Analysis of Specific IgE Distribution Characteristics of Dust Mite Allergen Components in Patients With Atopic Dermatitis
Abstract
Objective: To investigate the distribution characteristics of specific immunoglobulin E (IgE) to dust mite components in patients with atopic dermatitis (AD), aiming to provide a reference basis for the development of microarray protein chips for allergen component discrimination diagnosis and personalized desensitization therapy.
Methods: A total of 55 patients who visited Huishan District People’s Hospital of Wuxi City, Xishan People’s Hospital of Wuxi City, and People’s Hospital of Wuxi City from January 2023 to December 2023 were selected. The results of serum total IgE (total IgE) and specific IgE detection were analyzed.
Results: Among the 55 patients, 54 (98.2%) were positive (cutoff value > 0.35 Ua/mL, the normal value for serum total IgE is under 150–400 IU/mL) for serum total IgE antibodies, 52 (94.6%) were positive for Dermatophagoides pteronyssinus (Dp)–specific IgE antibodies, and 50 (90.9%) were positive for Dermatophagoides farinae (Df)–specific IgE antibodies. The protein chip results were consistent with the existing Phadia detection results.
Conclusion: The development of chips based on CRD (component-resolved diagnosis) cannot only rely on IgE binding rate to assist in precise treatment of patients but also choose personalized desensitization therapy, providing more practical recommendations for preventing cross-allergy, avoiding allergic environments, or ingesting sensitizing foods for susceptible individuals.
1. Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease, with its etiology primarily attributed to damage to the barrier and immune dysregulation triggered by environmental allergens, including house dust mites (HDMs) [1]. Its clinical manifestation includes recurrent eczematous lesions and severe pruritus [2]. In central and northern Sweden, 20% of AD patients exhibit high sensitivity to HDM allergens [3]. Among HDMs, Dermatophagoides pteronyssinus (Dp) and Dermatophagoides farinae (Df) are predominant species, and their bodies and feces serve as major environmental allergens [4]. In basic experiments, extracts from dust mites can induce AD-like skin lesions in mice [5].
Upon entry into the body, dust mite allergens not only trigger type I hypersensitivity reactions mediated by IgE, but also IgE-mediated late phase reaction and IgE-mediated delayed-type hypersensitivity reaction. Total IgE is a traditional marker for allergic diseases, but its specificity is relatively poor [6]. Conventional in vitro IgE testing is based on crude extracts composed of sensitizing and non-sensitizing molecules. Crude extracts fail to provide information on IgE-specific recognition and the presence of cross-reactivity can affect the detection of total IgE. Specific IgE (sIgE) testing has become one of the most important diagnostic tools for IgE-mediated immediate-type allergies [7]. It has been reported that Der p has 34 components, and Der f has 37 components (http://www.allergen.org/).
Component-resolved diagnosis (CRD) is a novel method for allergy diagnosis [8]. It utilizes purified recombinant allergens to identify patients’ sensitization profiles. CRD can effectively differentiate true sensitization from cross-reactivity-induced sensitization [9] and provide a broad spectrum of IgE profiles to specific allergens for sensitized individuals. In recent years, researchers have employed microarray technology to perform serological diagnosis using recombinant protein spots on solid carriers, yielding reliable detection results with minimal serum requirements. Protein chips have become an important tool for allergen CRD due to their high detection throughput, low sample demand, and simplicity of operation [10].
We prepared recombinant antigen protein chips for Der p and Der f allergens for patients who tested positive for serum IgE, Der p, and Der f. using a chemiluminescent immunoassay–based microarray, we detected the specific IgE levels of 32 Der p components and 36 Der f components in the serum of AD patients. By screening the positivity rate of allergic populations through CRD, analyzing the major components of allergens in the population, we validated the effectiveness of this chip, providing a more convenient, efficient, and precise measurement chip for clinical testing.
2. Materials and Methods
2.1. Serum
Samples from AD patients were provided by Huishan District People’s Hospital of Wuxi City, Xishan People’s Hospital of Wuxi City, and People’s Hospital of Wuxi City. Diagnosis of AD complied with the diagnostic criteria established by Xu et al., including basic features such as dry skin, eczematous skin lesions, and intense pruritus [11]. Among the 55 patient samples, there were 29 males and 26 females, with ages ranging from 3 to 60 years (mean age: 21.44 ± 18.59 years). Total IgE levels for Der p and Der f, as measured by Phadia, were as follows: Der p total IgE = 13.49 ± 24.57 IU/mL, Der f total IgE = 19.14 ± 31.60 IU/mL. A total of 54 samples tested positive and 1 sample tested negative. This study was approved by the hospital’s ethics committee [HYLL20220901001].
2.2. Chip
Recombinant proteins provided by our research group were used to prepare specific IgE chips for Der p and Der f allergen components, synthesized by Jiangsu Sanlian. The preparation process is as follows.
2.2.1. Reagents and Solutions
Restriction enzymes XhoI, NotI, and AvrII (NEB, R0146M, R0189M, R0174L); Zeocin (Invitrogen™, R25001); SMD1168 yeast strain (Invitrogen™, C17500); Yeast expression vector pGAPZα A (Invitrogen™, V20520); Anti–His-tag mAb (MBL, D291-3); Horseradish peroxidase (HRP)–conjugated goat antimouse IgG (H + L) (Beyotime, A0216); Alexa Fluor® 555-conjugated antimouse IgG antibody (CST, 4409S); Alexa Fluor® 488-conjugated antihuman IgE antibody (Novus, DDXCH07A488-100); Protein spot solution (1 part PBS, 30 parts glycerol, 0.05 parts Triton X-100) (prepared in the laboratory); Bovine serum albumin (Sigma, SRE0096); Mouse IgG (Proteintech, B900620); Human IgE (Abcam, ab229797).
2.2.2. Synthesis of Der p and Der f Allergen Protein Gene Sequences
For the 32 Der p allergen components: Der p 1, Der p 2, Der p 3, Der p 4, Der p 5, Der p 6, Der p 7, Der p 8, Der p 9, Der p 10, Der p 11, Der p 13, Der p 14, Der p 15, Der p 18, Der p 20, Der p 21, Der p 23, Der p 24, Der p 25, Der p 26, Der p 28, Der p 29, Der p 30, Der p 31, Der p 32, Der p 33, Der p 36, Der p 37, Der p 38, Der p 39, and Der p 40. The coding region reference sequences were obtained from the GenBank database. These sequences were optimized for expression in yeast and Escherichia coli, with XhoI and NotI restriction enzyme sites added at the 5′ and 3′ ends. Each sequence was then inserted into the pUC57-Amp plasmid (GENEWIZ).
For the 36 Der f allergen components: Der f 1, Der f 2, Der f 3, Der f 4, Der f 5, Der f 6, Der f 7, Der f 8, Der f 10, Der f 11, Der f 13, Der f 14, Der f 15, Der f 16, Der f 18, Der f 20, Der f 21, Der f 22, Der f 23, Der f 24, Der f 25, Der f 26, Der f 27, Der f 28, Der f 29, Der f 30, Der f 31, Der f 32, Der f 33, Der f 34, Der f 35, Der f 36, Der f 37, Der f 38, Der f 39, and Der f 40. The coding region reference sequences were obtained from the GenBank database. These sequences were optimized for expression in yeast and Escherichia coli, with XhoI and NotI restriction enzyme sites added at the 5′ and 3′ ends. Each sequence was then inserted into the pUC57-Amp plasmid (GENEWIZ).
2.2.3. Detection of Clinical Samples
The specific IgE content for the 32 Der p and 36 Der f components in the serum was detected using a chemiluminescent immunoassay-based microarray (indirect method). The detection instrument used was the SLXP-001 fully automatic biological chip reader (Jiangsu Sanlian Biological Engineering Co., Ltd.). The specific steps were as follows: (1) Add 70 μL of serum to the microarray cup, diluted 1:2, and incubate at 30°C for 20 min. The total volume of the sample solution added was 140 μL. (2) After incubation, remove the microarray and wash it, then incubate the microarray in a solution containing HRP–conjugated antihuman IgE antibody at 30°C for 10 min (3) After incubation, remove the microarray and wash it, then place it in a luminol substrate solution, and read the grayscale values using a charge coupled device (CCD) camera. Image analysis and result interpretation were performed using biological chip image and data analysis software.
2.2.4. Performance Analysis of the Chip Detection
Using the Phadia detection results as the standard, the positive agreement rate of the protein chip was calculated as the number of cases correctly identified as positive by the protein chip method (i.e., consistent with the Phadia results) divided by the total number of positive cases detected by Phadia. The negative agreement rate was calculated as the number of cases correctly identified as negative by the protein chip method (i.e., consistent with the Phadia results) divided by the total number of negative cases detected by Phadia. (The cutoff value for chip values was set at 250).
2.3. Statistical Analysis
Original scanning data were exported from the SLXP-001 fully automatic biological chip reader, and the grayscale values of the 32/36 recombinant proteins in 55 serum samples were calculated. Results were expressed as mean ± standard deviation (x ± s).
3. Results
3.1. Positive Rates of Dust Mite Allergen Component-Specific IgE
The signal values of 32 and 36 recombinant antigens were detected in 55 samples using the Der p and Der f protein chips, respectively. The serum positivity rates for these two mite recombinant antigens were calculated. See Figures 1 and Figure 2.
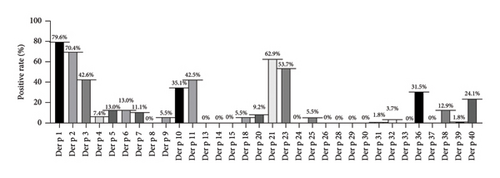
From Figure 1, it can be observed that the positivity rates of major allergens (> 40%) in Der p were as follows: Der p 1 (79.6%), Der p 2 (70.4%), Der p 21 (62.9%), Der p 23 (53.7%), Der p 3 (42.6%), and Der p 11 (42.5%); the positivity rates of moderate allergens (20%∼40%) were Der p 10 (35.1%), Der p 36 (31.5%), and Der p 40 (24.1%), while the rest were minor allergens. (Der f, allergens from Df; Der p, allergens from Dp).
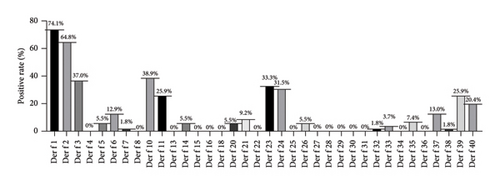
From Figure 2, it can be observed that the positivity rates of major allergens (> 40%) in Der f were as follows: Der f 1 (74.1%), Der f 2 (64.8%); the positivity rates of moderate allergens (20%∼40%) were Der f 10 (38.9%), Der f 3 (37%), Der f 23 (33.3%), Der f 24 (31.5%), Der f 11 (25.9%), and Der f 39 (25.9%), while the rest were minor allergens.
3.2. Cross-Reactivity Rates of Dust Mite Allergen Components
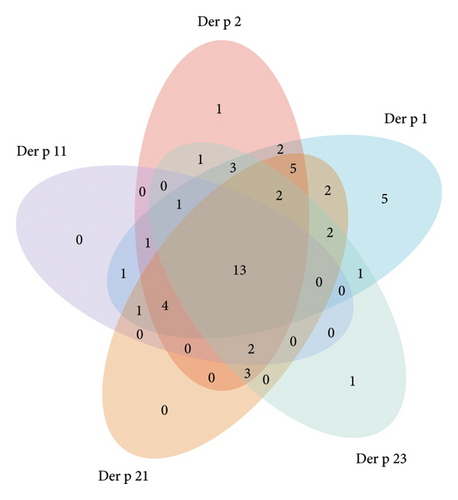
Further analysis was conducted on the five recombinant allergens with high positivity rates in Der p and Der f. The specific and shared positive cases were visualized. The results showed that 22.2% (12 cases) of patients exhibited positive reactions to all five allergens (Der p 1, Der p 2, Der p 11, Der p 21, and Der p 23), increasing susceptibility to allergic diseases. Additionally, 57.4% (31 cases) of patients exhibited simultaneous allergic reactions to Der p 1 and Der p 2, while 35.2% (19 cases) of patients exhibited allergic reactions to the three most common IgE-positive allergens: Der p 1, Der p 2, and Der p 21 (Figure 3).
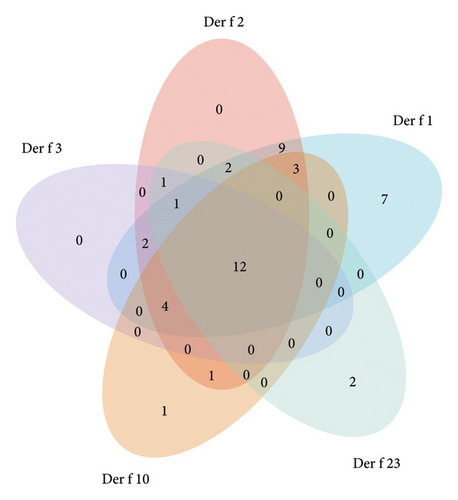
In the analysis of Der f results, 24.1% (13 cases) of patients exhibited positive reactions to all five allergens (Der f 1, Der f 2, Der f 3, Der f 10, and Der f 23), increasing susceptibility to allergic diseases. Additionally, 61.1% (33 cases) of patients exhibited simultaneous allergic reactions to Der f 1 and Der f 2, while 35.2% (19 cases) of patients exhibited allergic reactions to the three most common IgE-positive allergens: Der f 1, Der f 2, and Der f 3 (Figure 4).
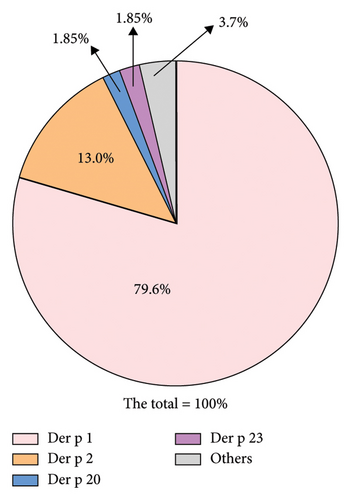
In addition to the population allergic to the three main allergens (Der p 1, Der p 2, and Der p 23), excluding duplicate data caused by cross-reactivity, among those not allergic to Der p 1, 13% of the population exhibited allergies to Der p 2, with the remaining 1.85% exhibiting allergies to Der p 20 and 23, respectively. Statistically, the sum of the positive rates for these four allergens approached 96.3% (Figure 5).
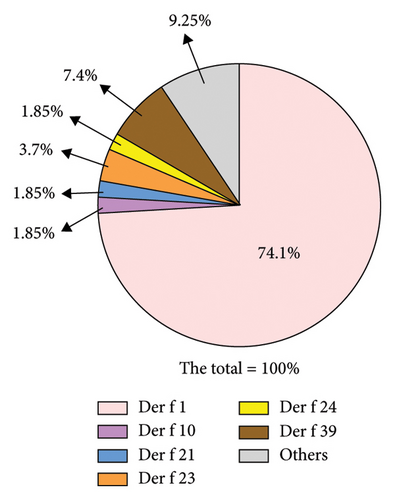
In patients diagnosed with Der f sensitization, the cases of positivity for Der f 1 and Der f 2 almost completely overlap. Among patients who are not allergic to Der f 1, 7.4% exhibit sensitization to Der f 39, while 3.7% are allergic to Der f 23. The proportions of patients allergic to Der f 10, Der f 21, and Der f 24 are all 1.85%. Statistically, the combined positivity rate of these 6 components—Der f 1, Der f 10, Der f 21, Der f 23, Der f 24, and Der f 39—covers 91.75% of positive patients (Figure 6).
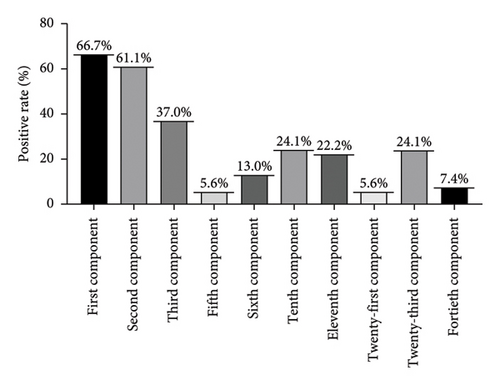
As two predominant mite species, the percentage of cross-reactivity rates for various components in Der p and Der f are shown in Figure 7 and Table 1. The highest positivity rate is observed in the first component (Der p 1, Der f 1) at 66.7%, followed by the second component (Der p 2, Der f 2) at 61.1%. The third component (Der p 3, Der f 3) accounts for 37.0% of positivity, while the tenth (Der p 10, Der f 10) and twenty-third (Der p2 3 and Der f2 3) components each account for 24.1%. The positivity rate for the eleventh (Der p 11, Der f 11) component is 22.2%.
| Number of allergen | Positive (n) | Positive rate (%) |
|---|---|---|
| 1 | 5 | 9.30 |
| 2 | 4 | 7.40 |
| 3 | 8 | 14.80 |
| ≥4 | 35 | 64.80 |
The distribution of allergen types in AD patients shows that among 54 patients with positive specific IgE antibodies, the sensitivity to one allergen is observed in 5 and 9 cases (9.3% and 16.7%), respectively, while sensitivity to two allergens simultaneously is observed in 4 and 3 cases (7.4% and 5.6%). Sensitivity to three allergens is observed in 8 and 11 cases (14.8% and 20.4%), and sensitivity to four or more allergens is observed in 35 and 26 cases (64.8% and 48.1%), respectively (Tables 1 and 2).
| Number of allergen | Positive (n) | Positive rate (%) |
|---|---|---|
| 1 | 9 | 16.70 |
| 2 | 3 | 5.60 |
| 3 | 11 | 20.40 |
| ≥4 | 26 | 48.10 |
4. Discussion
Through the development of a comprehensive and easily detectable protein microarray for specific IgE against Der p and Der f, we amalgamated the sensitivity of ELISA allergen detection with the advantages of microarray technology. This facilitated precise diagnosis of sensitivities to allergen species in positive sera, enabling a one-time screening of patients’ susceptibility to dozens of specific allergens. Multiarray allergen technology can differentiate more information on specific patter of sensitization from various cross-sensitivities and provide meaningful recommendations to avoid dietary and immunotherapeutic interventions [12].
Prior studies have indicated that serum IgE in HDM sensitization patients shows significantly elevated levels against Der p 1, 2, and 10 components [13]. Analysis of population data from various regions reveals notable differences in the positivity rates of IgE against these three allergens due to geographical, age-related, and clinical factors: Der p 1 (64%–100%), Der p 2 (62%–91%), and Der p 10 (6%–59%) [14, 15]. Research has also suggested that inhalation of Der p 1 can induce eczema, indicating that skin contact may not be the sole pathway for dermatitis induction [16]. Active reduction of Der p 1 levels in beds and carpets significantly reduces the severity of AD in children and adults with moderate to severe symptoms [17].
In the serum of eczema patients in this study, high positivity rates were observed for Der p 1 (79.6%), Der p 2 (70.4%), Der p 3 (42.6%), Der p 10 (35.2%), Der p 11 (42.6%), Der p 21 (63.0%), and Der p 23 (53.7%). These findings are akin to the primary allergens identified in a clinical study of 722 cases in Germany [18]. Among patients with allergic rhinitis, the prevalence rates were 61.3% for Der p 1, 77.0% for Der p 2, and 50.8% for Der p 23. Der p 4, p 5, p 7, and p 21 were considered moderate allergens (with prevalence rates ranging from 20% to 40%), whereas our moderate allergens mainly included Der p 10, Der p 11, and Der p 21, with Der p 21 being particularly prominent among the primary allergens for our population.
The research indicates that among AD patients, the highest positivity rate for inhaled allergens in the inhalation group is dust mites [19]. In our experiment, the positivity rates for dust mite components were as follows: Der f 1 (74.01%), Der f 2 (64.8%), Der f 10 (38.9%), Der f 3 (37.0%), Der f 23 (33.3%), and Der f 24 (31.5%). It’s worth noting that the previous study utilized crude extracts, whereas we employed specific IgE detection. Hence, we hypothesize that specific IgE detection could reduce the likelihood of misdiagnosis or underdiagnosis. In a separate study aimed at determining the sensitization rates of allergic children to Der p 1, Der p 2, and Der p 10 components and investigating their clinical phenotypes, results showed a sensitization rate of 98.8% for Der p, 100% for Der f, 82.5% for Der p 1, 78.8% for Der p 2, and 8.8% for Der p 10. Additionally, Der p 10-sensitized patients exhibited a significant positive correlation with IgE levels against crab and shrimp, while total IgE levels showed significant positive correlations with Der p, Der f, and their components [20]. Further exploration of the positivity rates of various components in the combination of HDMs and dust mites revealed that the first component accounted for 66.7%, the second component for 61.1%, the third component for 37.0%, and the 10th and twenty-third components jointly for 24.1%, ranking fourth. The 11th component also represented a significant advantage at 22.2%.
Allergic diseases are prone to recurrent episodes, and over time, exposure to allergens may lead to sensitization to other allergens, a phenomenon known as cross-reactivity [21]. In the diagnosis of Der p sensitization, we found that 64.8% of the population tested positive for four or more allergens. The combined positivity rate for Der p 1, Der p 2, Der p 20, and Der p 23 approached 96.3%. Therefore, using a chip prepared with these four allergens can cover the majority of Der p-positive individuals, thereby saving on chip production costs to some extent. Similarly, in the diagnosis of Der f sensitization, where there are cross-reactive components, the proportion of individuals testing positive for four or more allergens reached 48.1%. Using crude extracts can confound the diagnosis of specific allergens. Our experiments showed that the combined positivity rate for Der f 1, Der f 10, Der f 21, Der f 23, Der f 24, and Der f 39 reached 91.75%. Thus, a combination chip incorporating these six allergens can be used for preliminary screening of patients’ sensitization to predominant allergens of Der f.
CCDs in plants and insect venoms are a common cause of irrelevant positive test results during in vitro allergy diagnosis. With the increase in molecular allergology and the introduction of recombinant allergens for CRD, the CCD problem is often believed to have been partly dissolved [22], and till now this irrelevant positive test have not been reported in dominant mite species.
In summary, the majority of AD patients exhibit positive reactions to specific IgE against dominant mite species—Der p and Der f. Developing chips based on CRD [23] not only aids in the precise diagnosis of patients but also enables personalized desensitization therapy selection. It provides more preventive measures against allergic reactions for sensitized patients by avoiding allergen exposure environments or consumption of allergenic foods. Identify selective allergens that elicit production of specific IgE. Future study will be needed to figure out the clinical implications of this technology as.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zixing Cui and Ling Fang designed the experiments. Min Sun and Juan Liu performed the experiments. Zixing Cui analyzed the data and wrote the manuscript. Ling Fang and Hua Guo revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by Scientific Research Program of Wuxi Health Commission (Grant M202244), top Talent Support Program for Young and Middle-Aged People of Wuxi Health Committee (Grant HB2023115), and Youth Project of National Natural Science Foundation of China (Grant 82302562).
Acknowledgments
This work was supported by Scientific Research Program of Wuxi Health Commission (Grant M202244), Top Talent Support Program for Young and Middle-Aged People of Wuxi Health Committee (Grant HB2023115), and Youth Project of National Natural Science Foundation of China (Grant 82302562). We sincerely thank all patients with atopic dermatitis and control subjects for their participation in this study.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.