, tapered distally to blunt tips.
Type locality. A seamount tentatively is named as M8 seamount in the tropical western Pacific (140°04′28″E, 10°37′50″N, 1,506 m).
Remarks. The species A. levensteini sp. nov. morphologically resembles A. nanhaiensis sp. nov. in possessing long and digitiform antennal scales, similar numbers of segment and elytra (Table 2). Phylogenetic analyses further support the sister relationship between these two new species. However, the notopodia are reduced to acicular lobes in A. levensteini, while they are fused with the corresponding neuropodia in A. nanhaiensis. Moreover, the nephridial papillae in A. levensteini are tapered to blunt tips, while in A. nanhaiensis, they are ovate or knob-like. The species A. sp.2 also has long and digitiform antennal scales. However, the nephridial papillae start at segment 3 in A. nanhaiensis sp. nov. and at segment 5 in A. levensteini sp. nov., while they start at segment 11 in A. sp.2.
3.3.3. Admetella multiseta sp. nov. (Figures 3, 5; and Table 2)
Zoobank LSID: http://zoobank.org:act:434146D8-5EF9-442C-AECC-84C88D800786
Material examined: Holotype. MBM286065, Dive 213, 140°11′39″E, 10°04′24″N, 938 m, 31 May 2019.
Etymology. Composite the Latin prefix multi- and the Latin noun seta (seta) refers to the well-developed notochaetae, a feature of the species.
Description. Holotype, well-preserved with 72 segments, length 120 mm, maximum width 42 mm (including chaetae) and 25 mm (excluding chaetae).
Body (Figures 3(a) and (3(g)) spindle shaped, tapering anteriorly and posteriorly, slightly arched dorsally, flattened ventrally. Parapodia (Figure 5(c)) elongated, two-thirds as long as width of body. Elytra attached to large inflated elytrophores (Figure 5(b)), 28 pairs, arranged on segments 2, 4, 5, and 7, then alternate segments to 23, and then on every third segment to segment 71; elytra large, delicate, translucent, and surface smooth without tubercles or papillae. Dorsal tubercles (Figure 5(b)) on cirrigerous segments inflated, continuous as inflated ridge to base of cylindrical cirrophores of dorsal cirri.
Prostomium (Figure 5(a)) bilobed, much wider than long. A pair of ocular areas prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 5(a)) with large and cylindrical ceratophore, inserted in middle of prostomium. Ceratophores of lateral antennae (Figure 5(a)) formed by continuations of anterolateral borders of prostomium; styles thinner and shorter than styles of median antenna. Median antenna and lateral antennae cylindrical, tapering distally to slender tips, with brown slight subterminal enlargements. Paired, flattened, subtriangular antennal scales (Figure 5(a)) attached behind ceratophores of lateral antennae; antennal scales broad basally, tapering to blunt tips. Palps (Figure 5(a)) smooth, stout, tapered with fine tips. Facial tubercle (Figure 5(a)) bulbous between bases of palps. Pharynx (Figures 5(d), 5(e), and 5(f)) strong, with a pair of folds at lateral bases, with 36 distal papillae (18 dorsal and 18 ventral), and two pairs of light-brown interlocking jaws without teeth.
First or tentacular segment achaetous, with two pairs of tentacular cirri inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small processes; styles long, and smooth, with brown slight subterminal enlargements. Second segment with rudimentary parapodia, achaetous, with dorsal transverse nuchal fold between elytrophores (Figure 5(a)). The third and fourth segments with biramous parapodia not elongated, notopodia reduced to tapering acicular process, notochaetae absent; neuropodia similar to following ones with bundles of neurochaetae. Starting from segment 5, parapodia (Figure 5(c)) gradually elongated, flattened transversely, and biramous, with numerous notochaetae and neurochaetae. Notopodia (Figures 5(g) and 5(h)) cylindrical, slightly shorter than neuropodia, with long digitiform acicular processes and numerous notochaetae forming fan-shaped bundles. Notochaetae (Figures 5(i) and 5(k)) slightly slenderer than neurochaetae, long, flexible, transparent, flattened with faint spinous rows, and tapered to blunt hooded tips. Neuropodia (Figures 5(g) and 5(h)) elongated, postchaetal lobes subtriangular, and prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figures 5(j) and 5(l)) numerous, forming fan-shaped bundles; long, flexible, transparent, and flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Styles of dorsal cirri (Figure 3(a)) translucent, long, extending beyond tips of chaetae, with slight subterminal enlargements. Ventral cirri on segments 2–5 elongated, extending beyond tips of neuropodia; on segment 2 similar to ventral tentacular cirri, much thicker, and longer than subsequent ventral cirri. Starting from segment 6, ventral cirri (Figures 5(c), 5(g), and 5(h)) smaller, subulate, and inserted in middle of elongated neuropodia.
Pygidium (Figure 5(b)) with a pair of anal cirri. Nephridial papillae (Figure 5(c)) on ventral bases of parapodia, starting from segment 6, small, tapered distally to blunt tips.
Type locality. A seamount tentatively named as M5 seamount in the tropical western Pacific (140°11′39″E, 10°04′24″N, 938 m).
Remarks. Both morphological and phylogenetic analyses firmly supported the sister-group relationship between the new species A. multiseta sp. nov. and A. undulata sp. nov. Both new species exhibit short, triangular antennal scales, a characteristic that is also present in three previously recognized Admetella species. Notably, Admetella undulata sp. nov. distinctly differs from the other four species by the absence of notopodia in the third segment, whereas they are present in the latter. Furthermore, both A. multiseta sp. nov. and A. undulata sp. nov. exhibit well-developed notochaetae, whereas in A. hastigerens and A. longipedate, these structures are not observed (Table 2). Lastly, the two new species vary significantly from A. brevis in the numbers of segments and elytra, with the former exhibiting lower numbers compared to the considerably higher values in A. brevis.
3.3.4. Admetella nanhaiensis sp. nov. (Figures 3, 6; and Table 2)
Zoobank LSID: http://zoobank.org:act:B9BE227F-9A67-4FC9-BF89-F0903EB965D5
Material examined: Holotype. MBM286808, Dive 320, 116°35′45″E, 15°05′59″N, 1,499 m, 2 August 2022.
Etymology. The specific name is derived from Nanhai, the Chinese name of the South China Sea, where the species was discovered.
Description. Holotype, well-preserved with 49 segments, length 27.2 mm, and width 15.8 mm (including chaetae) and 11.4 mm (excluding chaetae).
Body (Figure 3(d)) oval shaped, tapering anteriorly and posteriorly, slightly arched dorsally, and flattened ventrally. Parapodia elongated (Figure 6(d)), as long as two-thirds width of body. Elytra (mostly missing) attached to distinct inflated elytrophores (Figure 6(c)), 20 pairs, arranged on segments 2, 4, 5, and 7, then alternate segments to 23, and then on every third segment to segment 47. Dorsal tubercles (Figure 6(c)) on cirrigerous segments distinctly inflated.
Prostomium (Figure 6(a)) bilobed, much wider than long. A pair of ocular areas large, prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 6(a)) with large and cylindrical ceratophore, inserted in middle of prostomium, styles detached. Ceratophores of lateral antennae (Figure 6(a)) formed by continuations of anterolateral borders of prostomium. Median antenna and lateral antennae cylindrical, tapering distally to slender tips, with slight subterminal enlargements. Paired, flattened, and elongated antennal scales attached behind ceratophores of lateral antennae; antennal scales (Figure 6(a)) slender, slightly broad basally, tapering to blunt tips, and tending to curl around the base of lateral antennae. Palps (Figure 6(a)) smooth, stout, and tapered with fine tips. Facial tubercle (Figure 6(a)) bulbous between bases of palps. Pharynx (Figure 6(a)) slightly extended, pink in color.
First or tentacular segment (Figure 6(a)) achaetous, with two pairs of tentacular cirri inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small tapering processes; styles long, smooth, with slight subterminal enlargements. Second segment with rudimentary parapodia, achaetous, with dorsal transverse nuchal fold between elytrophores. Third and fourth segments with parapodia not elongated, without notochaetae; notopodia reduced to short, slender acicular processes, free on segment 3, and fused with dorsal part of neuropodia on segment 4; neuropodia similar to the following ones with bundles of neurochaetae. Starting from segment 5, parapodia (Figure 6(d)) gradually elongated, flattened transversely, and biramous. Notopodia (Figures 6(e) and 6(f)) cylindrical, much shorter than neuropodia, with long digitiform acicular processes and bundles of notochaetae. Notochaetae (Figures 6(g) and 6(h)) slightly slenderer than neurochaetae, delicate, flexible, transparent, flattened with faint spinous rows, and tapered to blunt hooded tips. Neuropodia (Figures 6(e) and 6(f)) elongated, postchaetal lobes subtriangular, and prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figures 6(i) and 6(j)) numerous, forming fan-shaped bundles; delicate, flexible, transparent, and flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Dorsal cirri (Figure 3(d)) translucent, long, and extending beyond tips of chaetae, with slight subterminal enlargements. Ventral cirri on segment 2 similar to ventral tentacular cirri, much thicker, and longer than subsequent ventral cirri; gradually shorter posteriorly, not extending neuropodia on segment 4. Starting from segment 5, ventral cirri (Figures 6(d), 6(e), and 6(f)) smaller, subulate, and inserted in middle of elongated neuropodia.
Pygidium (Figure 6(b)) blunt, anal cirri detached. Nephridial papillae (Figure 6(d)) starting from segment 3, ovate or knob-like, located on ventral bases of parapodia.
Type locality. The Zhenbei Seamount in the South China Sea (116°35′45″E, 15°05′59″N, 1,499 m).
3.3.5. Admetella undulata sp. nov. (Figures 3 and 7; and Table 2)
Zoobank LSID: http://zoobank.org:act:1E480662-9C57-4AE6-9643-D21ADEC22D5F

Material examined: Holotype. MBM286809, Dive 320, 116°35′53″E, 15°06′08″N, 1,642.0 m, 2 August 2022.
Etymology. The Latin adjective undulatus (undulate) refers to the undulate appearance of parapodia during swimming.
Description: type specimens with 67 segments, length 95.0 mm, and width 27 mm (including chaetae) and 18 mm (excluding chaetae).
Body (Figure 3(b)) spindle shaped, tapering anteriorly and posteriorly, slightly arched dorsally, and flattened ventrally. Parapodia elongated, as long as width of body (Figures 3(b) and 7(e)). Elytra (mostly missing) attached to large inflated elytrophores (Figure 7(d)), 26 pairs, arranged on segments 2, 4, 5, and 7, then alternate segments to 23, and then on every third segment to segment 65; elytra large, delicate, translucent, surface smooth without tubercles or papillae. Dorsal tubercles (Figure 7(d)) on cirrigerous segments inflated, continuous as inflated ridge to base of cylindrical cirrophores of dorsal cirri.
Prostomium (Figure 7(a)) bilobed, much wider than long. A pair of ocular areas prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 7(a)) with large and cylindrical ceratophore, inserted in middle of prostomium, styles missing. Ceratophores of lateral antennae (Figure 7(a)) formed by continuations of anterolateral borders of prostomium; styles thinner, cylindrical, and tapering distally to slender tips, with brown slight subterminal enlargements. Paired, flattened, and subtriangular antennal scales attached behind ceratophores of lateral antennae; antennal scales (Figure 7(a)) broad basally, tapering to blunt tips. Palps (Figure 7(a)) smooth, stout, and tapered with fine tips. Facial tubercle (Figure 7(a)) bulbous between bases of palps. Pharynx not extended.
First or tentacular segment achaetous, with two pairs of tentacular cirri (Figure 7(c)) inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small tapering processes; styles long, and smooth, with brown slight subterminal enlargements. Second segment with rudimentary parapodia, achaetous, with dorsal transverse nuchal fold between elytrophores (Figure 7(a)). Third and fourth segments with parapodia not elongated (Figure 7(c)); notopodia achaetous, absent on segment 3, reduced to short acicular processes on segment 4; neuropodia similar to following ones with bundles of neurochaetae. Starting from segment 5, parapodia gradually elongated (Figures 7(e) and 7(f)), flattened transversely, and biramous, with numerous notochaetae and neurochaetae. Notopodia (Figures 7(g) and 7(h)) cylindrical, much smaller than neuropodia, with long digitiform acicular processes and numerous notochaetae forming fan-shaped bundles. Notochaetae (Figure 7(i)) equal to neurochaetae in width, long, flexible, transparent, flattened with faint spinous rows, and tapered to blunt hooded tips. Neuropodia (Figures 7(g) and 7(h)) elongated, postchaetal lobes truncate, and prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figure 7(j)) are numerous, forming fan-shaped bundles; long, flexible, transparent, and flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Styles of dorsal cirri (Figure 7(d)) translucent, long, and extending beyond tips of chaetae, with slight subterminal enlargements. Ventral cirri (Figure 7(c)) on segments 2–5 elongated, extending beyond tips of neuropodia; on segment 2 similar to ventral tentacular cirri, much thicker, and longer than subsequent ventral cirri. Starting from segment 6, ventral cirri (Figures 7(e), 7(f), 7(g), and 7(h)) smaller, subulate, and inserted in middle of elongated neuropodia.
Anus (Figure 7(b)) ventral, anal cirri missing. Nephridial papillae (Figures 7(e) and 7(f)) on ventral bases of parapodia, starting from segment 6, small, cirriform, tapered distally to fine tips.
Type locality. The Zhenbei Seamount in the South China Sea (116°35′53″E, 15°06′08″N, 1,642 m).
3.3.6. Admetella sp.1 (Figures 3, 8; and Table 2)
Material examined: MBM286810, Dive 221, 140°09′26″E, 10°03′06″N, 2,000 m, 9 June 2019.

Description. Specimen not well-preserved, with 45 segments, length 40.4 mm, and width 23.4 mm (including chaetae) and 15.8 mm (excluding chaetae).
Body (Figure 3(e) and 3(i)) oval shaped, tapering anteriorly and posteriorly, slightly arched dorsally, and flattened ventrally. Parapodia elongated, as long as width of body (Figure 3(e)). Elytra (all missing) attached to distinct inflated elytrophores (Figure 8(c)), 19 pairs, arranged on segments 2, 4, 5, and 7, then alternate segments to 23, and then on every third segment to segment 44. Dorsal tubercles (Figure 8(c)) on cirrigerous segments distinctly inflated.
Prostomium (Figure 8(a)) bilobed, much wider than long. A pair of ocular areas large, prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 8(a)) with large and cylindrical ceratophore, inserted in middle of prostomium, styles missing. Ceratophores of lateral antennae (Figure 8(a)) cylindrical, much smaller than median antenna, and formed by continuations of anterolateral borders of prostomium; styles of lateral antennae missing. Antennal scales broken off, with scars of attachment visible (Figure 8(a)). Palps (Figure 8(a)) smooth, stout, and tapered with fine tips. Facial tubercle bulbous between bases of palps. Pharynx not extended.
First or tentacular segment achaetous, with two pairs tentacular cirri inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small tapering processes; styles missing. Second segment with rudimentary parapodia, achaetous, with dorsal transverse nuchal fold between elytrophores (Figure 8(a)). Third segment possess short, digitiform parapodia, achaetous, with notopodia reduced to short, digitiform acicular processes. Several following parapodia (Figure 3(e)) gradually longer, biramous, both rami with slender acicular processes. Chaetae mostly missing in segments 2–8, small amount of notochaetae visible on left parapodium of segment 6 and right parapodium of segment 7. Starting from segment 9, parapodia with numerous notochaetae and neurochaetae. Notopodia (Figures 8(e) and 8(f)) cylindrical, much shorter than neuropodia, with long digitiform acicular processes and bundles of notochaetae. Notochaetae (Figure 8(g)) equals to neurochaetae in width, flexible, transparent, flattened with faint spinous rows, and tapered to blunt hooded tips. Neuropodia (Figures 8(e) and 8(f)) elongated, postchaetal lobes subtriangular, and prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figures 8(h) and 8(i)) numerous, forming fan-shaped bundles; flexible, transparent, flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Dorsal cirri (mostly missing, Figure 3(e)) translucent, long, and extending beyond tips of chaetae, with slight subterminal enlargements. Ventral cirri (Figure 3(e)) on segments 2–5 elongated, with slight brown subterminal enlargements, extending beyond tips of neuropodia; on segment 2 similar to ventral tentacular cirri, much thicker, and longer than subsequent ventral cirri. Starting from segment 6, ventral cirri (Figures 8(d), 8(e), and 8(f)) smaller, subulate, inserted in middle of elongated neuropodia.
Pygidium (Figure 8(b)) blunt, anal cirri detached. Nephridial papillae (Figure 8(d)) starting from segment 9, ovate or knob-like, located on ventral bases of parapodia.
Locality. A seamount tentatively named as M5 seamount in the tropical western Pacific (140°09′26″E, 10°03′06″N, 2,000 m).
Remarks. The damaged antennal scales of the examined specimen rendered species-level identification challenging. Nonetheless, the remaining key characteristics aligned with those observed in Admetella. To augment morphological investigation, the mitochondrial genome and nuclear genes (18S, 28S, and ITS) were successfully sequenced and deposited in GenBank. Phylogenetic analyses confirmed the sister-group relationship between A. sp.1 and A. sp.2. Admetella sp.1 distinguishes itself from A. sp.2 by exhibiting nephridial papillae starting in the ninth segment, as opposed to the eleventh segment in A. sp.2, whereas in the remaining four species, these papillae occur in segments 3–6.
3.3.7. Admetella sp.2 (Figures 3, 9; and Table 2)
Material examined. MBM286811, Dive 227, 140°05′37″E, 10°37′55″N, 1,707 m, 15 June 2019.
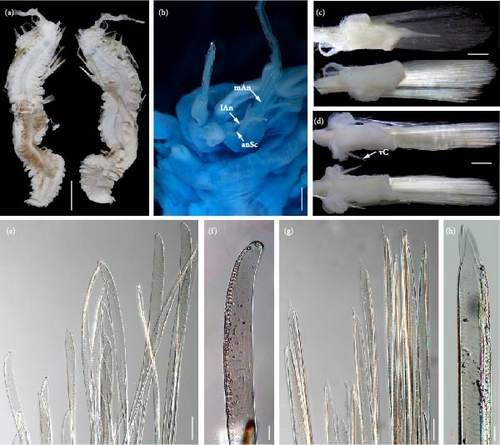
Description. Specimen seriously broken during preservation (Figure 9(a)), with more than 56 segments, length 74.1 mm, and width 33.2 mm (including chaetae) and 25.6 mm (excluding chaetae).
Body oval shaped (Figures 3(f) and 3(j)), tapering anteriorly and posteriorly, slightly arched dorsally, and flattened ventrally. Parapodia elongated as long as width of body (Figure 3(f)). Elytra (all missing) attached to inflated elytrophores, around 22 pairs, arranged on segments 2, 4, 5, 7, then alternate segments to 23, and then on every third segment to segment 53. Dorsal tubercles on cirrigerous segments inflated (Figure 3(f)).
Prostomium (Figure 9(b)) bilobed, much wider than long. A pair of ocular areas large, prominent, rounded, colorless, and located in posterolateral region. Median antenna (Figure 9(b)) with large and cylindrical ceratophore, inserted in middle of prostomium, styles missing. Ceratophores of lateral antennae (Figure 9(b)) cylindrical, much smaller than median antenna, formed by continuations of anterolateral borders of prostomium; styles of lateral antennae with slight brown subterminal enlargements. Antennal scales (Figure 9(b)) attached behind ceratophores of lateral antennae, flattened, slender, and tapering to blunt tips. Palps (Figure 9(b)) smooth, stout, and tapered with fine tips. Facial tubercle bulbous between bases of palps. Pharynx not extended.
First or tentacular segment achaetous, with a small projecting process and two pairs tentacular cirri inserted laterally into prostomium; tentaculophores cylindrical, basal of dorsal tentaculophores with small tapering processes; styles missing. Second segment with rudimentary parapodia, achaetous, with dorsal transverse nuchal fold between elytrophores. Third and fourth segments with biramous parapodia not elongated, notopodia reduced to short, digitiform acicular processes, without notochaetae; neuropodia with bundles of neurochaetae. Starting from segment 5, parapodia (Figure 3(f)) gradually elongated, flattened transversely, and biramous, with numerous notochaetae and neurochaetae. Notopodia (Figures 9(c) and 9(d)) cylindrical, much shorter than neuropodia, with long digitiform acicular processes and bundles of notochaetae. Notochaetae (Figures 9(e) and 9(f)) equals to neurochaetae in width, flexible, transparent, flattened with faint spinous rows, and tapered to blunt hooded tip. Neuropodia (Figures 9(c) and 9(d)) elongated, postchaetal lobes truncate, and prechaetal lobes extending as long digitiform acicular processes. Neurochaetae (Figures 9(g) and 9(h)) numerous, forming fan-shaped bundles; flexible, transparent, and flattened with spinous regions consisting of transverse rows of spines; tips bare, tapered to two tips. Dorsal cirri (Figure 3(f)) translucent, long, extending beyond tips of chaetae, with slight brown subterminal enlargements. Ventral cirri (Figure 3(f)) on segment 2 similar to ventral tentacular cirri, much thicker, and longer than subsequent ventral cirri. Ventral cirri on segments 3 and 4 with slight brown subterminal enlargements. Starting from segment 6, ventral cirri (Figures 9(c) and 9(d)) smaller, subulate, inserted in middle of elongated neuropodia.
Pygidium blunt, anal cirri detached. Distinct nephridial papillae (Figure 9(a)) starting from segment 11, located on ventral bases of parapodia, small, tapered distally to blunt tips.
Locality. A seamount tentatively named as M8 seamount in the tropical western Pacific (140°05′37″E, 10°37′55″N, 1,707 m).
Remarks. The specimen sustained mutilation as a result of tissue sampling by molecular researchers during the unfreezing procedure from an ultra-low temperature condition (−80°C). A significant portion of the parapodia and chaetae had dislodged from the body. Furthermore, the preservation of the head was inadequate. As a result, definitive species identification of the specimen proved unfeasible. However, the sequencing of the mitochondrial genome and nuclear genes (18S, 28S, and ITS) were successfully completed and subsequently uploaded to GenBank, serving as a supplementary resource for the morphological investigation.
4. Discussion
4.1. Morphological Comparison of Admetella and Bathyadmetella
Members of the genus Admetella have frequently been observed in the East Pacific, offshore from Hawaii, as well as in the western Pacific, yet none of the observed species have been formally described. Through our examination of polynoid specimens collected from three seamounts in the tropical western Pacific, we identified six distinct species belonging to the genus Admetella, indicating a remarkable species diversity within the Pacific Ocean. This underscores the crucial need for continued research efforts in the future.
Pettibone [4] conducted a comprehensive review of the genus Admetella, utilizing 12 specimens, which included the type specimens of A. hastigerens Chmaberlin, 1919 [6] and A. dolichopus Chamberlin, 1919 [6]. She concluded that all previously described species of Admetella were synonymous with A. longipedate McIntosh, 1885 [2], suggesting a lack of distinctiveness among them. However, Fauchald [26] contested this view, asserting that A. hastigerens constitutes a valid species due to its absence of acicula in the first segment, which contrasts with the presence of this character in A. longipedate. The third recognized species, A. brevis Levenstein, 1978 [7] is likewise a distinct species, differentiating from A. longipedate in its reduced numbers of segments and elytra, prolonged abdominal antennae, cone-shaped dorsal tubercles, and the occurrence of acicular lobes on the tentacular segment.
Pettibone [4] originally established the genus Bathyadmetella, grounded solely on a fragmented specimen retrieved from the Pacific Ocean adjacent to the mouth of the Columbia River. Subsequently, Uschakov [3] categorized both genera under the polynoid subfamily Admetellinae, thus affirming their phylogenetic proximity. Bathyadmetella exhibits numerous similarities with Admetella, including the presence of antennal scales, achaetous second segment accompanied by rudimentary parapodia, and nephridial papillae conspicuously shorter than the ventral cirri. A fundamental distinguishing characteristic between the two genera lies in the presence of paired tentacular sheathes located between the palps and tentacular cirri, observed in Bathyadmetella but absent in Admetella. Pettibone [4] highlighted the shape of antennal scales as another principal differentiator, wherein Admetella possessed short and triangular scales versus long and digitiform scales in Bathyadmetella. However, our study reveals that both morphological types are present among the six species of Admetella (Table 2). Consequently, the shape of antennal scales alone cannot serve as a definitive taxonomic marker distinguishing the two genera; however, it remains a significant characteristic in delineating species within the genus Admetella. The short and triangular antennal scales observed in A. multiseta sp. nov. and A. undulata sp. nov. seem to have evolved from the long and digitiform ones. This closely related phylogenetic relationship between the two species is corroborated by their morphological similarity, further emphasizing the evolutionary links shared by these species.
Apart from the aforementioned distinctions, Bathyadmetella exhibits distinct differences from Admetella, notably in the morphology of its notopodia featuring short acicular processes and the conspicuous absence of notochaetae, in contrast to Admetella which possesses elongated acicular lobes and clusters of notochaetae. Notably, the taxonomic status of Bathyadmetella was initially established solely on the basis of a single, inadequately preserved specimen. Our comprehensive examination revealed that the notochaetae exhibit a tendency to detach or break off easily, particularly in species characterized by diminished notopodia and a scarcity of notochaetae. Therefore, a thorough taxonomic reassessment of Bathyadmetella is imperative, underlining the need for intensified sampling efforts to validate and refine its classification.
- (1)
Tentacular sheaths present; notopodia with short acicular lobes……………………….……………..…………………………………………………………
-
Bathyadmetella Pettibone, 1967……………………..………………………………………………………B. commando
-
Tentacular sheaths absent; notopodia with long acicular lobes…………………………….………………………………………………………………………
-
Admetella McIntosh, 1885………………………………………………………………………………..2
- (2)
Antennal scales short, triangular………………….……………………………………………………..3
-
Antennal scales long, digitiform…………………..……………………………………………………..7
- (3)
Acicula present on first segment……………………………………………………………A. longipedata
-
Acicula absent on first segment…………………………………………………………………………..4
- (4)
Notopodia absent on segment 3……………………………………………………A. undulata sp. nov.
-
Notopodia present on segment 3…………………………………………………………………………..5
- (5)
Relatively small body size with 45 segments………………………………………………..……A. brevis
-
Large body size with 57–72 segments………………………………………………………..……………6
- (6)
Notochaetae start on segment 5………………………………………………………A. multiseta sp. nov.
-
Notochaetae absent……………………………………………….……………………….…A. hastigerens
- (7)
Notopodia reduced to acicular lobes; nephridial papilla tapered to blunt tips…………………………………………………………..A. levensteini sp. nov.
-
Notopodia fused with neuropodia; nephridial papilla ovate or knob-like……………………………………………………………………A. nanhaiensis sp. nov.
4.2. Phylogenetic Relationships of Polynoid Groups
The group Polynoidae, occupying the second largest position among polychaete families, encompasses nine subfamilies and 174 genera [27, 28]. Despite numerous phylogenetic studies leveraging morphological and/or molecular datasets [8, 9, 29, 30, 31, 32, 33, 34, 35], the relationships among numerous subfamilies remain unresolved. This situation is primarily attributed to the intricacies of polynoid phylogeny, coupled with inadequate data in most studies (e.g., 16S + COI + 18S + 28S sequences), and the inclusion of DNA sequences from misidentified species. Notably, Gonzalez et al. [31] conducted a study on the phylogeny of Polynoidae utilizing transcriptomic data, which yielded significant insights into the systematics of the group. However, this approach is constrained by the scarcity of transcriptomic data for numerous taxonomic groups.
To address this gap, we combined reliable sequences of five representative polynoid subfamilies sourced from GenBank with novel sequences of Admetella. Consequently, we presented the first phylogenetic analyses of Admetella, leveraging 13 mitochondrial protein-coding genes, along with 18S−28S rRNA and ITS genes. Our analyses conclusively demonstrate that the deep-sea genus Admetella is monophyletic and stands as the sister group to the deep-sea clade encompassing Lepidonotopodinae and Macellicephalinae.
According to the finding, the monophyly of Polynoidae was confirmed, with a sister-group relationship established to the family Sigalionidae. Furthermore, the phylogenetic relationships among the primary polynoid groups were elucidated. The subfamily Lepidonotopodinae, which dominates deep-sea chemosynthetic habitats, was confirmed as monophyletic, consistent with prior research [8, 30, 36, 37]. Despite low support in the ML analysis, the monophyly of Macellicephalinae was upheld. Macellicephalinae, a prolific subfamily primarily inhabiting deep-sea environments, encompasses 28 genera and 80 species. Unfortunately, sequencing was limited to two species residing in the water columns of inland anchialine caves, necessitating the inclusion of additional species to comprehensively assess the monophyly of Macellicephalinae. A recent transcriptome-based phylogenetic investigation revealed that Lepidonotopodinae is nested within Macellicephalinae [31]. Nevertheless, our study established a sister-group relationship between Lepidonotopodinae and Macellicephalinae, highlighting the importance of intensive taxonomic sampling within Macellicephalinae to validate this finding.
The monophyly of the subfamily Polynoinae is robustly supported by our results. The two prevalent genera, Harmothoe and Eunoe, exhibit a close relationship. The genus Harmothoe may belong to a paraphyletic group, necessitating a taxonomic reevaluation. The clade comprised of A. gelatinosa and Drieschia cf. elegans has been acknowledged as the sister group to Polynoinae. A. gelatinosa was initially described under the name Polynoe gelatinosa and subsequently reassigned to Halosydna and then Alentia by various authors. The taxonomic status of A. gelatinosa and Drieschia cf. elegans remains unresolved. It is recommended that these species continue to be provisionally classified within Polynoinae incertae sedis pending a taxonomic revision.
4.3. Character Evolution among Polynoid Groups
The discovery of a new species of Admetella provides us with an opportunity to delve into the evolution of pivotal characteristics and habitats of Admetellinae and allied taxonomic groups. For this purpose, the ASR analyses were performed through stochastic character mapping on the topology previously derived from BI analyses. Therefore, the accuracy of the inferred ancestral state depends on the reliability of the phylogenetic analyses and the inclusion of more representative taxa.
The presence of antennal scales constitutes an uncommon trait within Polynoidae, uniquely observed in Admetellinae, and is hence regarded as a synapomorphy of this subfamily. McIntosh [2], in the initial description of Admetella, hypothesized that the antennal scales in Admetella might be homologous to the scales, colloquially known as papal sheaths or tentacular lobes, located at the base of tentacles in Sigalionidae. However, upon closer inspection, it becomes evident that these two features differ significantly in their number and anatomical placement; Admetellinae possesses a single pair of antennal scales situated above the lateral antennae, whereas Sigalionidae exhibits two pairs of papal sheaths within their tentacular segment. Given the substantial phylogenetic distance between these two groups, it is plausible to consider this similarity as a case of morphological convergence.
Lateral antennae constitute a pivotal feature for distinguishing subfamilies and genera, exhibiting a discernible correlation with habitat types, as evinced by our analysis. The shallow-water-dwelling groups, Polynoinae and Lepidonotinae, consistently exhibit lateral antennae, in contrast to Macellicephalinae and Lepidonotopodinae, which primarily inhabit the deep sea and do not possess them. Our analysis reveals that the presence of lateral antennae represents an ancestral trait within Polynoidae, which was subsequently lost as polynoids adapted to deep-sea environments. Serving as an intermediary between the aforementioned groups, Admetella, a deep-sea taxon endowed with lateral antennae, occupies an intermediate position within the phylogenetic tree. The distinct lateral antennae observed in Branchinotogluma segonzaci and Thermopolynoe branchiate imply that the acquisition of lateral antennae occurred secondarily, at least twice, within the subfamily Lepidonotopodinae.
Polynoids inhabiting both deep-sea and cave environments have evolved from a shared shallow-water ancestor. These organisms exhibit inherited traits such as the absence of eyes and elongation of dorsal cirri, adaptation attributed to their living in environments devoid of light. The distinctive elongated cirri observed in Admetella species (Figure 3) serve as a mechanism for enhancing perception range and sensitivity. Shared morphological and biological similarities have been noted among obligate deep sea and subterranean fauna, including annelids and crustaceans [9, 32, 33, 38, 39, 40, 41, 42].
Our analysis indicates that swimming ability has independently evolved four times within the family Polynoidae, exemplified by distinct lineages: Branchipolynoe adapted to hydrothermal vents, Drieschia residing in shallow waters, Pelagomacellicephala and Gesiella found in anchialine caves, and Admetella inhabiting deep-sea seamounts. Gonzalez et al. [9] analyzed convergent adaptations in polynoid motility patterns, confirming the correlation between the elongated dorsal cirri and enhanced swimming ability. Moreover, Admetella species’ active swimming ability is facilitated by its flattened body shape and the elongation of its parapodia and chaetae.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Authors’ Contributions
Xuwen Wu and Qi Kou contributed equally to this work.
Acknowledgments
We thank the assistance of the crew of R/V KeXue and ROV FaXian for sample collection. We also thank Mr. Shaoqing Wang for taking the photos on board. This work was supported by the National Natural Science Foundation of China (grant number: 41930533), the Shandong Provincial Natural Science Foundation (grant number: 2023HWYQ-101), the Taishan Scholars Program (grant number: tsqn:202306288), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number: XDB42000000), and the Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Beibu Gulf University (grant number: 2022KA05).
Open Research
Data Availability
The materials described in this study are available at the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS) at Institute of Oceanology, Qingdao, China. Voucher IDs: Admetella multiseta sp. nov., MBM286065; A. levensteini sp. nov., MBM286807; A. nanhaiensis sp. nov., MBM286808; and A. undulata sp. nov., MBM286809. The sequences that support the findings of this study have been deposited in NCBI GenBank (Table 1). The registration of Admetella multiseta, A. levensteini, A. nanhaiensis, and A. undulata, in Zoobank, are as follows: urn:lsid:zoobank.org:act:434146D8-5EF9-442C-AECC-84C88D800786, urn:lsid:zoobank.org:act:E7A7E60D-6C7D-4FA2-BC9E-D308BDAD15F7, urn:lsid:zoobank.org:act:B9BE227F-9A67-4FC9-BF89-F0903EB965D5, urn:lsid:zoobank.org:act:1E480662-9C57-4AE6-9643-D21ADEC22D5F, respectively. The publication LSID: urn:lsid:zoobank.org:pub:8023FDA4-A583-40F5-BE6E-971E180E782B.




