Gut Microbiota, Metabolites, Circulating Cytokines and Growth Factors, Plasma Proteins, and Risk of Intracranial Aneurysms: A Two-Sample Mendelian Randomization Study
Abstract
Background: Increasing evidence implicates the gut microbiota, metabolites, circulating cytokines and growth factors, and plasma proteins as potential susceptibility factors for intracranial aneurysm (IA). However, due to their complexity, the causal relationship between these factors and IA remains unclear. Our goal was to determine whether these factors are causally associated with IA, UIA, and SAH and provide suggestions for the prevention and treatment of these cerebrovascular diseases.
Methods: Utilizing data from genome-wide association studies (GWAS), we conducted a large-scale Mendelian randomization (MR) analysis between these factors and diseases using five different models (Wald ratio, IVW, MR-Egger, weighted median, and MRPRESSO). Several sensitivity analyses were also applied to ensure the robustness of the results.
Results: Our MR analysis revealed several significant causal relationships between 18 gut microbiota taxa (genus.Bilophila-SAH, beta[95%CI] = −1.08[−1.61 ~ −0.54]), 55 blood metabolites (7-alpha-hydroxy-3-oxo-4-cholestenoate-IA, beta[95%CI] = −2.78[−4.47 ~ −1.08]), 2 cytokines (IL-6-UIA, beta[95%CI] = 0.73[0.34 ~ 1.39]), 45 plasma proteins (RELT-UIA, beta[95%CI] = −0.8[−1.22 ~ −0.38]), and IA, UIA, and SAH. Many of these were reported for the first time.
Conclusions: In conclusion, our study provides reference of the potential causal effects of gut microbiota, blood metabolites, cytokines, and plasma proteins on IA, UIA, and SAH. These findings may contribute to a better understanding of the pathogenesis and potential therapeutic targets for these cerebrovascular diseases.
1. Introduction
Intracranial aneurysm (IA) is a localized and pathological expansion of the intracranial artery wall that poses a risk of rupture [1]. It primarily occurs in the circle of Willis, and the incidence rate in the general population is approximately 3% [2]. Unruptured UIAs are typically asymptomatic, and about 85% of spontaneous subarachnoid hemorrhage (SAH) cases are caused by ruptured aneurysms [3]. SAH is a common critical illness in neurosurgery, with a mortality rate of up to 30%–50% and a disability rate of around 30% [2]. Globally, SAH affects 6.67 out of 100,000 people every year, with nearly half a million individuals suffering from it [4]. Given the significant incidence rate and associated mortality, it is imperative to gain a better understanding of the pathogenesis of UIA and develop preventive strategies.
In light of the notable augmentation in the role attributed to gut microbiota, a group of microorganisms that colonize the human gut [5], and microbial metabolites within central nervous system ailments, there has been a burgeoning recognition of the potential of targeting gut microbiota as a therapeutic intervention for such disorders, including stroke [6]. It has been shown that intestinal bacteria have a significant impact on host health because they impact metabolism and immunity [7]. The depletion of gut microbiota by antibiotics reduces the incidence of IAs in mice, according to a recent study [8]. Therefore, we can speculate that an intestinal microbiota imbalance may become a high-risk factor for IAs and increase the risk of developing them.
With the improvement of bioanalytical techniques and the development of new techniques, hundreds of metabolites can now be measured systematically and simultaneously [9]. Given the complex and diverse biochemical processes involved in the development of diseases, studying the relationship between metabolic abnormalities and human diseases has sparked great interest [10]. It is important to understand that cytokines play a key role in the immune system’s response to inflammation [11]. Low-grade inflammation has been suggested to increase mutation rates and increase mutated cell proliferation by supplying trophic signals [12]. Stratilová et al. reported that there is macrophage infiltration in ruptured and UIA tissues in humans, and white blood cell infiltration is more significant in ruptured aneurysms, indicating a close correlation between vascular inflammatory response and aneurysm rupture [13]. Research has shown that increased levels of IL-4 in genes have a protective effect on the risk of ischemic stroke, providing important new ideas for potential therapeutic targets for preventing ischemic stroke [14].
Plasma proteins play a core role in a series of biological processes that are often dysregulated in diseases and are the main source of many indications for treatment targets [15]. Plasma proteins are particularly associated with circulatory diseases such as stroke, as they have physical contact with blood vessels [16]. Genome-wide association study (GWAS) on plasma protein levels has identified genetic variations associated with proteins [17], which provides an opportunity to explore drug targets using MR analysis. Recent research has revealed serum protein biomarkers for predicting IAs and their rupture by establishing a mass spectrometry-based serum marker screening method [18]. However, the causal nature of the association between IA and plasma proteins is still unclear [19].
The MR study is a method that allows the use of genetic variants as instrumental variables (IVs) to explore the causal association between exposure and outcome. The advantage of MR is less residual confounding and reverse causation than traditional observational studies [20]. Therefore, an MR study can yield the causal association between the exposure (gut microbiota, blood metabolites, cytokines, and plasma proteome) and outcomes (IA, SAH, and UIA). Using newly released GWAS summary data for these factors and diseases, we aimed to analyze the causal connection between intestinal gut microbiota, blood metabolites, cytokines, and plasma proteome for IA, UIA, and SAH through the MR method.
2. Methods
2.1. Study Design
We first extracted GWAS summary statistics of gut microbiota, blood metabolites, cytokine, pQTLs of plasma proteins, and IA, UIA, and SAH from the respective consortiums. Then, we performed a large-scale two-sample MR analysis between them to explore the effect of gut microbiota, blood metabolites, cytokine and plasma proteins on IA, UIA, and SAH.
2.2. Acquisition of GWAS Summary Data and Ethical Review
GWAS summary data for gut microbiota abundance were obtained from a GWAS of host genetic variation conducted by the MiBioGen consortium, which has been the largest multiethnic genome-wide meta-analysis of the gut microbiome until now [21]. It demonstrated 122,110 host genetic variants associated with the abundance levels of 211 taxa (9 phyla, 16 classes, 20 orders, 35 families, and 131 genera) by using 16S ribosomal RNA gene sequencing in 18,340 participants, over 85% of which were of European ancestry. The summary statistics and IVs for circulating levels of cytokines and growth factors were derived from a GWAS including 8293 European individuals [22]. Detailed information was obtained from original studies. The genome-wide association data of IA, UIA, and SAH were from a study that performed a cross-ethnic, GWAS in 10,754 cases and 306,882 controls, revealed a polygenic architecture, and explained over half of the disease heritability [23]. The pQTLs of plasma proteins were obtained from Nature journal, which measured 2994 plasma proteins in 3301 European participants using the SomaLogic SomaScan platform and reported a total of 10,572,788 genetic variants [17]. Details of data sources are presented in Table 1. All the data used in this study were obtained from previously published research, and all of the original studies had obtained ethical approval from the respective institutions. Therefore, this study did not require an independent ethical review.
| Traits | Author | Year | Sample | Population | PMID |
|---|---|---|---|---|---|
| Gut microbiota | Kurilshikov et al. | 2021 | 18,340 | Mixed | 33462485 |
| Metabolites | Shin et al. | 2014 | 7824 | Europe | 24816252 |
| Circulating cytokines and growth factors | Ahola-Olli et al. | 2017 | 8293 | Europe | 27989323 |
| Plasma proteins | Benjamin B. Su et al. | 2018 | 3301 | Europe | 29875488 |
| IA, UIA, and SAH | Bakker et al. | 2020 | 317,636 | Mixed | 33199917 |
2.3. Selection of IVs
To ensure data robustness and the accuracy of results, the selection of IVs used in our study should comply with three crucial principles: (1) IVs were associated with exposure variables. Considering the small number of eligible IVs of cytokines at a genome-wide significant level (p < 5 × 10−8), a relatively more comprehensive threshold (p < 1 × 10−5) was applied to select IVs to keep consistency. Regarding pQTLs of plasma proteins, due to their characteristics, we still filtered using a genome-wide significant threshold. (2) There was no association between IVs and outcome variables. (3) The IVs were not associated with confounders. Finally, SNPs with linkage disequilibrium were excluded (clump window = 1000 kb, r2 = 0.001) using 1000 genomic European reference panels.
2.4. Mendelian Randomization (MR) Analysis
We conducted MR analysis using gut microbiota, blood metabolites, cytokine, and plasma proteins as the exposure and IA, UIA, and SAH as the outcome with R packages “TwoSampleMR” (version 0.5.6), “MR” (version 0.8.0), and “MR pleiotropy residual sum and outlier (MRPRESSO)” (version 1.0), respectively. If only a single SNP was available, we used the Wald ratio model to estimate its effect; otherwise, we used the inverse-variance weighted (IVW) model as the primary model [24]. The Cochran Q test in IVW was applied to test for the presence of heterogeneity in selected SNPs, and if there was significant heterogeneity (p < 0.05), we used a random-effects model; otherwise, we used a fixed-effects model. In this study, we considered a causal relationship to exist if the result was significant (p < 0.05) in the IVW model. We also used the other models as references, and if the positive results were replicated in these models, we thought the results were more reliable.
Moreover, to ensure the robustness of primary analyses, we applied several sensitivity analyses. First, MR-Egger, weighted median, weighted mode, and simple mode, as supplementary methods for IVW, were more robust to directional pleiotropy. Furthermore, MR-Egger regression was performed to assess the presence of directional pleiotropy, in which p-values for intercept less than 0.05 were considered statistically significant [25]. In addition, we used the MRPRESSO test to identify the possible pleiotropic outliers and reassessed the causal effect estimates after removing outliers [26]. A “leave-one-out” sensitivity analysis was applied where the MR was performed again but leaving out each SNP in turn to identify potentially influential SNPs. We also conducted Steiger’s directional test to ensure the consistency of the SNP’s effect direction on exposure and outcome.
2.5. Statistical Analyses
In this formula, EAF is the effect allele frequency of IVs, BETA is the effect size of IV on exposure, SE is the standard error of IVs on exposure, and N is the sample size of exposures.
Normally, when F is greater than 10, we consider the IV to be strong.
3. Results
3.1. The Causal Effects of Gut Microbiota on IA, UIA, and SAH
For the 211 taxa in the MiBioGen consortium, each group exposed genetic variations used as an IV ranging from 3 to 17 SNPs. In MR analysis, we identified 5, 8, and 5 gut microbiomes with causal effects on IA, UIA, and SAH, respectively, based on the IVW results (see Figure 1). Among them, the Bilophila genus had causal effects on the three disease subtypes (p = 0.002, p < 0.001, p = 0.02, respectively), and the direction was consistently negative (b = −0.43, b = −0.63, b = −1.08, respectively). Clostridiaceae and Porphyromonadaceae have negative causal effects on IA and UIA (b = −0.54 and -0.63, b = −0.42 and -0.67), while Streptococcus has positive causal effects on IA and SAH (b = 0.31, b = 0.39). Detailed results are given in Table 2.
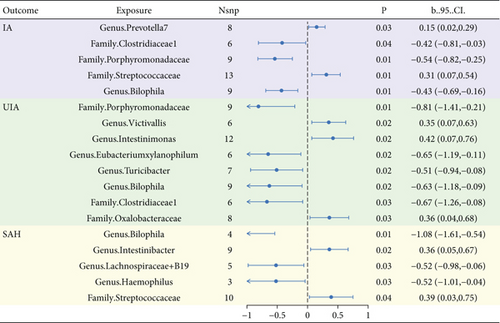
| Gut microbiota | SNPs | b_IVW | P_IVW | Q | p | Intercept | p |
|---|---|---|---|---|---|---|---|
| IA | |||||||
| Genus.Prevotella7 | 8 | 0.15 | 0.03 | 6.39 | 0.50 | −0.09 | 0.29 |
| Family.Clostridiaceae1 | 6 | −0.42 | 0.04 | 6.93 | 0.23 | −0.06 | 0.45 |
| Family.Porphyromonadaceae | 9 | −0.54 | 0.00 | 5.17 | 0.74 | −0.01 | 0.83 |
| Family.Streptococcaceae | 13 | 0.31 | 0.01 | 12.22 | 0.43 | −0.01 | 0.81 |
| Genus.Bilophila | 9 | −0.43 | 0.00 | 6.08 | 0.64 | 0.02 | 0.76 |
| UIA | |||||||
| Genus.Bilophila | 4 | −1.08 | 0.00 | 0.55 | 0.91 | 0.01 | 0.95 |
| Genus.Intestinibacter | 9 | 0.36 | 0.02 | 1.86 | 0.98 | −0.01 | 0.88 |
| Genus.Lachnospiraceae | 5 | −0.52 | 0.03 | 1.08 | 0.90 | 0.03 | 0.63 |
| Genus.Haemophilus | 3 | −0.52 | 0.03 | 1.12 | 0.57 | 0.06 | 0.51 |
| Family.Streptococcaceae | 10 | 0.39 | 0.04 | 3.79 | 0.92 | −0.04 | 0.64 |
| SAH | |||||||
| Family.Porphyromonadaceae | 9 | −0.81 | 0.01 | 11.18 | 0.19 | −0.09 | 0.30 |
| Genus.Victivallis | 6 | 0.35 | 0.02 | 4.16 | 0.53 | 0.08 | 0.66 |
| Genus.Intestinimonas | 12 | 0.42 | 0.02 | 5.77 | 0.89 | −0.01 | 0.79 |
| Genus.Eubacterium xylanophilum | 6 | −0.65 | 0.02 | 3.07 | 0.69 | 0.04 | 0.71 |
| Genus.Turicibacter | 7 | −0.51 | 0.02 | 5.95 | 0.43 | −0.10 | 0.23 |
| Genus.Bilophila | 9 | −0.63 | 0.02 | 10.03 | 0.26 | 0.06 | 0.64 |
| Family.Clostridiaceae1 | 6 | −0.67 | 0.03 | 2.20 | 0.82 | 0.10 | 0.37 |
| Class.Clostridia | 3 | 1.28 | 0.03 | 2.86 | 0.24 | −0.16 | 0.40 |
| Family.Oxalobacteraceae | 8 | 0.36 | 0.03 | 8.47 | 0.29 | 0.15 | 0.06 |
3.2. The Causal Effects of Blood Metabolites on IA, UIA, and SAH
We conducted MR analysis on 468 blood metabolites, and the IVW results showed 18, 20, and 17 serum metabolites with causal relationships to IA, UIA, and SAH, respectively, which was shown in Figure 2.1-Palmitoylglycerophosphoethanolamine exhibits a positive causal relationship with IA, UIA, and SAH, and an increase in their levels increases the risk of disease (b = 0.79, b = 1.55, and b = 1.42, respectively). 3-Indoxyl sulfate, 1-linoleoylglycerophosphoethanolamine, 1-arachidonoylglycerophosphocholine, isovalerylcarnitine, and 1-stearoylglycerophosphoethanolamine were found to have causal effects with IA and SAH, but the difference is that 3-indoxyl sulfate and 1-arachidonoylglycerophosphocholine have a positive effect on the disease, while others have a reverse effect. Pyridoxate, androsterone sulfate, X-12644, and 7-alpha-hydroxy-3-oxo-4-cholestenoate (7-Hoca) had positive causal effects on both IA and UIA, except for 7-Hoca. Detailed results are given in Table 3.
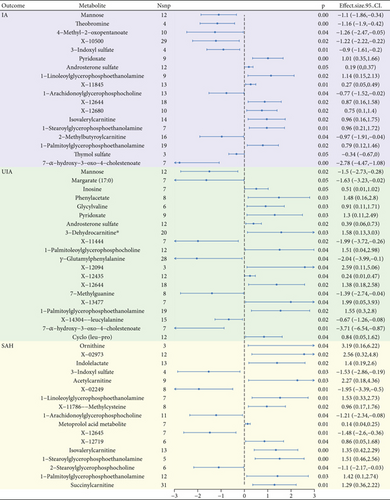
| Metabolite | SNPs | b_IVW | P_IVW | Q | p | Intercept | p |
|---|---|---|---|---|---|---|---|
| IA | |||||||
| Mannose | 12 | −1.10 | 0.00 | 14.31 | 0.22 | 0.03 | 0.18 |
| Theobromine | 4 | −1.16 | 0.00 | 2.55 | 0.47 | −0.09 | 0.67 |
| 4-Methyl-2-oxopentanoate | 10 | −1.26 | 0.04 | 5.38 | 0.80 | 0.02 | 0.73 |
| X-10500 | 29 | −1.22 | 0.02 | 24.58 | 0.65 | −0.02 | 0.30 |
| 3-Indoxyl sulfate | 4 | −0.90 | 0.01 | 2.34 | 0.50 | −0.11 | 0.53 |
| Pyridoxate | 9 | 1.01 | 0.00 | 3.97 | 0.86 | 0.01 | 0.80 |
| Androsterone sulfate | 12 | 0.19 | 0.05 | 7.62 | 0.75 | −0.01 | 0.40 |
| 1-Linoleoylglycerophosphoethanolamine | 9 | 1.14 | 0.02 | 17.75 | 0.02 | −0.02 | 0.66 |
| X-11845 | 13 | 0.27 | 0.01 | 15.11 | 0.24 | 0.03 | 0.22 |
| 1-Arachidonoylglycerophosphocholine | 13 | −0.77 | 0.04 | 19.01 | 0.09 | 0.02 | 0.08 |
| X-12644 | 18 | 0.87 | 0.02 | 20.15 | 0.27 | −0.02 | 0.30 |
| X-12680 | 10 | 0.75 | 0.02 | 7.19 | 0.62 | −0.04 | 0.19 |
| Isovalerylcarnitine | 14 | 0.96 | 0.02 | 23.60 | 0.04 | −0.02 | 0.22 |
| 1-Stearoylglycerophosphoethanolamine | 7 | 0.96 | 0.01 | 7.35 | 0.29 | 0.03 | 0.23 |
| 2-Methylbutyroylcarnitine | 16 | −0.97 | 0.04 | 8.97 | 0.88 | −0.02 | 0.48 |
| 1-Palmitoylglycerophosphoethanolamine | 19 | 0.79 | 0.02 | 14.77 | 0.68 | 0.01 | 0.53 |
| Thymol sulfate | 3 | −0.34 | 0.05 | 1.82 | 0.40 | 0.07 | 0.61 |
| 7-Alpha-hydroxy-3-oxo-4-cholestenoate | 7 | −2.78 | 0.00 | 6.82 | 0.34 | −0.07 | 0.16 |
| SAH | |||||||
| Ornithine | 3 | 3.19 | 0.04 | 0.59 | 0.74 | −0.02 | 0.94 |
| X-02973 | 12 | 2.56 | 0.02 | 9.18 | 0.60 | 0.03 | 0.60 |
| Indolelactate | 13 | 1.40 | 0.02 | 13.72 | 0.32 | −0.02 | 0.47 |
| 3-Indoxyl sulfate | 4 | −1.53 | 0.03 | 5.68 | 0.13 | −0.16 | 0.67 |
| Acetylcarnitine | 9 | 2.27 | 0.03 | 17.71 | 0.02 | 0.00 | 0.94 |
| X-02249 | 8 | −1.95 | 0.01 | 11.90 | 0.10 | −0.08 | 0.23 |
| 1-Linoleoylglycerophosphoethanolamine | 7 | 1.53 | 0.01 | 9.16 | 0.16 | −0.03 | 0.58 |
| X-11786--methylcysteine | 8 | 0.96 | 0.02 | 7.28 | 0.40 | −0.02 | 0.71 |
| 1-Arachidonoylglycerophosphocholine | 11 | −1.21 | 0.04 | 18.55 | 0.05 | 0.04 | 0.06 |
| Metoprolol acid metabolite | 7 | 0.14 | 0.01 | 4.05 | 0.67 | −0.02 | 0.51 |
| X-12645 | 7 | −1.48 | 0.01 | 6.13 | 0.41 | −0.10 | 0.14 |
| X-12719 | 6 | 0.86 | 0.04 | 13.76 | 0.02 | 0.06 | 0.58 |
| Isovalerylcarnitine | 13 | 1.35 | 0.00 | 16.24 | 0.18 | −0.05 | 0.04 |
| 1-STearoylglycerophosphoethanolamine | 5 | 1.51 | 0.00 | 3.53 | 0.47 | 0.07 | 0.17 |
| 2-Stearoylglycerophosphocholine | 6 | −1.10 | 0.04 | 2.83 | 0.73 | 0.04 | 0.54 |
| 1-Palmitoylglycerophosphoethanolamine | 12 | 1.42 | 0.03 | 7.17 | 0.79 | −0.03 | 0.68 |
| Succinylcarnitine | 31 | 1.29 | 0.01 | 33.14 | 0.32 | −0.01 | 0.28 |
| UIA | |||||||
| Mannose | 12 | −1.50 | 0.02 | 9.09 | 0.61 | 0.03 | 0.39 |
| Margarate (17 : 0) | 7 | −1.63 | 0.05 | 6.00 | 0.42 | −0.11 | 0.20 |
| Inosine | 7 | 0.51 | 0.05 | 1.86 | 0.93 | 0.04 | 0.45 |
| Phenylacetate | 8 | 1.48 | 0.03 | 4.97 | 0.66 | 0.03 | 0.68 |
| Glycylvaline | 6 | 0.91 | 0.03 | 1.69 | 0.89 | 0.08 | 0.42 |
| Pyridoxate | 9 | 1.30 | 0.03 | 7.83 | 0.45 | −0.03 | 0.47 |
| Androsterone sulfate | 12 | 0.39 | 0.02 | 4.37 | 0.96 | 0.00 | 0.98 |
| 3-Dehydrocarnitine∗ | 20 | 1.58 | 0.03 | 25.99 | 0.13 | −0.05 | 0.21 |
| X-11444 | 7 | −1.99 | 0.02 | 2.55 | 0.86 | 0.03 | 0.56 |
| 1-Palmitoleoylglycerophosphocholine∗ | 12 | 1.51 | 0.04 | 9.87 | 0.54 | 0.00 | 0.95 |
| Gamma-glutamylphenylalanine | 28 | −2.04 | 0.04 | 47.47 | 0.01 | 0.01 | 0.67 |
| X-12094 | 3 | 2.59 | 0.04 | 0.59 | 0.74 | 0.06 | 0.61 |
| X-12435 | 12 | 0.24 | 0.04 | 11.94 | 0.37 | 0.06 | 0.12 |
| X-12644 | 18 | 1.38 | 0.02 | 18.88 | 0.34 | 0.00 | 0.88 |
| 7-Methylguanine | 8 | −1.39 | 0.04 | 2.72 | 0.91 | 0.07 | 0.20 |
| X-13477 | 7 | 1.99 | 0.04 | 5.23 | 0.51 | −0.05 | 0.39 |
| 1-Palmitoylglycerophosphoethanolamine | 19 | 1.55 | 0.02 | 19.10 | 0.39 | 0.04 | 0.12 |
| X-14304--leucylalanine | 15 | −0.67 | 0.02 | 5.56 | 0.98 | 0.00 | 0.96 |
| 7-Alpha-hydroxy-3-oxo-4-cholestenoate | 7 | −3.71 | 0.01 | 5.25 | 0.51 | −0.07 | 0.36 |
| Cyclo(leu-pro) | 12 | 0.84 | 0.04 | 10.89 | 0.45 | 0.02 | 0.66 |
3.3. The Causal Effects of Cytokines on IA, UIA, and SAH
A total of 28 cytokines were included in the MR analysis as exposures, and the number of SNPs for each inflammatory factor ranged from 3 to 18. The IVW results showed that IL-6 had a causal effect on UIA, and MIG had a causal effect on SAH. An increase in IL-6 levels increases the risk of developing UIA (b = 0.73, p = 0.03), while an increase in MIG levels reduces the risk of developing SAH (b = −0.27, p = 0.02).
3.4. The Causal Effects of Plasma Proteome on IA, UIA, and SAH
We conducted an MR analysis using 2994 plasma proteins as exposure. To reduce false-positive results, we performed FDR correction on the p values of the results using the Benjamini–Hochberg method. Results indicated that 20, 9, and 16 plasma proteins had casual relationships with IA, UIA, and SAH, respectively, after FDR correction (Figure 3). In total, eight plasma proteins (RELT, ATP1B2, NTN1, ASIP, CST7, LRRN1, SEMA4D, and SEMA5A) showed direct negative causality with IA, while 12 proteins (RARRES1, HP, FTH1.FLT, APOL1, LYZ, SULT1E1, IL1R2, TGFBI, BGN, HAVCR1, PMEPA1, and CCL21) had positive causality. Six plasma proteins (RELT, CFHR5, GPX7, SVEP1, PPT1, and SEMA4D) could reduce the risk of UIA, while three proteins (TXNDC12, ERAP2, and TGFBI) elevated it. The expression of three plasma proteins (CRP, PATE4, and NAAA) was negatively correlated with SAH, whereas 13 proteins (HP, ERAP2, RARRES1, IL18R1, APOL1, FTH1.FLT, SPARCL1, PAM, IL1R2, ADAM23, BGN, PMEPA1, and MFGE8) were positively correlated. Detailed results are given in Table 4.
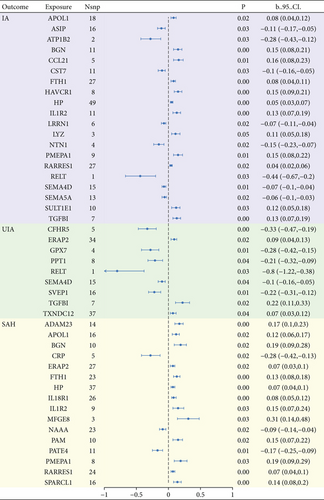
| Protein | SNPs | b_IVW | P_IVW | Q | p | Intercept | p |
|---|---|---|---|---|---|---|---|
| IA | |||||||
| RELT.14112.40.3 | 1 | −0.43507 | 0.000315 | NA | NA | NA | NA |
| ATP1B2.7218.87.3 | 2 | −0.27831 | 0.000484 | NA | NA | NA | NA |
| RELT.5115.31.3 | 1 | −0.26303 | 0.000193 | NA | NA | NA | NA |
| NTN1.6649.51.3 | 4 | −0.151 | 0.000267 | 3.795678 | 0.284389 | −0.0139 | 0.717668 |
| ASIP.5676.54.3 | 16 | −0.10657 | 0.000452 | 7.523554 | 0.941463 | −0.00164 | 0.945661 |
| CST7.3302.58.1 | 11 | −0.10147 | 0.000419 | 12.81478 | 0.234213 | 0.007311 | 0.810031 |
| LRRN1.11293.14.3 | 6 | −0.07316 | 0.000163 | 2.221531 | 0.81772 | −0.02216 | 0.324774 |
| SEMA4D.5737.61.3 | 15 | −0.06716 | 4.36E − 05 | 16.57862 | 0.279329 | 0.014678 | 0.291079 |
| SEMA5A.13132.14.3 | 13 | −0.06449 | 0.000243 | 8.431317 | 0.750582 | −0.00481 | 0.699149 |
| RARRES1.8398.277.3 | 27 | 0.042084 | 0.000249 | 27.06173 | 0.406102 | 0.001434 | 0.877262 |
| HP.3054.3.2 | 49 | 0.046125 | 9.72E − 06 | 42.59529 | 0.693231 | 0.005055 | 0.584155 |
| FTH1.FTL.3031.66.1 | 27 | 0.077502 | 1.91E − 05 | 20.90068 | 0.747072 | 0.004787 | 0.744917 |
| APOL1.11510.31.3 | 18 | 0.081307 | 0.000181 | 15.18213 | 0.582365 | 0.01898 | 0.220531 |
| LYZ.4920.10.1 | 3 | 0.113496 | 0.000708 | 4.495949 | 0.105613 | 0.070758 | 0.589837 |
| SULT1E1.9878.3.3 | 10 | 0.117608 | 0.000422 | 14.51882 | 0.10503 | 0.053772 | 0.145938 |
| IL1R2.14133.93.3 | 11 | 0.130608 | 1.13E − 05 | 6.997895 | 0.725644 | 0.016075 | 0.509038 |
| TGFBI.3283.21.1 | 7 | 0.133737 | 1.93E − 05 | 2.809193 | 0.832386 | 0.029706 | 0.234288 |
| BGN.13690.26.3 | 11 | 0.145288 | 2.16E − 05 | 8.814379 | 0.549806 | 0.031027 | 0.324452 |
| HAVCR1.9021.1.3 | 8 | 0.147666 | 2.14E − 06 | 13.99148 | 0.051333 | 0.012216 | 0.769596 |
| PMEPA1.6936.7.3 | 9 | 0.150301 | 4.46E − 05 | 9.317761 | 0.316203 | 0.036258 | 0.324237 |
| CCL21.2516.57.3 | 5 | 0.156529 | 3.16E − 05 | 0.851375 | 0.931431 | 0.011664 | 0.720098 |
| UIA | |||||||
| RELT.14112.40.3 | 1 | −0.79818 | 0.0002 | NA | NA | NA | NA |
| RELT.5115.31.3 | 1 | −0.46424 | 0.000206 | NA | NA | NA | NA |
| CFHR5.3666.17.4 | 5 | −0.33217 | 4.29E − 06 | 2.74418 | 0.601506 | −0.20652 | 0.205717 |
| CFHR5.7885.17.3 | 5 | −0.30428 | 0.000176 | 1.515288 | 0.82393 | 0.081922 | 0.553416 |
| GPX7.8345.27.3 | 4 | −0.28455 | 2.68E − 05 | 3.01062 | 0.389991 | −0.07613 | 0.318757 |
| SVEP1.11109.56.3 | 16 | −0.21633 | 1.85E − 05 | 24.75519 | 0.053329 | −0.01873 | 0.663814 |
| PPT1.9244.27.3 | 8 | −0.20655 | 0.000348 | 8.367677 | 0.301291 | 0.070794 | 0.101902 |
| SVEP1.11178.21.3 | 19 | −0.19844 | 4.78E − 06 | 26.02181 | 0.09926 | −0.0167 | 0.64727 |
| SEMA4D.5737.61.3 | 15 | −0.10288 | 0.000433 | 12.3824 | 0.575622 | 0.04107 | 0.088577 |
| TXNDC12.4815.25.3 | 37 | 0.074506 | 0.000348 | 28.19355 | 0.820231 | −0.00113 | 0.943613 |
| ERAP2.8960.3.3 | 34 | 0.085177 | 0.00011 | 21.88248 | 0.930185 | 0.011003 | 0.537962 |
| TGFBI.3283.21.1 | 7 | 0.220431 | 8.25E − 05 | 2.320072 | 0.888036 | 0.029231 | 0.493877 |
| SAH | |||||||
| CRP.4337.49.2 | 5 | −0.2791 | 0.000174 | 4.51122 | 0.341219 | −0.15367 | 0.127555 |
| PATE4.8065.245.3 | 11 | −0.16737 | 6.88E − 05 | 14.95092 | 0.133861 | 0.007752 | 0.879241 |
| NAAA.3173.49.2 | 23 | −0.09143 | 0.000236 | 26.67035 | 0.224015 | 0.004157 | 0.84602 |
| HP.3054.3.2 | 37 | 0.066361 | 1.84E − 05 | 43.46042 | 0.183446 | −0.01533 | 0.301483 |
| ERAP2.8960.3.3 | 27 | 0.066479 | 0.000167 | 26.80466 | 0.419632 | −0.00138 | 0.926676 |
| RARRES1.8398.277.3 | 24 | 0.067016 | 2.21E − 05 | 12.57445 | 0.960623 | 0.004198 | 0.738915 |
| IL18R1.3446.7.2 | 26 | 0.083609 | 3.64E − 07 | 20.25061 | 0.733559 | −0.02029 | 0.192336 |
| APOL1.11510.31.3 | 16 | 0.115062 | 0.000145 | 14.53238 | 0.485595 | 0.02449 | 0.257345 |
| FTH1.FTL.3031.66.1 | 23 | 0.126905 | 8.44E − 07 | 12.94555 | 0.934669 | −0.00301 | 0.895769 |
| SPARCL1.4467.49.2 | 16 | 0.142066 | 1.94E − 06 | 24.06543 | 0.063991 | 0.003801 | 0.88487 |
| PAM.5620.13.3 | 10 | 0.147237 | 0.00014 | 7.187171 | 0.617639 | 0.027348 | 0.434068 |
| IL1R2.14133.93.3 | 9 | 0.153026 | 0.000474 | 7.234299 | 0.511578 | 0.019812 | 0.550454 |
| ADAM23.7049.2.3 | 14 | 0.166693 | 2.81E − 07 | 7.382665 | 0.88137 | −0.00208 | 0.927699 |
| BGN.13690.26.3 | 10 | 0.185797 | 0.000206 | 7.290568 | 0.606895 | 0.078402 | 0.095368 |
| PMEPA1.6936.7.3 | 8 | 0.18743 | 0.000355 | 5.645324 | 0.581718 | 0.099634 | 0.116923 |
| MFGE8.4455.89.2 | 3 | 0.312426 | 0.000408 | 2.131378 | 0.34449 | −0.0222 | 0.948517 |
4. Discussion
We used the MR method to investigate the potential association between the exposure (gut microbiota, blood metabolites, cytokines, and plasma proteome) and outcomes (IA, SAH, and UIA). Our MR analysis identified five gut microbiota species with causal effects on IA, 8 gut microbiota species with causal effects on UIA, and 5 gut microbiota species with causal effects on SAH. A growing number of studies indicate that environmental factors play a more important role in the pathophysiology of IAs than genetic factors [27, 28]. The gut microbiota may affect the formation and rupture of IA by regulating local inflammation and affecting blood pressure [29]. Li et al. found that a total of 145 genera of microorganisms were differentially enriched between UIA patients and the control group [30]. These reports are consistent with our conclusions. However, the specific mode of action of these gut microbiota on aneurysms still needs further research and investigation. Moreover, there are studies indicating that antibiotic consumption can significantly reduce the incidence of IA [8]. Our results suggest that the genus.Prevotella7 is a risk factor for IA. However, our study revealed that family.Clostridiaceae1 has a protective effect against both IA and SAH, although the underlying mechanisms require further investigation. It has been demonstrated that antibiotic use may affect intestinal Clostridium and thus lead to antibiotic-associated diarrhea, but whether it increases the risk of IA and SAH is unclear [31]. He et al. reported that family.Porphyromonadaceae and genus.Bilophila are common protective bacterial features in SAH and UIA [32], which is consistent with our findings. Additionally, our results indicate that family.Porphyromonadaceae and genus.Bilophila are also a protective factors for IA. We observed an increased abundance of family.Streptococcaceae is associated with an elevated risk of IA and UIA. Piccirilli et al. previously reported a case of fungal aneurysm in the posterior inferior cerebellar artery (PICA) caused by group B Streptococcus, emphasizing the importance of considering the connection between gut microbiota and aneurysms in clinical practice [33]. It has been demonstrated that arterial hypertension is a significant risk factor for SAH [34]. A study led by Li et al. used the MRM method to investigate the causal relationship between gut microbiota and hypertension and found a correlation between the abundance of genus.Eubacteriumxylanophilum and hypertension [35]. Our results found that genus.Eubacteriumxylanophilum has a negative causal relationship with SAH, which may be through its influence on blood pressure. Genus.Intestinimonas is negatively correlated with the risk of ischemic stroke [36]. However, our results suggest that it increases the risk of SAH while reducing the risk of ischemic stroke, but the specific reasons remain unclear. Our study found that genus.Lachnospiraceae is a protective factor for UIA. It has been reported that genus.Lachnospiraceae is abundant in the bodies of humans and mice with obesity and hyperglycemia and promotes diet-induced obesity by producing long-chain fatty acids [37]. An epidemiological study of a Chinese population showed that as the body mass index (BMI) increases by one unit, the incidence of UIA decreases by 12.5% [38], which reasonably explains our results. Research has indicated a positive correlation between the abundance of genus.Victivallis and the risk of UIA [39]. Our findings also suggest that it is positively associated with the risk of SAH. Other known possible causes of SAH include smoking, high blood pressure, hyperhomocysteinemia, and Marfan syndrome [40, 41]. Additionally, we have reported two risk factors for SAH (class.Clostridia, family.Oxalobacteraceae) and one protective factor (genus.Turicibacter), as well as one risk factor for UIA (genus.Intestinibacter) and one protective factor (genus.Haemophilus). However, the specific mechanisms underlying these associations remain unclear, and further research is needed to elucidate them. Although IA patients have been found to have intestinal microbiota imbalance, there is currently no consensus. These unresolved issues hinder the inference of a causal relationship between gut microbiota and the occurrence and rupture of IAs. In the treatment of IAs, targeted eradication of bacteria may be used in the future to reduce the risk of IA occurrence and rupture.
A study carried out by So-Youn Shin et al. reported the most comprehensive exploration of genetic loci influencing human metabolism to date, including 7824 adult individuals from two European population studies and genome-wide significant associations at 145 metabolic loci and their biochemical connectivity regarding more than 400 metabolites in human blood [42]. This laid the foundation for our study. Our results identified 18, 20, and 17 plasma metabolites that are likely related to IA, UIA, and SAH, respectively, with the majority of them being reported for the first time. Substances belonging to the arachidonic acid class are believed to be beneficial in preventing and improving age-related decline in brain and cardiovascular function [43], as well as combating oxidative stress in the nervous system [44] and exhibiting anti-inflammatory properties [45]. Our findings also indicate a negative correlation between 1-arachidonoylglycerophosphocholine and the risk of IA and SAH. Guo et al., in their dual-sample MR analysis involving 486 serum metabolites, identified 1-linoleoylglycerophosphoethanolamine as a risk factor for lacunar stroke [46], which we found to be a risk factor for both IA and SAH. Furthermore, their research demonstrated that 1-palmitoleoylglycerophosphocholine is a protective factor against lacunar stroke but becomes a risk factor inUIA; however, the reasons for this difference remain unclear. Deep vein thrombosis (DVT) is a common complication of SAH [47], and it has been reported that 1-stearoylglycerophosphoethanolamine can prevent the formation of DVT [48]. Our results indicate a positive correlation between 1-stearoylglycerophosphoethanolamine and the risk of IA and SAH, which may be related to coagulation function. Indoxyl sulfate (IS) is a low molecular weight protein-bound uremic toxin primarily associated with the progression of cardiac or arterial diseases in patients with chronic kidney disease (CKD) [49], and its metabolism is influenced by various factors, including the gut microbiota [50]. Studies have shown that IS can cause vascular endothelial dysfunction by inducing endothelial cell apoptosis, oxidative stress, and inflammatory damage [51]. However, our results indicate that 3-indoxyl sulfate can reduce the risk of IA and SAH, which conflicts with previous findings. A metabolomics study revealed an association between gamma-glutamylphenylalanine and salt-sensitive hypertension [52], while our research found that gamma-glutamylphenylalanine can decrease the risk of UIA, possibly achieved through blood pressure modulation. Isovalerylcarnitine has been shown to promote cell apoptosis by activating relevant enzymes [53], and other studies have indicated that isovalerylcarnitine is a risk factor for rheumatoid arthritis [54]. Pentimalli et al. found a significant correlation between the level of cell apoptosis and the rupture of aneurysms [55], suggesting that isovalerylcarnitine may increase the risk of IA and SAH by influencing cell apoptosis. Inflammation has been reported to mediate the formation and development of IAs [56]. Mannose is an anti-inflammatory substance that can inhibit inflammation by inducing regulatory T cells and suppressing effector T cells and inflammatory macrophages, among others [57]. Wang et al. found that D-mannose can inhibit inflammatory reactions in the central nervous system [58]. Our results also indicate that mannose has a protective effect against IA and UIA. In addition to the substances mentioned above, we have identified dozens of blood metabolites associated with IA, UIA, and SAH, but currently, research on these metabolites is limited, and further investigations are still needed [59, 60].
The plasma proteome, which circulates throughout the body and samples the health status of various organs, is an ideal matrix for studying disease mechanisms and biomarkers [61]. C-C motif chemokine Ligand 21 (CCL21), an important chemotactic factor belonging to the CC subfamily, is a small molecular weight protein with chemoattractant and cell migration functions [62]. A study by Guedj et al. demonstrated that the inflammatory factor CCL21 in human abdominal aortic aneurysm can trigger the production of chemokines by smooth muscle cells, thereby facilitating the recruitment of immune cells and the development of atherosclerosis [63]. Our findings also suggest that CCL21 may promote the occurrence and progression of IA. C-reactive protein (CRP), a highly conserved plasma protein, is involved in systemic inflammatory response [64]. Romero, Cataneo, and Cataneo conducted a prospective evaluation of patients with SAH from aneurysmal rupture and found that higher levels of CRP in the blood were associated with poorer clinical outcomes and the occurrence of delayed ischemic neurological deficits [65]. However, our results indicate a negative correlation between CRP levels and the risk of SAH, which requires further experimental verification. Studies have shown that the variation in hepatitis A virus cellular receptor 1 (HAVCR1) is a genetic risk factor for coronary heart disease and ischemic stroke [66]. Interestingly, we have found that it is also a risk factor for IA haptoglobin (HP), an acute-phase protein that binds free Hb and has been implicated in various physiological processes [67]. Humans can have three phenotypes of Hp: Hp1-1, Hp2-1, and Hp2-2. It has been discovered that Hp2-2 is an independent risk factor for poor prognosis in SAH [68]. Our results further confirm that HP increases the risk of SAH. Leng et al. have identified associations between Interleukin 18 Receptor 1 (IL8R1) and Interleukin 1 Receptor Type 2 (IL1R2) with SAH [69], and our findings corroborate these observations, indicating that IL8R1 is a risk factor for SAH while IL1R2 is a risk factor for both IA and SAH. N-acylethanolamine acid amidase (NAAA) is a cysteine hydrolase involved in inflammation control through the hydrolysis of substances such as palmitoylethanolamide (PEA) [70]. Research has demonstrated that NAAA inhibitors can alleviate blood–brain barrier (BBB) disruption and secondary damage following traumatic brain injury (TBI) [71]. However, our results suggest that NAAA is a protective factor for SAH, and the underlying mechanisms require further investigation. Our research has found that Netrin 1 (NTN1) can reduce the risk of IA. It has been reported that Netrin-1 regulates iron death through the PPARγ/Nrf2/GPX4 signaling pathway following SAH to alleviate early brain injury [72]. Fiore et al. revealed a certain correlation between the Semaphorin 5A (SEMA5A) gene and remodeling of the cranial vascular system [73], while our results indicate that SEMA5A can lower the risk of IA SPARC Like 1 (SPARCL1) has been identified as a risk factor for IA. Peters et al. observed significant upregulation of the cell adhesion-related protein SPARC through molecular pathological analysis of IAs [74]. Sulfotransferase Family 1E Member 1 (SULT1E1) is a Phase II drug-metabolizing enzyme that catalyzes the sulfonation of estrogens and can regulate inflammatory response and lipid metabolism in human endothelial cells through PPARγ [75]. Our study also suggests that SULT1E1 is one of the risk factors for IA. Ruigrok et al. previously reported an association between transforming growth factor beta induced (TGFBI) and IA [76], and our results confirm that TGFBI is a risk factor for IA. Epithelial–mesenchymal transition (EMT) plays an important role in the formation and development of IAs [77]. Yuan et al. discovered that thioredoxin domain containing 12 (TXNDC12) can promote EMT by activating β-catenin [78]. Our findings suggest that TXNDC12 can increase the risk of UIA, possibly through promoting EMT. Additionally, we have identified over 20 plasma proteins that are causally related to IA, UIA, or SAH, but their underlying mechanisms remain unclear.
However, our study also inevitably has the following limitations: (1) The GWAS summary data we utilized predominantly originated from European populations, and the results may exhibit certain variations when applied to other ethnic groups. (2) Most of the identified gut microbiota, blood metabolites, and plasma proteomic factors showing causal relationships with IA, UIA, and SAH have not been validated through wet lab experiments. Detecting the prevalence of these diseases in populations with the corresponding characteristics could better establish this causal relationship, and we hereby call for further studies. (3) Due to the limited availability of IVs, we employed a relatively relaxed IV screening threshold (p < 1 × 10 − 5) for gut microbiota and blood metabolites. Additionally, we did not perform FDR correction for the abovementioned results. (4) The results may be affected by a variety of covariates such as blood pressure and lipids, and our study was not corrected for covariates due to technical limitations, which remain to be investigated further.
5. Conclusion
In conclusion, we conducted a comprehensive study using the MR method to investigate the causal relationships between gut microbiota, blood metabolites, cytokines, and plasma proteome with IA, UIA, and SAH. Through our analysis, we discovered numerous potential biomarkers associated with IA, UIA, and SAH, providing some reference for assessing the risk of related diseases and facilitating future research.
Nomenclature
-
- GWAS
-
- genome-wide association study
-
- IA
-
- intracranial aneurysm
-
- MR
-
- Mendelian randomization
-
- SAH
-
- subarachnoid hemorrhage
-
- SNP
-
- single nucleotide polymorphism
-
- UIA
-
- unruptured intracranial aneurysm
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
A.M., Z.X., and M.T.: conceptualization, methodology, validation, investigation, supervision, software, visualization, writing—original draft, and writing—reviewing and editing. A.A., X.C., W.W., M.A., Q.Y., H.Q., Z.W., and Y.W. participated in the coordination of data acquisition and data analysis and reviewed the manuscript. A.M., Z.X., and M.T. contributed equally to this work and shared the first authorship.
Funding
This work was supported by the Youth Science and Technology Innovation Talent Program (2023TSYCCX0054), the Xinjiang Uygur Autonomous Region Regional Collaborative Innovation Program (2022E01068), and the Key Projects of Science and Technology Department of Xinjiang Uygur Autonomous Region (2022D01D70).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data used to support the findings of this study are included within the tables in the article. The MR analysis used summary GWAS data publicly available from GWAS.




