Antiemetic Efficacy and Safety of Palonosetron on Days 1 and 5 with Aprepitant and Dexamethasone during Bleomycin, Etoposide, and Cisplatin Chemotherapy in Patients with Germ Cell Tumor: A Prospective Study
Abstract
Objective. BEP (bleomycin, etoposide, and cisplatin) chemotherapy is a standard regimen for germ cell tumors (GCTs); however, their high emetogenicity is problematic. The control of chemotherapy-induced nausea and vomiting (CINV) is crucial to complete the treatment. We conducted this study to explore the efficacy and safety of antiemetic therapy with dexamethasone and aprepitant for 5 days in combination with palonosetron 0.75 mg on days 1 and 5 in BEP. Methods. This open-label single-arm study was prospectively conducted in 4 hospitals. Chemotherapy-naïve men with GCT who were scheduled to receive the BEP regimen were eligible. The primary endpoint was the complete response (CR) rate of CINV. Results. A total of 19 patients were enrolled. Overall, 16 (84.2%) patients experienced some nausea, whereas only 4 (21.1%) patients had grade 1 emetic events. Overall CR of CINV was achieved in 9 (47.4%) patients. Although 14 (73.7%) patients experienced 22 adverse events after palonosetron administration, severe adverse events (grade 3 or more) attributable to it did not occur. Conclusion. The results suggest that aprepitant, palonosetron, and dexamethasone antiemetic therapy for patients with GCT receiving BEP is safe, whereas the efficacy of additional palonosetron administration on day 5 for prevention of delayed CINV remains unclear. This trial is registered with UMIN000008110.
1. Introduction
Although malignant germ cell tumors (GCTs) are rare, chemotherapy is highly effective and has the potential to cure even metastatic disease. BEP (bleomycin, etoposide, and cisplatin) is a standard regimen for GCTs; however, cisplatin, a key drug, is categorized as having a high emetogenic risk [1] and is given for 5 consecutive days. Chemotherapy-induced nausea and vomiting (CINV) is one of the significant adverse events associated with anticancer chemotherapy and the control of CINV is crucial to complete the treatment.
Currently, a three-drug regimen for prophylactic CINV containing aprepitant in addition to a 5-hydroxytryptamine-3 receptor antagonist (5HT3-RA) and dexamethasone is recommended [1]. Palonosetron is a second-generation 5HT3-RA and is a long-acting agent. Before the aprepitant era, several studies showed that palonosetron was superior to granisetron, which is in the first generation in terms of controlling delayed CINV [2]. However, there is no definitive evidence about the difference in efficacy between the two generations for highly emetogenic chemotherapy. Suzuki et al. reported results of the TRIPLE study, which compared 2 generations of 5HT3-RAs in a 3-drug regimen for prophylactic CINV [3]. Although they found no significant differences between the 2 generations of 5HT3-RAs in terms of the complete response of CINV as a primary endpoint, the second-generation 5HT3-RA was significantly superior for controlling delayed CINV as a secondary endpoint.
Because BEP includes administration of cisplatin for 5 consecutive days, acute and delayed CINV can overlap. Although the three-drug antiemetic regimen is recommended for multiday chemotherapy in the NCCN guidelines for antiemesis [1], they mention that it is difficult to determine the specific regimen for each day. Indeed, there are few reports and a lack of evidence on antiemetic therapy for cisplatin-based chemotherapy for GCTs. Einhorn et al. [4] and Adra et al. [5] reported trials on antiemetic therapy when administering multiday chemotherapy for GCTs with administration of palonosetron three times (0.25 mg IV on days 1, 3, and 5). Their acute and delayed complete response (CR) rates were 34% and 61% and 29.6% and 46.3%, respectively. It seemed that the CR rates were insufficient in both studies. On the other hand, Hamada et al. [6] and Ioroi et al. [7] reported overall CR rates of 90.0% and 62.5%, respectively, with palonosetron 0.75 mg administration on day 1. Although these CR rates were relatively favorable, there was a considerable difference between the two trials. Therefore, the best regimen is still unknown.
In 2012, when we planned this study, we hypothesized that multiple doses of palonosetron would be more effective than the previous antiemetic regimen containing second-generation 5HT3-RA antagonists for prevention of delayed CINV. On the other hand, there are concerns about drug accumulation due to repeated palonosetron administration. However, according to a simulation study of the human plasma concentration, repeated administration of palonosetron is considered acceptable because the maximum concentration and the area under the concentration time curve after the administration on days 1 and 5 were 1.09 times and 1.11 times as high as those after a single administration on day 1, respectively [8].
Therefore, we conducted the APDATE (aprepitant, palonosetron, and dexamethasone antiemetic therapy) study to explore the efficacy and safety of antiemetic therapy with dexamethasone and aprepitant for 5 days in combination with palonosetron on days 1 and 5.
2. Methods
2.1. Study Design
This open-label single-arm study was prospectively conducted in multiple institutions (4 hospitals) belonging to the Sapporo Medical University Urologic Oncology Consortium.
Chemotherapy-naïve men aged 20 years or more with GCT who were scheduled to receive the BEP regimen and with ECOG performance status 0–2 were eligible. Men who had brain metastasis or nausea/vomiting of more than grade 1 in the Common Terminology Criteria for Adverse Events (CTCAE) were excluded.
In the BEP regimen (repeated after 21 days), the following were administrated: cisplatin 20 mg/m2 intravenously (IV) on days 1–5, etoposide 100 mg/m2 IV on days 1–5, and bleomycin 30 mg/body IV on days 1, 8, and 15. The antiemetic agent regimen was as follows: palonosetron 0.75 mg IV on days 1 and 5, dexamethasone 9.9 mg IV on days 1–5 and 8.0 mg per os (P.O.) once daily on days 6–8, and aprepitant 125 mg on day 1 and 80 mg on days 2–5 P.O. once daily.
Rescue therapy: the use of drugs other than palonosetron, dexamethasone, and aprepitant was permitted for nausea/vomiting when patients needed it.
Patients wrote a diary from BEP day 0 to BEP day 10. The diary contained 6 queries including the Multinational Association of Supportive Care in Cancer Antiemesis Tool (MASCC Antiemesis Tool: MAT) [9] as follows: Q1, in the 24 hours since chemotherapy, did you have any nausea? (yes/no); Q2, if you had nausea, please circle or enter the number that most closely resembles your experience (0 (none)–10 (as much as possible)); Q3, in the 24 hours since chemotherapy, did you have any vomiting? (yes/no); Q4, if you vomited, how many times did it happen? (No.); Q5, the amount of food intake (normal, decreasing, little); Q6, the actual amount of food intake (0–100%) and the contents of supplementary food (if any).
We evaluated the efficacy and safety of the antiemetic regimen in this study from BEP day 1 to day 10. The primary endpoint was the CR rate of CINV. The secondary endpoints were the complete control (CC) rate of CINV, the frequency of rescue, the amount of food intake, and incidence of adverse events.
We obtained written informed consent from all patients. This study was approved by the Institutional Review Board for Clinical Research at Sapporo Medical University in May 2012 (#24-4) and the ethical review board at each hospital. The study was carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. This study was registered with the University hospital Medical Information Network Clinical Trials Registry in Japan (UMIN000008110).
2.2. Assessment
We evaluated nausea/vomiting events using the MAT. The CR rate is calculated by (the cases without vomiting or using rescue) divided by (the cases for analysis). The CC rate is calculated by (those who did not show any vomit or use rescue drugs or did not have any nausea or only had a little) divided by (the cases for analysis). We evaluated the CR rates and CC rates in the overall time (days 1–10), acute phase (days 1–5), and delayed phase (days 6–10). We evaluated the adverse effects using the CTCAE v4.0.
Apart from the CTCAE, the degree of nausea was evaluated by classifying it into three grades by the numerical rating scale of diary Q2 (mild, 1–3 points; moderate, 4–6 points; severe, 7–10 points).
We could also evaluate the amount of food intake for each meal by using the self-written diary. Anorexia was evaluated by the answer to the diary Q5 and the amount of food intake. If the patients answered “decreasing” or “little” to Q5, the men who ate half or more were classified as having grade 1 anorexia and the others were classified as having grade 2. Anorexia needing intravenous infusion was classified into grade 3.
3. Results
3.1. Patients
Totally, 22 patients were recruited from May 2012 through March 2020 from 4 hospitals. Finally, 19 patients were eligible because 3 were excluded from this study due to protocol violations (the diary was not written by 1, 1 took prohibited medicine before entry, and 1 was administered prohibited medicine).
Table 1 shows the patients’ characteristics. Although germ cell tumor generally tends to develop in young men, the median age seemed to be relatively high in this study. Table 2 shows the incidence of CINV events and their grades. Sixteen (84.2%) patients experienced some nausea, whereas vomiting was observed only in 4 patients (21.1%). Of the 4 patients having a total of 6 emetic events, 2 vomited only once (both on day 6), 1 did so twice on day 6, and the remaining 1 did so once daily on days 7 and 8 during the study period (Supplement 1). All of their CTCAE grades were grade 1.
| Age (years), median (range) | 37 (22–53) |
| Disease, n (%) | |
| Testicular cancer | 18 (94.7%) |
| Extragonadal germ cell tumor | 1 (5.3%) |
| Histopathology, n (%) | |
| Seminoma | 8 (42.1%) |
| Nonseminoma | 11 (57.9%) |
| IGCCC, n (%) | |
| Good prognosis | 14 (73.7%) |
| Intermediate prognosis | 5 (26.3%) |
| Alcohol consumption, n (%) | |
| Present | 11 (57.9%) |
| None | 6 (31.6%) |
| Unknown | 2 (10.5%) |
| Smoking history, n (%) | |
| Smoking | 12 (76.4%) |
| Exsmoker | 2 (15.0%) |
| None | 5 (8.6%) |
- IGCCC, International Germ Cell Consensus Classification.
| Nausea CTCAE grade, n (%) | |
| Overall | 16 (84.2%) |
| G1 | 6 (31.6%) |
| G2 | 7 (36.8%) |
| G3 | 3 (15.8%) |
| Vomiting CTCAE grade, n (%) | |
| G1 | 4 (21.1%) |
| Using rescue therapy | 9 (47.4%) |
- G, grade.
Rescue therapy was needed in nearly half of the cases. Of them, metoclopramide was the only administered drug in all except for one administered olanzapine and oral 5HT3-RA.
3.1.1. Primary and Secondary Endpoints
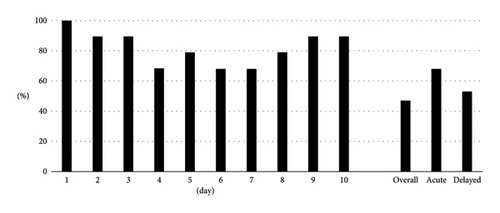
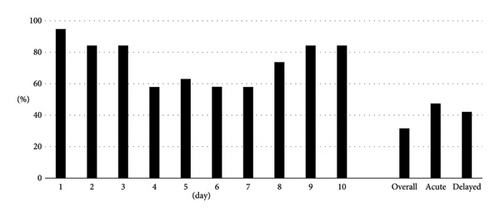
Overall, CR and CC were achieved in 9 (47.4%) and 6 (31.6%) patients, respectively. The CR rates in the acute and delayed phases were 68.4% and 52.6%, respectively. The CC rates in the acute and delayed phases were 47.4% and 42.1%, respectively. We also demonstrated CR rates (Figure 1) and CC rates (Figure 2) by date as additional information.
3.2. Incidence and Severity of Nausea
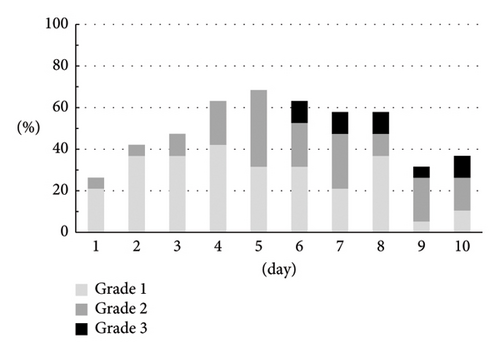
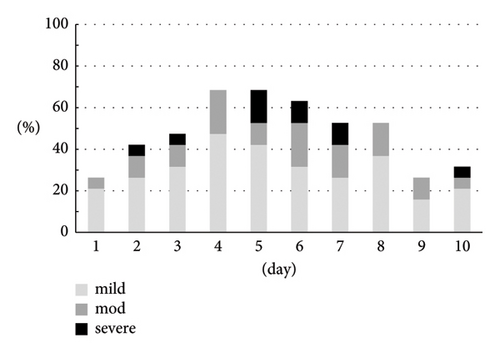
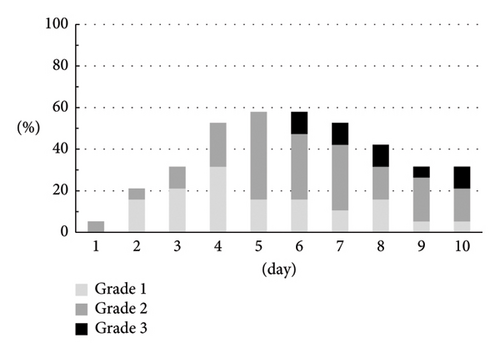
The CTCAE grade and the severity classification by the numerical rate scaling of nausea are shown in Figures 3(a) and 3(b). Both assessments indicated similar trends. There were 8 (42.1%) patients who experienced grade 2 (5 men) or grade 3 (3 men) and moderate (3 men) or severe (5 men) nausea.
3.3. Anorexia
The patients complaining of anorexia gradually increased until day 5 (Figure 3(c)). In spite of finishing cisplatin administration, anorexia was sustained and 3 patients (15.8%) needed intravenous injection on day 6 or later.
3.4. Adverse Events
Table 3 summarizes the adverse events after palonosetron administration and grades in this study. Although 14 (73.7%) patients experienced 22 adverse events, severe adverse events (grade 3 or more) attributable to palonosetron did not occur.
| Overall | G1 | G2 | G3–5 | |
|---|---|---|---|---|
| Constipation | 11 (57.9%) | 7 (36.8%) | 4 (21.1%) | 0 (0.0%) |
| Hiccups | 6 (31.6%) | 2 (10.5%) | 4 (21.1%) | 0 (0.0%) |
| Diarrhea | 3 (15.8%) | 3 (15.8%) | 0 (0.0%) | 0 (0.0%) |
| Hypertension | 1 (5.3%) | 0 (0.0%) | 1 (5.3%) | 0 (0.0%) |
| Headache | 1 (5.3%) | 1 (5.3%) | 0 (0.0%) | 0 (0.0%) |
- G, grade.
4. Discussion
In general, it is considered that there is no significant difference in the antiemetic effect among 5HT3-RAs. Although they are effective for early CINV, there is no definitive evidence on the antiemetic effect in the delayed phase. Suzuki et al. demonstrated a better antiemetic effect on delayed CINV for palonosetron than for granisetron [3]. However, that result was not the primary endpoint. Because BEP chemotherapy is a multiday emetogenic anticancer agent regimen, we conducted this study with the expectation of a better antiemetic effect with additional palonosetron on day 5.
The primary endpoint was the CR rate and it was 47.4% in this study. From the reports of cisplatin-based chemotherapy for GCTs with triple antiemetic agents, Hamada et al. [6] reported a 90% CR rate in the first course of chemotherapy, Ioroi et al. [7] reported 62.5%, Shimura et al. [10] reported 50%, Albany et al. [11] reported 42%, Olver et al. [12] reported 41%, and Adra et al. [5] reported 24%. The CR rate in Hamada’s study was higher than those in the others. Although the actual reason is unknown, the small number of participants in the studies might have affected the results. On the other hand, a retrospective comparative study by Shimura et al. adopted a protocol similar to that in our study, including administration of palonosetron 0.75 mg on day 1 and day 5 of BEP chemotherapy. Interestingly, our results were comparable to those of their study. Overall, four studies on Japanese men administered palonosetron 0.75 mg on day 1 of BEP chemotherapy demonstrated higher acute CR rates than a study using a different dosage and schedule for the palonosetron regimen (0.25 mg IV on days 1, 3, and 5) or other 5HT3-RAs, suggesting that palonosetron 0.75 mg on day 1 provides a better effect for prevention of CINV in BEP. However, further studies are needed to clarify the superiority of the administration method. In the present study, the CR rate in the delayed phase was low as compared to the early phase, which is discordant with other reports except for Shimura’s. However, the CR rates in the delayed phase were comparable among these studies, suggesting that this trend may reflect the higher CR rate in the acute phase in the two studies.
This study was conducted with the expectation that the overall CR rate would improve. However, despite good CR rates in the acute phase, all emetic events occurred after day 6 in this study, so the efficacy of additional palonosetron 0.75 mg on day 5 of BEP chemotherapy was equivocal. Of course, we could not reach a conclusion because of the lack of controls. Moreover, although the antiemetic regimen for BEP chemotherapy in this study adequately controlled emesis, it seemed that the efficacy against nausea was insufficient. Einhorn et al. investigated the correlation between nausea and the quality of life (QOL) and concluded that nausea itself did not always interfere with the QOL in patients with BEP chemotherapy [4]. Therefore, the completion of BEP chemotherapy may require better control of emesis rather than nausea.
We evaluated nausea by the CTCAE grade and a numerical rating scale in the present study. Although the former was determined by objective data like food intake and intravenous injection needs and the latter by subjective data, both showed similar trends. Similarly, the nausea grade determined using the CTCAE and anorexia grade were almost the same because both were determined on the basis of food intake and intravenous injection. These findings suggest that these two evaluation tools may not always be necessary simultaneously, but the diary on food intake was effective for adding objective information on the nausea grade of the CTCAE.
Although we administered palonosetron 0.75 mg on day 1 and day 5, adverse effects were mild and tolerable. There were no adverse effects of grade 3 or more, although constipation was the most frequent one. It was likely to be induced by palonosetron but we could not determine the extent of the increase because there were no controls.
This study has some limitations. First, the number of patients in this study was small. It was difficult to collect more patients due to the low prevalence of the disease, although we extended the period of this study to 5 years. Second, this study had a single arm. Therefore, we could not compare the efficacy and safety between this antiemetic regimen and the conventional one. However, it is difficult to divide patients with GCT into two treatment arms. Shimura et al. reported and evaluated the efficacy of antiemetic therapy with two arms, but there were only nine patients in each group. On the other hand, the prospective setting was a strong point in this study. There were previously only two prospective studies of a three-drug antiemetic regimen using palonosetron for patients with GCT treated by BEP in Japan. In addition, to the best of our knowledge, there were no prospective studies using palonosetron 0.75 mg administration on BEP days 1 and 5 anywhere in the world.
5. Conclusions
This study showed the efficacy and safety of palonosetron 0.75 mg administration twice for patients with GCT treated by BEP chemotherapy. Although the safety of palonosetron 0.75 mg administration on day 1 and day 5 was sufficient because there were no severe adverse events attributable to it, the efficacy of additional palonosetron administration on BEP day 5 remains to be investigated. Although this study included a small number of cases, it is important to demonstrate an antiemetic therapy option in multiday chemotherapy for germ cell tumor because the guidelines indicate that multiday chemotherapy antiemetic therapy should be considered on an individual regimen basis. In addition, in BEP chemotherapy, there are almost no reports of antiemetic regimens in which 0.75 mg of palonosetron was administered twice in one course. We hope that the list of antiemetic therapies for BEP chemotherapy summarized in this study will be helpful in future research planning.
Ethical Approval
This study was approved by the Institutional Review Board for Clinical Research at Sapporo Medical University in May 2012 (#24-4) and the ethical review board at each hospital. The study was carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. This study was registered with the University hospital Medical Information Network Clinical Trials Registry in Japan (UMIN000008110).
Consent
Written informed consent was obtained from all individual participants included in the study.
Disclosure
This manuscript was submitted as a preprint in the link https://www.researchsquare.com/article/rs-1715703/v1.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
F.F., T.S., and T.T. drafted the original manuscript and made the figures. F.F. and T.S. made the tables. H.K. conceived the idea of the study and designed it. F.F., T.S., T.T., H.H., N.I., M.O., Y.K., K.H., and K.K. were responsible for the acquisition and analysis of data. N.M. supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors have approved the final version of the manuscript to be published.
Acknowledgments
We thank Kim Barrymore for editing a draft of this manuscript.
Open Research
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.




